Abstract
Objectives
Abnormalities of bone metabolism could be followed in gastrectomized patients as a late complication. Nowadays, many biochemical and radiologic measurements are applied to detect these abnormalities. The aim of our study is to determine the valuable parameter as an appropriate screening test during long-term follow-up periods and define the usefulness of new biochemical markers for bone metabolism by comparing with traditional markers.
Methods
Fifteen patients who had undergone partial gastrecomy were chosen randomly and fifteen healthy controls were compared. Then, several biochemical and radiologic tests were measured. We excluded subjects who proved to have other causes of bone metabolism abnormalities. Ten patients and 10 controls were finally selected.
Results
Comparing the data with those of a corresponding control group, the lumbar bone density measured by quantitative computed tomography (QCT) was statistically significantly lower in the patient group (p<0.01) The urinary deoxypyridinoline, a biochemical marker for bone resorption, was statistically higher in the patient group (p <0.025). Osteocalcin, Procollagen I C-terminal peptide (PICP) and Type I collagen C-terminal telopeptide (ICTP) were slightly but not significantly higher in the patient group. The serum parathyroid hormone (PTH) and 25-hydroxy vitamin D levels were similar in both groups.
Conclusion
We co suggest that urinary deoxypyridinoline and QCT are appropriate parameters as screening tests for the detection of bone metabolism abnormalities in gastrectomized patients during long-term follow-up. Urinary deoxypyridinoline may be a simple and rapid test which could replace cumbersome 24-hour urinary hydroxyproline.
Keywords: Gastrectomy, Bone metabolism, Bone density
INTRODUCTION
The development of metabolic bone disease that followed after gastrectomy has been reported many years ago, but the incidence of bone metabolism changes after gastrectomy has tended to have quite different results according to authors1–8). Furthermore, the mechanisms of these metabolic changes were not apparent. Either a vitamin D or a calcium deficiency or, perhaps, even both together, contributes to reduced bone mass9).
In the past, although numerous measurements have been used for quantitative methods of bone mass and biochemical markers of bone metabolism in patients with gastrectomy, great divergences were reported in the incidences and results of postgastrectomy bone disease2–8,13,14).
Therefore, we tried to determine what appropriate screening tests during long-term follow-up periods might be valuable for gastrectomized (subtotal gastrectomy) men, comparing with age and a sex-matched control group, and also to define the usefulness of new biochemical markers for bone metabolism by comparing with the old traditional markers.
Materials and Methods
1. Materials
We studied 15 male patients, who had undergone subtotal gastrectomy (with Billroth II anastomosis) because of peptic ulcers and gastric cancers without metastasis or local recurrence, by imaging study during follow-up periods. The patients were referred to our hospital outpatient department for a routine examination after informed consent. Fifteen healthy males with a similar age distribution served as a control group. All participants in the study had their history investigated, physical examination and 12-panel blood chemistry, including liver and renal function tests, after 12-hour fasting periods.
We excluded participants who had any other endocrine, malignant or metabolic bone disease or who had received any medication well known to influence bone metabolism or had abnormal liver or renal functions. Finally, 10 patients and 10 controls participated in our study. All participants had a normal nutritional status, lived at home, had work and led normal life styles, including average physical activity and sexual activity. Also, no one had a history of fracture in the past or present.
2. X-ray & Bone Chemistry
Plain lumber AP and lateral X-ray films were taken. The bone mineral density (BMD) of the lumber spine was measured by quantitative computed tomography (QCT) with the single energy technique (80 kVp) using Gadolinium enhanced computed tomography (GECT) 9800 highlights (software Imagix, University of California, San Francisco, U.S.A).
3. Biochemical markers for bone metabolism
Serum Procollagen I C-terminal propeptide (PICP) and Type I collagen C-terminal telopeptide (ICTP) levels were determined using a commercially available radioimmunoassay (RIA) kit (125I-labelled, Orion Diagnostica, Finland). The levels of intact parathyroid hormone (PTH) and osteocalcin were determined by a commercially available RIA kit (125I-labelled, Nichols, U.S.A.). The levels of 25-hydroxy vitamin D were determined by a RIA kit (3H-labelled, Nichols, U.S.A.). The spot urinary deoxypyridinoline (DPD) excretion was measured by a ELISA (Metra biosystems, U.S.A.) without limitation of diet.
4. Blood chemistry
Blood chemistry parameters, including liver and renal function tests, calcium, phosphorus and alkaline phosphatase were measured with routine laboratory technique (Hitachi 747 automatic analyzer).
5. Statistical analysis
The Student’s t-test was used for statistical evaluation. The data in the text and table are given as the mean± standard deviation (SD).
RESULTS
1. Subjects
The age of patients and controls were 49.3± 9.2 years old and 49.7± 7.7 years old respectively. The interval since the operation (subtotal gastrectomy) was 113± 23.7 months (78~151 months). The height was 168.4± 6.13 cm in the patient groups and 168.5± 6.79 cm in the control group. The weight of the two groups was 61.0± 9.07 kg and 68.7± 7.06 kg, respectively. The weight of the patient group was significantly lower than that of the control group (p<0.025). The body mass index (BMI) of patients and controls was 21.56± 3.44 kg/m2 and 24.18± 1.92 kg/m2 (Table 1).
Table 1.
Comparison of age, height, weight and BMI*
| Patient (n = 10) | Control (n = 10) | |
|---|---|---|
| Age (year) | 49. ± 9.2 | 49.7 ± 7.7 |
| Height (cm) | 168.4 ± 6.13 | 168.5 ± 6.79 |
| Weight (kg) | 61.0 ± 9.07** | 68.7 ± 7.06 |
| BMI (kg/m2) | 21.56 ± 3.44*** | 24.18 ± 1.92 |
Body mass index
p-value < 0.0025
p-value < 0.05
2. X-ray and Bone density measurement
There was no evidence of radiologic signs of osteopenia. Vertebral bone density measured by QCT was 107.81± 19.25 mg/cm3 K2HPO4 in patients and 131.27± 20.44 mg/cm3 K2HPO4 in controls. The BMD of gastrectomized patients was significantly lower than that of the controls (p<0.01) (Table 2)(Figure 1).
Table 2.
Biochemical data in gastrectomized patients
| Patient (n = 10) | Control (n = 10) | Normal value | |
|---|---|---|---|
| Calcium(mg/dl) | 8.78 ± 0.319 | 9.1 ± 0.386 | 8.5–11.0 |
| Phosphorus(mg/dl) | 3.12 ± 0.35 | 3.06 ± 0.46 | 2.5–4.8 |
| Alkaline phosphates(KA-u) | 5.4 ± 1.6 | 4.4 ± 1.2 | 3.0–10.0 |
| Intact PTH(pg/ml) | 20.26 ± 7.86 | 18.10 ± 7.97 | 10.0–65.0 |
| 25-hydroxyvitamin D(nm/dl) | 32.74 ± 12.25 | 29.56 ± 10.9 | 25.7–15.8 |
| Osteocalcin(ng/ml) | 4.17 ± 1.25 | 3.40 ± 0.78 | 2.3–13.8 |
| PICP(ug/1) | 128.72 ± 61.19 | 118.85 ± 29.7 | 38.0–202.0 |
| ICTP(ug/1) | 3.99 ± 1.68 | 3.24 ± 0.82 | 1.8–5.0 |
| DPD(nM DPD/mM creatnine) | 6.83 ± 3.19* | 4.45 ± 1.2 | 2.5–5.0 |
| BMD(mg/cm3K2HPO4) | 107.81 ± 19.25** | 131.27 ± 20.44 | Age matched |
p-value < 0.025
p-value < 0.01
Figure 1.
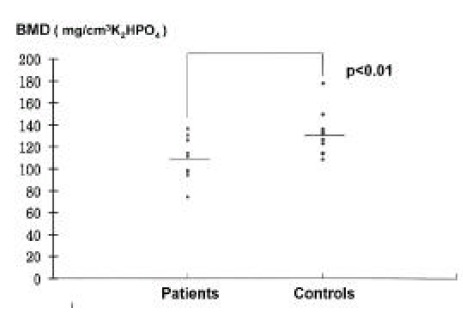
Bone mineral density in patients and controls. Horizontal bar; mean values.
3. Blood chemistry and Biochemical parameters of bone metabolism
No difference was found in the serum calcium and alkaline phosphatase levels between the groups (Table 2). The spot urinary DPD excretion were 6.83± 3.19 nM DPD/mM creatinine in patients and 4.45± 1.2 nM DPD/mM creatinine in controls. There was statistically significant increase in the patient group (p<0.025)(Figure 2). The serum osteocalcin and PICP levels were slightly but not significantly higher in the patient group (Figure 3, 4). The serum PTH and 25-hydroxy vitamin D levels were similar in both groups. There was no statistical difference in the serum ICTP levels in both groups although serum ICTP levels in patients were higher than in controls (Figure 5).
Figure 2.
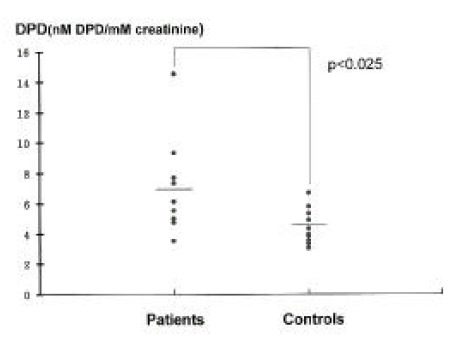
Urinary DPD in patients and controls. Horizontal bar; mean values
Figure 3.
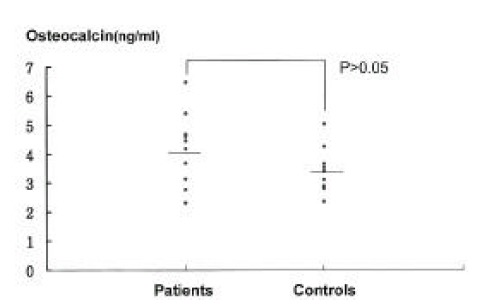
Serum Osteocalcin levels in patients and controls Horizontal bar; mean values.
Fig. 4.
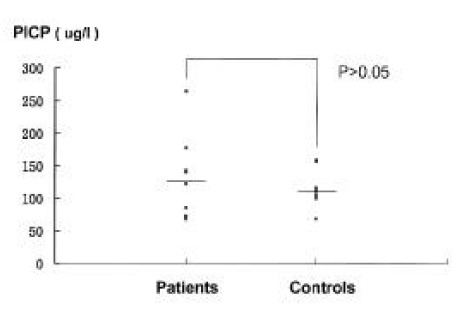
Serum PICP levels in patients and controls. Horizontal bar; mean values
Figure 5.
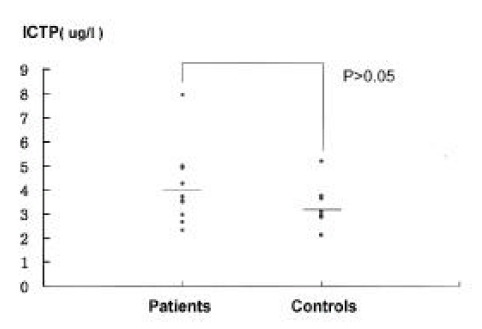
Serum ICTP levels in patients and controls. Horizontal bar; mean values.
DISCUSSION
Metabolic bone diseases have long been associated with gastric surgery. The incidence of metabolic bone disease after gastrectomy has been estimated about 1-42% even though there was great discrepancy between authors1–8). A reduced bone mass has been reported as occurring 5 or more years after gastrectomy7). In our study, the average interval since operation was 113± 23.7 months. Osteomalacia is most commonly reported, but osteoporosis or combination of osteomalacia and osteoporosis, and less well-characterized forms of osteopenia, have been described1,7,8).
In fact, the pathogenesis of osteoporosis has not been explained as satisfactorily as that of osteomalacia7). Osteoporosis is a generalized bone disorder, characterized by a decrease in the quantity of bone but no change in quality. It occurs most frequently in postmenopausal women and the aged. Osteomalacia is a failure of the organic matrix to mineralize normally. Therefore, osteoporosis was thought to be present if there were radiological abnormalities only, particularly if it was confined to the axial skeleton. If a biochemical disturbance existed, osteomalacia was diagnosed. In certain cases, these diagnoses were supported by bone biopsy.
Although the etiology of postgastrectomy bone disease remains unclear, either a vitamin D or a calcium deficiency or, perhaps, even both together, contribute to a reduced bone mass9). Proposed causes of a vitamin D or a calcium deficiency have included malabsorption of vitamin D or calcium7), a decreased intake of vitamin D or calcium11), inadequate contact between nutrients and their absorptive sites (either because of altered anatomy or rapid transit)23) and reduced exposure to sunlight23). In our study, all participants had a normal nutritional status, lived at home, had work and led normal life styles, including average physical activity and sexual activity. On the basis of the above facts, it is difficult to assume that either reduced exposure to sunlight or a decreased intake of vitamin D or calcium contributed to a vitamin D or a calcium deficiency. Also no patient has complained of steatorrhea or indigestion or other gastrointestinal symptoms to suggest malabsorption syndrome clinically. Therefore, the loss of gastric acid secretion necessary for solubilization of dietary calcium or the by-pass of the critical absorption surface of the duodenum could be responsible12).
An apparent relationship between bone mass and body weight indicates that a poor nutritional state contributes to the condition24,25). In our study, weight and BMI in the patient group are significantly lower than those of the control (Table 1). According to Korean statistics26), BMI in the normal weight group ranges from 20 to 25 and lean group is definded when BMI is less than 20. We compared 4 patients whose BMI are less than 20 with patients whose BMI are above 20. We could not find any difference in blood chemistries and biochemical parameters of bone metabolism. Furthermore, bone densitometry using QCT did not reveal any difference between the two groups. Therefore, lean body weight in 4 out of 10 patients did not influence low bone density in the patient group.
Biochemical parameters of bone metabolism were divided into bone formation (osteoblastic activity) and bone resorption (osteoclastic activity) markers. The serum alkaline phosphatase has been a sensitive marker for bone formation in the past. Serum calcium and phosphorus values seldom lead to detection of postoperative bone disease or indicate specific types of osseous lesion27). However, serum alkaline phosphatase levels, reasonably good as screening tests for osteomalacic bone disease, reported as being elevated in 10 to 30% of affected persons and are a more reliable index to postgastrectomy bone disease27). In our study, whereas serum calcium was slightly lower in the patient group, phosphorus, alkaline phosphatase, intact PTH and 25-hydroxy vitamin D were slightly higher than the control. However, there was no statistical significance between the two groups in terms of mean serum calcium, phosphorus, alkaline phosphatase, intact PTH and 25-hydroxy vitamin D.
It is difficult to state whether vitamin D deficiency in our study contributed to osteopenia because 25-hydroxy vitamin D is higher in the patient group. Slightly high PTH in the patient group could be a compensation for low calcium. Therefore, we can speculate that reduced bone mass in the patient group could be attributed to a combination of osteoporosis, induced by slightly increased PTH overactivity in compensation for relative calcium deficiency, and osteomalacia on the basis of slightly elevated alkaline phosphatase.
In recent times, new markers for bone metabolism introduced. The serum levels of osteocalcin bone-specific protein have become a most sensitive and specific test for osteoblastic activity5). Type I collagen accounts for about 90% of the organic matrix of mineralized bone. The synthetic rate of type I procollagen can be assessed by measuring the serum concentration of its carboxy terminal propeptide, PICP. The serum PICP levels reflect the synthesis of type I collagen and osteoblastic activities5,15). In our study, serum osteocalcin levels were slightly increased in the patients, but there was no statistical difference (Figure 3). Serum PICP levels, in general, tended to be increased in gastrectomized patients5,15). Our patients also had slightly increased PICP levels than the controls but there were not statistically significant (Figure 4). Recently developed serum ICTP, which is a carboxy terminal telopeptide of type I collagen, cross-linked via pyridinoline cross-links and liberated during the degradation of type I collagen and serum ICTP level, which reflects the activity of bone resorption may be of use in the diagnosis and follow-up of the metabolic status of the bone, especially in states associated with increased lysis of the bone (such as multiple myeloma, osteolytic metastasis, rheumatoid arthritis, immobilization and osteoporosis)17).
The 24-hour urinary hydroxyproline excretion in the fasting state was widely used as a marker of bone resorption till now5,7). The hydroxypyridinium derivatives pyridinoline (PYD) and deoxypyridinoline (DPD) are trifunctional crosslinks found exclusively in mature fibrillar collagens of skeletal tissues. Urinary DPD is considered to be derived from only skeletal collagens. In contrast to hydroxyproline as a conventional bone resorption marker, the pyridinium crosslinks are released during the breakdowm of mature collagen and, therefore, are excreted without interference from newly synthesized or intermediate products. Furthermore, urinary concentration appears not to be influenced by the effects of diet or physical exercise. Therefore, pyridinium crosslinks have also been used as a marker of bone resorption18–20). In our study, urinary DPD excretion and serum ICTP were measured. Although the ICTP level was slightly higher in the patient group, there was no statistical significance (Figure 4). However, urinary DPD excretions of gastrectomized patients were significantly more increased than those of controls (p<0.025)(Figure 1).
Roentgenologic differentiation of osteomalacia from osteoporosis in a patient without pseudofractures may be impossible. It is an accepted concept that at least 30–50% of the bone must be demineralized before detection is possible28). No patient in our study showed osteopenia by plain lumbar X-ray AP and lateral view. Therefore, detection of osteopenia needs bone densitometry. The fact that the degree of osteopenia tends to increase with the length of time after surgery points to the need for long-term surveillance14).
Noninvasive methods measuring bone mass are radiologic photodensitometry, single or dual photon absorptiometry, QCT and dual energy X-ray absorptiometry (DEXA). Invasive method is a bone biopsy in the iliac crest21,22). There are two kinds of QCT, i.e. single energy QCT and dual energy QCT. Dual energy QCT may measure most accurately bone mineralization, because vertebral trabecular bone measurements are summed with adjacent calcium deposits in the cortical bone, osteophytes, compression fracture and other factors. The X-ray source used in DEXA has several advantages over the isotope source used in DPA, which has led to discontinuation of DPA in favor of DEXA in recent years. DEXA has better image resolution and a faster scanning speed29). DEXA and dual energy QCT were not available in our institution during this study. In our study, we used single energy (80kVp) QCT with the addition of a mineral calibration phantom (K2HPO4). Using this technique, the bone mineral density of gastrectomy patients was significantly lower than that of controls (p<0.01.(Figure 1).
In conclusion, markers for bone metabolism and bone are necessary in order to bone diseases after gastrectomy, because routine biochemical chemistry and routine lumbar spine X-ray failed to detect these diseases. Therefore, we suggest that urinary DPD and bone densitometry like QCT are appropriate parameters for screening tests for the detection of bone metabolism abnormalities in the gastrectomized patient during a long-term follow-up period especially after 5 years.
Urinary DPD is, specially, a simple and rapid test which can replace the cumbersome 24-hour urinary hydroxyproline. Also, prophylactic administration of supplemental calcium and/or vitamin D, if indicated with monitoring of serum calcium, 25-hydroxy vitamin D and 24-hour urinary calcium might be reasonable after gastric surgery.
REFERENCES
- 1.Hall GH, Neale G. Bone rarefaction after partial gastrectomy. Ann Int Med. 1963;59:455–463. doi: 10.7326/0003-4819-59-4-455. [DOI] [PubMed] [Google Scholar]
- 2.Mellstrom D, Rundgren A. Long-term effects after partial gastrectomy in elderly men-a longitudinal population study of men between 70 and 75 years of age. Scand J. Gastroenterol. 1982;17:433–439. doi: 10.3109/00365528209182082. [DOI] [PubMed] [Google Scholar]
- 3.Aukee S, Alhava EM, Karjalainen P. Bone Mineral after partial gastrectomy II. Scand J Gastroenterol. 1975;10:165–169. [PubMed] [Google Scholar]
- 4.Paterson CR, Woods CG, Pulvertaft CN, Fourman P. Search for osteomalacia in 1228 patients after gastrectomy and other operations on the stomach. Lancet. 1965;27:1085–1088. doi: 10.1016/s0140-6736(65)90060-7. [DOI] [PubMed] [Google Scholar]
- 5.Resch H, Pietschmann P, Pernecker B, Krexner E, Willvonseder R. The influence of partial gastrectomy on biochemical parameters of bone metabolism and bone density. Clin Investig. 1992;70:426–429. doi: 10.1007/BF00235526. [DOI] [PubMed] [Google Scholar]
- 6.Blichert-Toft M, Beck A, Christiansen C, Transbol I. Effects of gastric resection and vagotomy on blood and bone mineral content. World J Surg. 1979;3:99–102. doi: 10.1007/BF01556392. [DOI] [PubMed] [Google Scholar]
- 7.Eddy RL. Metabolic bone disease after gastrectomy. Am J Med. 1971;50:442–449. doi: 10.1016/0002-9343(71)90333-0. [DOI] [PubMed] [Google Scholar]
- 8.Garrick R, Ireland AW, Posen S. Bone abnormalities after gastric surgery; a prospective histological study. Ann Int Med. 1971;75:221–225. doi: 10.7326/0003-4819-75-2-221. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura O, Sakamoto H, Furumoto T, Furumptp T, Nosaka K, Kouno K, Hisaki T, Koga S. Bone disorder in long-term survival after gastrectomy with reference to bone mineral content. Jpn J Surg. 1987;88:1684–1694. [PubMed] [Google Scholar]
- 10.Williams JA. Effects of upper gastro-intestinal surgery on blood formation and bone metabolism. Brit J Surg. 1964;51:125–135. doi: 10.1002/bjs.1800510208. [DOI] [PubMed] [Google Scholar]
- 11.Kim CB, Choi SH, Kim YI, Lee YS. The effects of gastrectomy on calcium balance. J Kor Surgical Society. 1994;46:472–480. [Google Scholar]
- 12.Filipponi P, Gregorio F, Cristallini S, Mannarelli C, Blass A, Scarponi AM, Vespasiani G. Partial gastrectomy and mineral metabolism:effect on gastrin-calcitonin release. Bone and Miner. 1990;11:199–208. doi: 10.1016/0169-6009(90)90059-o. [DOI] [PubMed] [Google Scholar]
- 13.Nilas L, Christiansen C. Influence of PTH and 1,25(OH)2D on calcium homeostasis and bone mineral content after gastric surgery. Calcif Tissue Int. 1985;37:461–466. doi: 10.1007/BF02557827. [DOI] [PubMed] [Google Scholar]
- 14.Klein KB, Orwoll ES, Lieberman DA, Meier DE, McClung MR, Parfitt AM. Metabolic bone disease in asymptomatic men after partial gastrectomy with Billroth II anastomosis. Gastroenterol. 1987;92:608–616. doi: 10.1016/0016-5085(87)90008-4. [DOI] [PubMed] [Google Scholar]
- 15.Melkko J, Niemi S, Risteli L, Risteli J. Radioimmunoassay of the carboxyterminal propeptide of human type I procollagen. Clin Chem. 1990;36:1382–1332. [PubMed] [Google Scholar]
- 16.Thompson GR, Neale G, Watts JM, Booth CC. Detection of vitamin-D deficiency after partial gastrectomy. Lancet. 1966;19:623–626. doi: 10.1016/s0140-6736(66)90823-3. [DOI] [PubMed] [Google Scholar]
- 17.Risteli J, Elomaa I, Niemi S, Novamo A, Risteli L. Radioimmunoassay for the pyridinoline cross-linked carboxyterminal telopeptide of type I collagen-a new serum marker of bone collagen degradation. Clin Chem. 1993;39:635–640. [PubMed] [Google Scholar]
- 18.Seibel MJ, Robins SP, Bilezikian JP. Urinary pyridinium crosslinks of collagen-specific markers of bone resorption in metabolic bone disease. Trends Endocrinol Metab. 1992;3:263–270. doi: 10.1016/1043-2760(92)90129-o. [DOI] [PubMed] [Google Scholar]
- 19.Robis SP, Woitage H, Hesley R, Ju J, Seyedin S, Seiebl MJ. Direct, enzyme-linked immunoassay for urinary pyridinoline as a specific marker for bone resorption. J Bone Miner Res. 1994;9:1643–1649. doi: 10.1002/jbmr.5650091019. [DOI] [PubMed] [Google Scholar]
- 20.Woitge H, Scheidt-Nave C, Leidig-Bruckner G, Duncan A, Nicol P, Ziegler R, Robins SP. Urinary hydroxypyridinium crosslinks of collagen in population-based screening for overt vertebral osteoporosis: results of a pilot study. J Bone Miner Res. 1994;9:1433–1440. doi: 10.1002/jbmr.5650090916. [DOI] [PubMed] [Google Scholar]
- 21.Health and public policy committee, American college of physicians; Philadelphia, Pennsylvania Radiologic methods to evaluate bone mineral content. Ann Inn Med. 1984;100:908–911. [PubMed] [Google Scholar]
- 22.Richardson ML, Genant HK, Cann CE, Ettinger B, Gordan GS, Kolb FO, Reiser UJ. Assessment of metabolic bone diseases by quantitative computed tomography. Clin Orthop. 1985;195:224–238. [PubMed] [Google Scholar]
- 23.Kaplan MM. Metabolic bone disease associated with gastrointestinal diseases. Dig Dis Sci. 1983;15:9–12. [Google Scholar]
- 24.Ott SM, Kilcoyne RF, Chesnut CH. Longitudinal changes in bone mass after one year as measured by different techniques in patients with osteoporosis. Calcif Tissue Int. 1986;39:133–138. doi: 10.1007/BF02555108. [DOI] [PubMed] [Google Scholar]
- 25.Mazess R, Barden Ettinger B, Johnstan, et al. Spine and femur density using dual-photon absorptiometry in US white women. Bone Miner. 1987;2:211–219. [PubMed] [Google Scholar]
- 26.Lee TH. Prevalence of obesity in Korean non-insulin dependent diabetic patients. Diabetes Res Clin Pract. 1996;32:71–80. doi: 10.1016/0168-8227(96)01251-x. [DOI] [PubMed] [Google Scholar]
- 27.Jones C, Williams TA, Nicholson G. In: Partial gastrectomy. Stammers FAR, Williams JA, editors. London: Butterworth & Co Ltd; 1963. p. P190. [Google Scholar]
- 28.Garn SM, Poznanski AK, Nagy JM. Bone measurement in the different diagnosis of osteopenia and osteoporosis. Radiology. 1971;100:509–518. doi: 10.1148/100.3.509. [DOI] [PubMed] [Google Scholar]
- 29.Pacific Roberto, Rupich Reta, Griffin Michael, Chines Arkadi, Noah Susman Louis V. Dual energy radiography versus quantitative computer tomography for the diagnosis of osteoporosis. J of Clinic Endoclinol and Metabol. 1990;70:705–709. doi: 10.1210/jcem-70-3-705. [DOI] [PubMed] [Google Scholar]


