Abstract
Objectives
Gastric resection may predispose gallstone formation. However, the mechanism has not been clearly understood. To evaluate the relationship between gastric resection and gallstone formation, we compared gallbladder(GB) motility in gastrectomized patients and control subjects.
Methods
We compared the GB volume and ejection fraction of the 46 gastrectomized patients with 37 healthy controls using real time ultrasonography.
Results
GB volume increased significantly in the gastrectomized group in fasting (30.2 ± 13.9 ml). The GB volume after a fatty meal was greater in the gastrectomized group (12.6 ± 6.4 ml) than in the control group (4.3 ± 3.3 ml) (p < 0.01). A significant reduction of ejection fraction was found in gastrectomized patients (56.9 ± 13.0%) in comparison with the control group (75.5 ± 16.1%) (p < 0.01). The GB ejection fraction had a poor correlation to the postoperative period (r = 0.232).
Conclusion
A gastrectomy appears to be a risk factor of GB dysmotility, which may play a major role in gallstone formation in gastrectomized patients.
Keywords: Gallbladder motility, Gastrectomy, Gallstone
INTRODUCTION
It has been reported that gastric resection increases the prevalence of gallstones and that this increase is more pronounced if a vagotomy is added1,2). Although the incidence of gallstones is thought to be increased in patients with a gastric resection or vagotomy1–3), the pathophysiologic mechanisms are still unclear. Vagal denervation is one of the postulated mechanisms for an increased risk of gallstones after gastrectomy due to delayed emptying and changes of bile composition. In animal studies, there were significant biochemical changes in bile composition4). However, the changes were not significant in human studies5,6). There were several different reports about these changes in bile and gallbladder (GB) motility7–10). However, these studies had some problems. First, the study populations were not constant. Second, most studies for physiologic GB contractions were performed only by the infusion of cholecystokinin, and were not carried out satisfactorily to determine GB motility11). Finally, most previous studies were retrospective.
In this study, we intended to find the risk of gallstones after a gastrectomy and/or vagotomy. We prospectively performed serial ultrasonographic studies12,13) on changes in GB volume and the incidence of gallstones and biliary sludges in patients who underwent gastrectomies.
MATERIALS AND METHODS
1. Materials
Forty six patients (31 male, 15 female) with gastric cancer who underwent gastrectomies and thirty seven cases of a normal control group (18 male, 19 female) were studied. Selection criteria for the present study included an absence of GB disease, no alterations in liver function tests and no use of anticholinergics. We excluded patients who were found by preoperative ultrasonography to have gallstones and those who had to fast over 10 days due to postoperative complications. None of the normal control group had a history of abdominal surgery.
The mean age of the test group was 53.3 ± 9.6 years (range 27–75). The surgical procedures were performed by one surgeon (SJK) and included a subtotal gastrectomy with Billroth II anastomosis in 39 cases and a total gastrectomy in 7 cases. Truncal vagotomy was performed in all cases for the purpose of extended lymphadenectomy around the right cardiac area. The mean period from gastric resection to the final examination was 18.3 ± 10.6 months, and the mean period of postoperative fasting was 6.7 ± 0.9 days (Table 1).
Table 1.
Characteristics of the subjects
| Patients (n = 46) | Controls(n = 37) | |
|---|---|---|
| Age (yr, mean ± SD) | 53.3 ± 9.6 | 46.3 ± 10.4 |
| Sex (M/F) | 31/15 | 18/19 |
| OP* type(n) | Subtotal gastrectomy: 39 Total gastrectomy: 7 |
|
| POD**(mo) | 18.3 ± 10.6 | |
| NPO***(day) | 6.7 ± 0.9 | |
OP*, operation; POD**, post-operation date; NPO***, duration of nothing by mouth
METHODS
The measurement of GB motility was done by one sonographer (HKL) using an Acuson 128 XP/10 sonographic machine equipped with a 3.5 mHz convex probe. GB volume was calculated by obtaining the width, height and length with real-time ultrasonography and multiplying the dimensions using the ellipsoidal method14). The real-time ultrasonography was conducted five times at 5-min intervals after an overnight fasting to obtain the average volume of the GB; it was conducted again 30 min. after a fatty meal which consisted of a blend of boiled rice, egg and beef, containing 750 kcal with 32% fat. Thereupon, these premeal and postmeal GB volumes were substituted into the following formula to calculate the ejection fraction (EF) of the GB15).
We looked for gallstones and/or GB sludges during real-time ultrasonography and compared the changes of GB motility according to gender, age, operation type, postoperative dates and presence or absence of gallstones and/or GB sludge.
All scores were expressed in terms of means and standard deviation of the mean (SD), and all data were analyzed statistically using Studant’s t-test and Pearson correlation with the level of p value less than 0.05.
RESULTS
1. Comparison of fasting and postprandial gallbladder volume
The average GB volume in the test group was 30.2 ± 13.9 ml during fasting and 12.6 ± 6.4 ml after feeding, which seemed to be significantly higher than the average volume of the control group (17.3 ± 15.8 ml while fasting and 4.3 ± 3.3 ml after feeding) (p < 0.01) (Figure 1).
Figure 1.
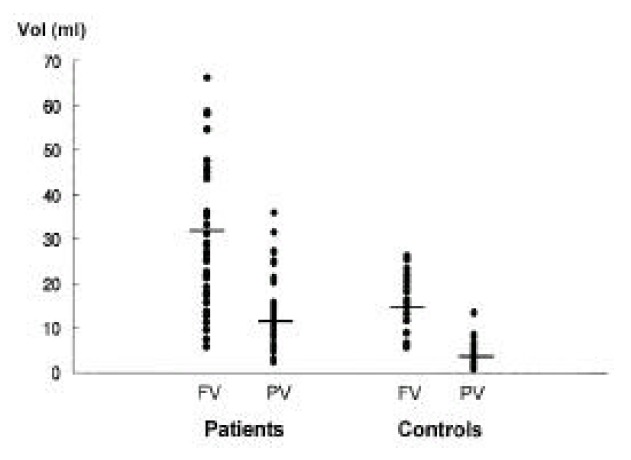
Fasting (FV) and postprandial (PV) GB volumes in patients and controls. Fasting GB volumes and volumes measured after a fatty meal were significantly higher in patients compared with controls (p < 0.01).
2. Comparison of gallbladder ejection fraction
The average ejection fraction of GB in the test group was 56.9 ± 13.0%, whereas that of the control group was 75.5 ± 16.1%. These results showed a significant reduction in EF for the test group (p < 0.01) (Figure 2).
Figure 2.
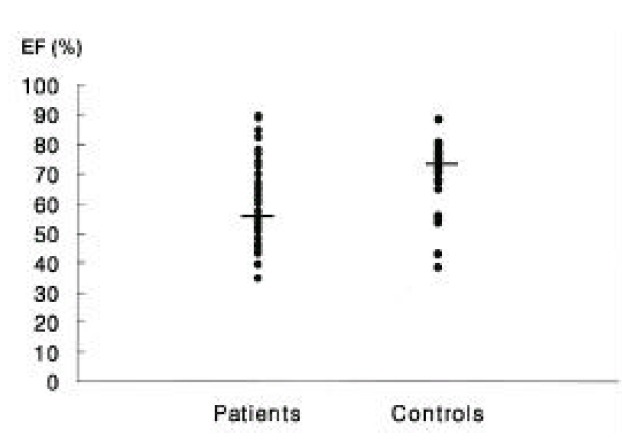
Ejection fractions (EF) in patients and controls. A significant reduction of ejection fraction was found in patients compared with controls (p < 0.01).
3. Comparison of gallbladder volume according to the type of gastric surgery
The average volume of a GB during fasting was significantly lower in subtotal gastrectomy patients (25.7 ± 10.6 ml) than in total gastrectomy patients (51.1 ± 79.3 ml) (p < 0.01) (Figure 3). The average volume of a postprandial GB was also higher (21.0 ± 9.4 ml) in subtotal gastrectomy patients than in total gastrectomy patients (11.3 ±5.2 ml). However, the ejection fraction was not significantly different between the two groups (57.5 ± 11.6% vs 59.7 ± 18.7%, respectively) (p > 0.05). GB sludge and gallstone formation did not seem to be related to the type of gastric surgery (p > 0.05).
Figure 3.
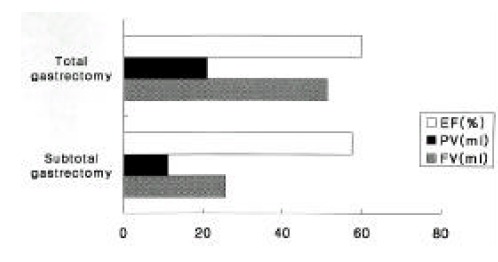
GB volumes and ejection fractions (EF) according to the type of gastric surgery. The average volumes of GB before (FV) and after (PV) meal were significantly lower in subtotal gastrectomy than in total gastrectomy (p < 0.01).
4. The changes in gallbladder motility according to postoperative period
The GB motility correlated poorly between the EF and the postoperative period (Figure 4). However, there was no correlation among GB volume (fasting and postprandial), gallstone formation and the postoperative period.
Figure 4.
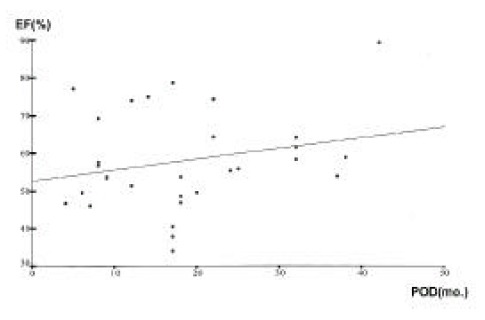
Changes in GB motility according to postoperative period (POD). The ejection fraction (EF) correlated well with postprandial period (r = 0.232).
5. Formation of gallstones and gallbladder sludges
The incidence of gallstone formation after a gastrectomy was 6.5% (3/46), and GB sludges developed in 25.6% (11/46) of the cases. The prevalence was not different between males and females, but there was no statistically significant difference (p > 0.05) in the average fasting volume of GB between the GB sludge group and the group without sludges (34.7 ± 16.4 ml, 28.8 ± 12.9 ml, respectively).
Also, there was no difference in the ejection fraction of the two groups (57.3 ± 11.9%, 56.8 ± 13,5%) (p > 0.05) (Figure 5).
Figure 5.
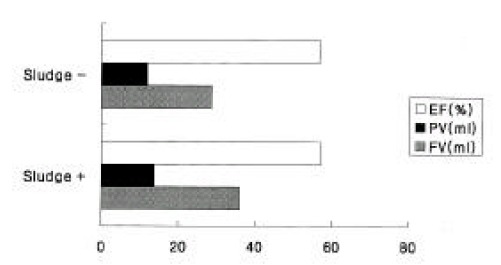
Changes in fasting (FV) and postprandial (PV) GB volumes and ejection fractions (EF) according to sludge formation. The GB volumes and ejection fractions were not statistically different between the two groups.
DISCUSSION
The increased incidence of gallstones in patients with gastrectomy has been reported. However, the pathophysiologic mechanism for gallstones after surgery is not yet clear1,2). The contraction of the GB is caused by the stimulation of the vagal nerve; impaired GB motor function could result from vagal denervation16). The effect of a gastrectomy on GB motor function has been reported with various results, but the frequency of gallstones was higher than in the control population1,18).
Vagal denervation is one of the postulated mechanisms for an increased risk of gallstones after a gastrectomy2,8,19). Vagotomy has been shown to cause biliary stasis and enhance gallstone formation8,19). Fisher et al. reported that GB contraction was decreased after an injection of anticholinergics20) and Kott et al. reported that the frequency of gallstones was decreased in severe myasthenia gravis patients who were taking anticholinesterase21). Some studies showed delayed emptying of the GB after a gastric resection or vagotomy2,3,7,8,22). Our results could also support the effect of the vagus nerve on GB motility.
Inoue et al. reported that the incidence of gallstones gradually increased during 5 years after a gastrectomy or vagotomy3). They injected exogenous cholecystokinin to induce a contraction of the GB and then investigated the significant changes in GB contraction, but there was no significant difference at the twelfth month after the operation compared with the normal control group. This finding was different from our results which showed a gradual increase in the ejection fraction of postoperative GB. GB motility has been shown to change after a truncal vagotomy because of impaired cholinergic activation, and a significantly increased plasma cholecystokinin response has been reported in vagotomized patients20,21). Meanwhile, the contraction of the GB could be induced by maintaining a plasma concentration of cholecystokinin above the physiologic level, after blocking the vagal nerve22). These results showed that vagal stimuli could modulate the response of cholecystokinin on GB motility. When cholecystokinin was infused in patients with a vagotomy or gastrectomy, some reports showed that GB contractilities were increased10), but some did not5). Besides, the GB susceptibility to choelcystokinin was reported to be increased in patients with vagal blocking20). Therefore, we thought that using a normal diet would be ideal to examine GB motility in patients with gastrectomies or vagotomies. Some other studies24,25) showed that preduodenal stimuli could evoke GB emptying, which explained the rationale to use a normal diet for the measurement of GB motility.
Studies on changes in bile composition after a gastrectomy have been investigated with a hypothesis that changes in bile composition could increase the incidence of gallstone formation. Tomkins et al. reported that cholesterol saturation in bile was increased after vagal denervation in animal experiments4). However, some investigators reported that the biliary cholesterol saturation was minimally increased6) or decreased in patients with gastrectomies5).
The incidence of gallstone formation after a gastrectomy has already been reported. Perzolla et al. reported the increased incidence of gallstones in patients with total gastrectomies with Roux-en-Y esophagojejunostomy17), and Rehnberg et al. reported the increased incidence after Billoth I gastrectomies2).
Our study showed results similar to Perzolla’s report, but we could not compare the incidence of gallstones between the types of gastric surgery because there was no Billroth I case in our study. Some studies reported an elevated occurrence of gallstones in men after gastrectomy2), but our study showed no difference between sexes. Most of the studies concerning the incidence of gallstones after gastric surgery have been retrospective1,2,26,27). We performed a prospective serial study on changes of GB motility. We could not follow up over a long period after surgery to tell the statistical significance for the development of gallstones and GB sludges, but we could observe GB sludges in 25.6% and gallstones in 6.5% after gastric surgery, which showed higher incidence than that of the normal population in Korea (4.1%)28). Our lower incidence of gallstones and sludges compared to western countries seemed to be related to our life-style, diet pattern, race, etc29,30).
We could not exclude the possibility that stomach cancer itself might influence our results because most of our study population underwent a gastrectomy due to stomach cancer. However, we thought our results concerning gastric surgery as a risk factor of GB motility were valuable because the incidence of early gastric cancer had been increasing and the survival rate after gastric surgery had also been improved31).
In this study, real-time ultrasonography was used to measure the volume of GB. Real-time ultrasonography can measure the volume and ejection fraction of the GB easily without using other complex equipment necessary for other tests, like cholangiography or radioisotope scanning12,32). As a measurement of GB volume, we could confirm the good reprodutibility of the ellipsoid method in diabetic patients33).
The result of this study showed that there was a significant difference in the volume and ejection fraction of the GB between the patients with gastrectomies and the control group. The incidence of gallstone disease seemed to increase in the patient group, and biliary stasis seemed to precede gallstone formation after gastric surgery.
In conclusion, gastric surgery appears to be a risk factor of GB dysmotility, which may play a major role in gallstone formation in patients with gastric surgery. Long-term follow-up studies would be necessary to investigate the prevalence of gallstones with a change in GB motility.
REFERENCES
- 1.Majoor CLH, Suren TJJ. Gallbladder complications following resection of stomach for peptic ulcer. Br Med J. 1947;2:8–11. doi: 10.1136/bmj.2.4513.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rehnberg O, Haglund U. Gallstone disease following antrectomy and gastroduodenostomy with or without vagotomy. Ann Surg. 1984;201:315–318. doi: 10.1097/00000658-198503000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue K, Fuchigami A, Higashide S, Sumi S, Kogire M, Suzuki T, Tobe T. Gallbladder sludge and stone formation in relation to contractile function after gastrectomy. Ann Surg. 1992;215:19–26. doi: 10.1097/00000658-199201000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tompkins R, Kraft A, Zimmerman E, Lichtenstein J, Zollinger R. Clinical and biochemical evidence of increased gallstone formation after complete vagotomy. Surgery. 1972;71:196–200. [PubMed] [Google Scholar]
- 5.Shaffer EA. The effect of vagotomy on gallbladder function and bile composition in man. Ann Surg. 1982;195:413–418. doi: 10.1097/00000658-198204000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witz M, Schneider A, Novis B, Engelberg M, Dinbar A. Bile composition and bile acid pool-size. Arch Surg. 1985;120:1306–1309. doi: 10.1001/archsurg.1985.01390350086018. [DOI] [PubMed] [Google Scholar]
- 7.Baxter J, Grime J, Critchley M, Jenkins S, Shields R. Relationship between gastric emptying of a solid meal and emptying of the gall bladder before and after vagotomy. Gut. 1987;28:855–863. doi: 10.1136/gut.28.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ihasz M, Griffith CA. Gallstones after vagotomy. Am J Surg. 1981;141:141–150. doi: 10.1016/0002-9610(81)90010-6. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrini CA, Lewin M, Patti MG, Thomas M, Ryan T, Way L. Gallbladder filling and response to cholecystokinin are not affected by vagotomy. Surgery. 1985;98:452–458. [PubMed] [Google Scholar]
- 10.Masclee A, Jansen J, Driessen W, Geuskens L, Lamers C. Effect of truncal vagotomy on cholecystokinin release, gallbladder contraction and gallbladder sensitivity to cholecystokinin in humans. Gastroenterology. 1990;98:1338–1344. doi: 10.1016/0016-5085(90)90354-4. [DOI] [PubMed] [Google Scholar]
- 11.Richer H. Physiologic consequences of vagotomy and gastric resection. Gastroenterol Clin North Am. 1994;23:193–213. [PubMed] [Google Scholar]
- 12.Kirshnamurthy GT, Bobba VR, Kingston E. Radionuclide ejection fraction: A technique for quantitative analysis of motor function of the human gallbladder. Gastroenterology. 1981;80:482–490. [PubMed] [Google Scholar]
- 13.Krook P, Allen F, Bush W. Comparison of real-time cholecystography and oral cholecystography. Radiology. 1980;132:145–148. doi: 10.1148/radiology.135.1.7360953. [DOI] [PubMed] [Google Scholar]
- 14.Dodds WJ, Groh W, Darweesh R. Sonographic measurement of gallbladder volume. AJR. 1985;145:1009–1011. doi: 10.2214/ajr.145.5.1009. [DOI] [PubMed] [Google Scholar]
- 15.Festi D, Frabboni R, Bazzoli F. Gallbladder motility in cholesterol gallstone disease. Gastroenterology. 1990;89:1779–1785. doi: 10.1016/0016-5085(90)90487-l. [DOI] [PubMed] [Google Scholar]
- 16.Brugge WR, Brand DL, Atkina HL. Gallbladder dyskinesia in chronic acalculous cholecystitis. Dig Dis Sci. 1986;31:461–467. doi: 10.1007/BF01320308. [DOI] [PubMed] [Google Scholar]
- 17.Pezzolla F, Lantone G, Guerra V, Misciagna G, Prete F, Giorgio I, Lorusso D. Influence of the method of digestive tract reconstruction on gallstone development after total gastrectomy for gastric cancer. Am J Surg. 1993;166:6–10. doi: 10.1016/s0002-9610(05)80573-2. [DOI] [PubMed] [Google Scholar]
- 18.Lorusso D, Misciagna G, Noviello MR, Tarantino S. Cholelithiasis after Billroth II gastric resection. Surgery. 1988;103:579–583. [PubMed] [Google Scholar]
- 19.Clave RA, Garpar MR. Incidence of gallbladder disease after vagotomy. Am J Surg. 1969;118:169–174. doi: 10.1016/0002-9610(69)90116-0. [DOI] [PubMed] [Google Scholar]
- 20.Fisher R, Rock E, Malmud L. Cholinergic effects on gallbladder emptying in humans. Gastroenterology. 1985;89:716–722. doi: 10.1016/0016-5085(85)90564-5. [DOI] [PubMed] [Google Scholar]
- 21.Kott E, Kerber G, Huberman M, Kott I. Myasthenia gravis: rarity of gallstone formation. Neurology. 1994;44:154–156. doi: 10.1212/wnl.44.1.154. [DOI] [PubMed] [Google Scholar]
- 22.Scott RB, Tan TM. Neural modulation of canine duodenal bile acid delivery in the interdigestive and postprandial periods. Am J Physiol. 1993;264:G357–G366. doi: 10.1152/ajpgi.1993.264.2.G357. [DOI] [PubMed] [Google Scholar]
- 23.Hopman W, Jansen J, Rosenbusch G, Lamers C. Role of cholecystokinin and the cholinergic system in the intestinal phase of gallbladder contraction in man (abstr) Gut. 1986;27:A603. doi: 10.1002/hep.1840110216. [DOI] [PubMed] [Google Scholar]
- 24.Ellenbogen S, Jenkins SA, Grime JS, Critchley M, Mackie C, Baxter J. Preduodenal mechanisms in initiating gallbladder emptying in man. Br J Surg. 1988;75:940–945. doi: 10.1002/bjs.1800751003. [DOI] [PubMed] [Google Scholar]
- 25.Devas HT, Yamagishi T. Evidence for a pyloro-cholecystic reflex for gallbladder contraction. Ann Surg. 1977;133:29–33. doi: 10.1097/00000658-197908000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turunen M, Antile L. Gallbladder disease following gastrectomy. Acta Chir Scand. 1964;127:134–137. [PubMed] [Google Scholar]
- 27.Fletcher DM, Clark CG. Gallstones and gastric surgery: a review. Br J Surg. 1968;55:895–899. doi: 10.1002/bjs.1800551205. [DOI] [PubMed] [Google Scholar]
- 28.Jung HW, Chun KS, Kim YS. Prevalence of gallstones in Korean. J Korean Academy Family Medicine. 1992;13:581–591. [Google Scholar]
- 29.Moon YM. Biliary stone in Korea. Korean J Gastroenterol. 1987;19:1–5. [Google Scholar]
- 30.Lee OC, Kim CFI, Hahm JS. Changing state and present features of gallstone disease. J Hanyang Med Col. 1994;14:355–363. [Google Scholar]
- 31.Cho HJ. A study of 26 cases of early gastric cancer. New Med J. 1990;33:26–34. [Google Scholar]
- 32.Mackie CR, Baxter JN, Grime JS. Gallbladder emptying in normal subject- A database for clinical cholescintigraphy. Gut. 1987;28:137–141. doi: 10.1136/gut.28.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hahm JS, Park JY, Park KG, Ahn YH, Lee MH, Park KN. Gallbladder motility in diabetes mellitus using real-time ultrasonography. Am J Gastroenterol. 1996;91:2391–2394. [PubMed] [Google Scholar]


