Abstract
We report a 46-year-old woman with primary biliary cirrhosis (PBC) presenting with Sjögren’s syndrome and systemic mononuclear inflammatory vasculopathy. Biopsy specimens of sural nerve showed findings consistent with vasculitic neuropathy. Perivascular inflammatory mononuclear cell infiltration was observed on muscle biopsy specimen. The findings of abdominal computed tomography and brain magnetic resonance imaging were suggestive of vasculitis. Clinical manifestations and radiologic findings were improved after high dose prednisolone therapy.
Keywords: systemic mononuclear inflammatory vasculopathy, Sjögren’s syndrome, primary biliary cirrhosis
INTRODUCTION
Primary biliary cirrhosis (PBC), which is characterized by chronic inflammation and fibrous obliteration of intra-hepatic bile ducts, is frequently related to a variety of disorders presumed to be autoimmune in nature, such as systemic lupus erythematosus, mixed connective tissue disease, CREST syndrome and Sjögren’s syndrome (SS)1). SS is known to be the most common autoimmune disorder associated with PBC1). Tsuneyama et al. reported that aberrant expression of PBC-specific antigen in the salivary gland might be related to coexistence of SS and PBC2). Alexander et al. suggested that about one third of primary SS patients suffered from inflammatory vascular disease1). The vasculitic lesions in SS can fit into four major categories: acute necrotizing vasculitis, leukocytoclastic vasculitis, mononuclear cell vasculitis and endarteritis obliterans3). The association between mononuclear inflammatory vasculopathy of the central nervous system and the peripheral nerve and muscle has been well documented in patients with SS4). PBC is rarely presented with vasculitis, and systemic mononuclear inflammatory vasculopathy in PBC has not been reported. To our knowledge, this is the first report of systemic mononuclear inflammatory vasculopathy associated with Sjögren’s syndrome in a patient with primary biliary cirrhosis.
CASE REPORT
A 46-year-old woman was admitted to our hospital because of abdominal pain and dyspnea. One month before admission, mild weakness and numbness developed in both lower legs and hands. She also complained of sicca symptoms and cognitive dysfunction, such as recent memory impairment and attention and concentration difficulties. She appeared acutely ill. Blood pressure was 120/80 mmHg, temperature 38.1 °C and respiration rate 22/min. On physical examination, there was tenderness on the lower abdomen. Auscultation of the lung revealed decreased breathing sound on both lower lung fields, but heart was normal. The liver and spleen were not palpable. Motor examination of the upper and lower extremities revealed a strength of 4+ power. Deep tendon reflexes were 2+ in both extremities, except 1+ in ankle jerk. Sensory examination showed decreased sensation to vibration and proprioception in a glove and stocking distribution on both lower legs. Laboratory findings were as follows: hematocrit 31.2%, white blood cell count 14,000/mm3 (87.8% neutrophils, 5.4% lymphocytes, 2.4% monocytes, 4.4% eosinophils), platelet count 313,000/mm3, total serum protein 5.0 g/dl, albumin 2.3 g/dl, alkaline phosphatase 701 U/L, ϒ -GTP 81 U/L, total cholesterol 94 mg/dl, calcium 7.0 mg/dl, phosphorus 4.3 mg/dl, sodium 126 mEq/L and potassium 3.9 mEq/L. The values for blood glucose, urea nitrogen, creatinine, AST, ALT, bilirubin, amylase, creatine kinase and lactate dehydrogenase were normal. Urine examination was normal. Tests for prothrombin time and activated partial thromboplastin time were within the normal range. Arterial blood gas analysis showed pH 7.50, PCO2 32 mmHg, PO2 52 mmHg, bicarbonate 25 mmol/L and O2 saturation 91 %. Immunologic studies showed circulating immune complex 2.70 μg/mℓ (normal<3.0 μg/mℓ), negative FANA, ANCA, and decreased level of CH50 (14.9 U/ml, normal 30.0–40.0 U/mℓ), C3 (27.6 mg/dl, normal 45–86 mg/dl) and C4 (8.3 mg/dl, normal 11–47 mg/dl). Tests for hepatitis B surface antigen, anti-HBs antibody and anti-HCV antibodies were negative. Lupus anticoagulant and anticardiolipin antibody were absent. Tests for antibodies to ENA and Ro were positive, but antibodies to La, Sm and nRNP were negative. Anti-mitochondrial antibody was positive (titer 1:320), but anti-LKM-1 and anti-smooth muscle antibody were negative. Rheumatoid factor and cryoglobulin were also negative. Results for other immunologic studies were as follows: ESR 99 mm/hr, CRP 168 mg/L, serum IgG 2,200 mg/dl, IgA 561 mg/dl, IgM 292 mg/dl and IgE less than 30.3 IU/ml. Thyroid function test showed euthyroid status. Cerebrospinal fluid examination showed pressure 17.5 cmH2O, protein 1,200 mg/dl, sugar 24 mg/dl and leukocytes 9/mm3. Bacterial culture for spinal fluid was negative. Electrocardiography demonstrated sinus tachycardia and inverted T wave in V4-V6 leads. Moderate amount of pericardial effusion was found on echocardiography, but ejection fraction was within normal limit. A chest radiography revealed mild cardiomegaly and both pleural effusion. On abdominal ultrasound, the echogenecity of the liver was slightly coarse, but others were unremarkable. Mottled hepatic uptakes were observed on hepatic scintigraphy. Abdominal CT demonstrated wall thickening of duodenum, terminal ileum and cecum, and multiple stricky densities in the mesentery. The results of Schirmer’s test were compatible with dry eye syndrome (right eye 2 mm/5 min, left eye 3 mm/5 min). The pathologic findings of the lower lip biopsy specimen and radiologic findings of salivary gland scan were consistent with SS (not shown). Electrophysiologic studies including nerve conduction velocity and electromyography demonstrated sensory predominant mixed peripheral polyneuropathy and polyradicular pattern sensory change. Brain MRI demonstrated diffuse, patchy increased signal intensities involving the brain stem, both basal ganglia and right thalamus on T2-weighted axial images, which were suggestive of multiple ischemic events related to vasculitis (Fig. 1). Sural nerve biopsy specimens demonstrated thickened vessel walls with mononuclear inflammatory cell infiltration in epineurium and perineurium (Fig. 2). Muscle biopsy of vastus lateralis showed muscle fiber necrosis and degeneration, variation in fiber size with internal nuclei and mononuclear inflammatory cell infiltration in endomysial and perimysial perivascular areas (Fig. 3). Needle aspirated liver biopsy specimen noted findings consistent with the early change of primary biliary cirrhosis (Figure 4). The diagnosis of SS associated with PBC presenting with systemic mononuclear inflammatory vasculopathy affecting the central and peripheral nervous system, muscle and gastrointestinal tracts was made. Prednisolone 50 mg/day (1 mg/kg/day) was given for one month, then slowly tapered. Her abdominal pain and dyspnea improved after initiation of steroid therapy. Weakness of both lower legs was also slowly improved. Follow up electrophysiologic studies and brain MRI findings after treatment with steroids for 4 months revealed improvement.
Fig. 1.
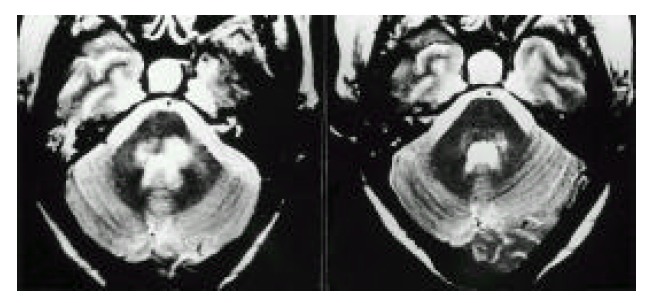
Axial T2-weighted (3,000/85/2) image at the level of middle cerebellar peduncle showed ill-defined hyperintense lesions (left). After treatment with prednisolone, these lesions nearly disappeared (right).
Fig. 2.
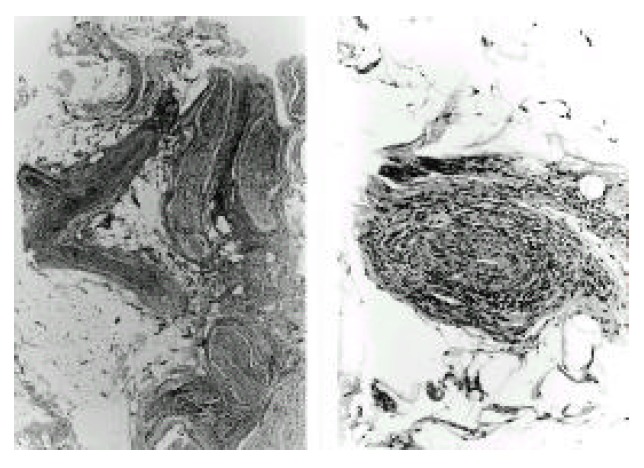
Sural nerve biopsy showed thickened blood vessels with marked inflammatory cell I infiltration in the epineurium and perineurium (left, ×40), and fibrinoid necrosis in the entire vessel wall (right, ×100). Hematoxylin and eosin.
Fig. 3.
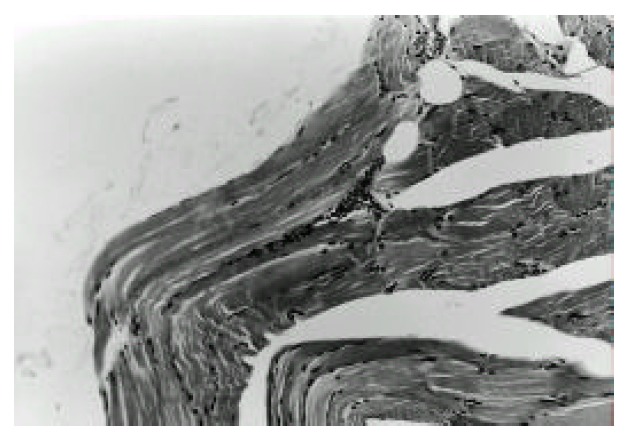
Muscle biopsy of vastus lateralis showing mild mononuclear inflammatory cell infiltration in endomysial perivascular area. Hematoxylin and eosin (×100).
Fig 4.
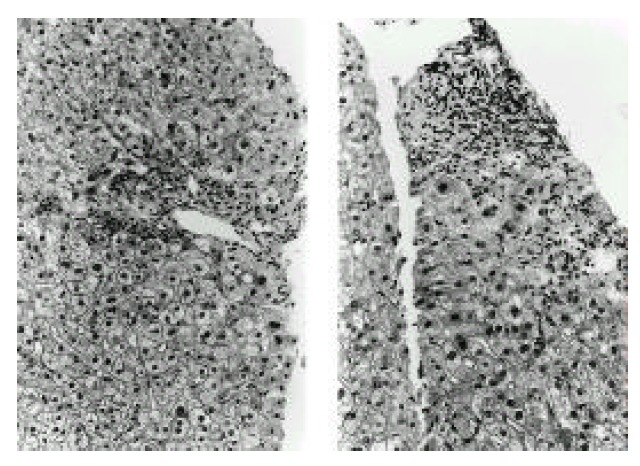
Liver biopsy demonstrated microscopic features consistent with primary biliary cirrhosis early stage: inflammatory cell infiltration, absence of bile duct in the portal area (left) and biliary piecemeal necrosis (right). Hematoxylin and eosin (×100).
DISCUSSION
Many patients with PBC are asymptomatic and symptoms of associated disorders are a commonly prominent aspect of the clinical manifestations. Our patient denied symptoms related to PBC, but she had typical symptoms of SS (xerostomia and xeroopthalmia) and clinical manifestations of systemic vasculitis involving the central and peripheral nervous system and gastrointestinal tracts. Our patient complained of changes in cognitive function characterized by recent memory impairment, attention and concentration difficulties. PBC patients associated with SS have more profound neuropsychiatric manifestations than PBC patients without SS5). Multiple vasculitic lesions involving the brain stem, both basal ganglias, right thalamus and cervicomedullary junction were demonstrated on brain MRI, but symmetrically hyperintense globus pallidi on T1 weighted images which are known to be characteristic findings of brain MRI in patients with PBC, were not observed6). The presence of abnormal brain MRI has suggested an organic etiology for cognitive and psychiatric dysfunction in this patient. Histologically, mononuclear inflammatory ischemic or hemorrhagic vasculopathy was a characteristic finding in CNS disease in SS. Mononuclear inflammatory vasculitis of CNS in patients with SS is frequently accompanied by vasculitis of the peripheral nervous system, muscle and skin4). Peripheral nervous system abnormalities have been demonstrated in patients with PBC as well as SS7). A disordered immune response, suggested by the presence of circulating immune complexes or vitamin E deficiency, are known to be possible causes of the neuropathy in patients with PBC8). Vitamin E deficiency can cause peripheral neuropathy, ataxia and proximal muscle weakness. Jeffrey GP et al. reported that thirty five of 80 (44%) patients with primary biliary cirrhosis had a biochemical deficiency of vitamin E8). In our case, although the level of vitamin E was not checked, pathologic findings of sural nerve biopsy specimens, suggesting vasculitic neuropathy and clinical improvement of neurologic symptoms after the administration of steroids made vitamin E deficiency a less likely possible cause of neuromuscular manifestations. As in our case, inflammatory vascular disease within the muscle, without clinical or laboratory evidence of frank myopathy, is known to be a prominent musculoskeletal symptom in patients with SS4). Thickening of small bowel walls, ascites and multiple sticky densities in the mesentery, which were demonstrated in our patient on abdominal ultrasound and CT, are consistent with radiologic findings of mesenteric vasculitis9–10). The pathogenesis of pericarditis and pleural effusion in connective tissue disease is thought to be associated with vasculitis which is supported by the deposition of immune complexes with activation of the complement system within blood vessels. Therefore, pericarditis and pleural effusion in our case might be an additional finding suggesting systemic vasculitis. Mechanisms that initiate and perpetuate systemic vasculitis in SS have not been clarified yet. Anti-Ro antibody has been suggested to play a role in mediating the vascular damage in CNS disease in SS4). Anti-Ro antibody was positive in our patient. The most effective therapy in patients with SS, presenting with systemic mononuclear inflammatory vasculopathy, has not been established, but moderate to high dose corticosteroids, alone or in conjunction with intravenous pulse cyclophosphamide therapy, are recommended as initial medical management4). In our patient, after institution of prednisolone starting at 1 mg/kg/day for one week, abdominal pain and dyspnea were much regressed. Vasculitis symptoms of the central and peripheral nervous system and muscle are more slowly improved.
In summary, this case suggests that systemic mononuclear inflammatory vasculopathy associated with SS may be an additional extra-hepatic manifestation of PBC.
REFERENCES
- 1.Lindor KD. Primary biliary cirrhosis. In: Feldman M, Scharschmidt BF, Sleisenger MH, editors. Gastrointestinal and liver disease. 6th ed. Philadelphia: WB Saunders; 1998. pp. 1275–83. [Google Scholar]
- 2.Tsuneyama K, Van De Water J, Yamazaki K, Suzuki K, Sato S, Takeda Y, Ruebner B, Yost BA, Nakanuma Y, Coppel RL, Gerschwin ME. Primary biliary cirrhosis and epithelitis: evidence of abnormal salivary gland immunohistochemistry. Autoimmunity. 1997;26:23–31. doi: 10.3109/08916939709009547. [DOI] [PubMed] [Google Scholar]
- 3.Tsokos M, Lazarou SA, Moutsopoulos HM. Vasculitis in primary Sjögren’s syndrome. Histologic classification and clinical presentation. Am J Clin Pathol. 1987;88:26–31. doi: 10.1093/ajcp/88.1.26. [DOI] [PubMed] [Google Scholar]
- 4.Alexander EL. Neurologic disease in Sjögren’s syndrome: mononuclear inflammatory vasculopathy affecting central/peripheral nervous system and muscle. A clinical review and update of immunopathogenesis. Rheum Dis Clin North Am. 1993;19:869–908. [PubMed] [Google Scholar]
- 5.Tarter RE, Hays AL, Carra J, Edwards KL, Van Thiel DH. Sjögren’s syndrome-Its contribution to neuropsychiatric syndrome in patients with primary biliary cirrhosis. Dig Dis Sci. 1989;34:9–12. doi: 10.1007/BF01536146. [DOI] [PubMed] [Google Scholar]
- 6.Uchino A, Hasuo K, Matsumoto S, Masuda K. Cerebral MR imaging in patients with primary biliary cirrhosis. Nippon Igaku Hoshasen Gakkai Zasshi. 1993;53:145–149. [PubMed] [Google Scholar]
- 7.Hendrickse MT, Triger DR. Autonomic and peripheral neuropathy in primary biliary cirrhosis. J Hepatol. 1993;19:401–407. doi: 10.1016/s0168-8278(05)80549-5. [DOI] [PubMed] [Google Scholar]
- 8.Jeffrey GP, Muller DP, Burroughs AK, Matthews S, Kemp C, Epstein O, Metcalfe TA, Southam E, Tazir-Melboucy M, Thomas PK. Vitamin E deficiency and its clinical significance in adults with primary biliary cirrhosis and other forms of chronic liver disease. J Hepatol. 1987;4:307–317. doi: 10.1016/s0168-8278(87)80539-1. [DOI] [PubMed] [Google Scholar]
- 9.Ko SF, Lee TY, Cheng TT, Ng SH, Lai HM, Cheng YF, Tsai CC. CT findings at lupus mesenteric vasculitis. Acta Radiol. 1997;38:115–120. doi: 10.1080/02841859709171253. [DOI] [PubMed] [Google Scholar]
- 10.Shiohira Y, Uehara H, Miyazato F, Matsumoto H. Vasculitis-related acute abdomen in systemic lupus erythematosus-ultrasound appearances in lupus patients with intra-abdominal vasculitis. Ryumachi. 1993;33:235–241. [PubMed] [Google Scholar]


