Abstract
Background
Matrix metalloproteinases (MMPs) have been implicated in the remodelling of extracellular matrix (ECM), including basement membrane. ECM remodelling is associated with pathological processes, including hepatic fibrosis, tumor invasion and metastasis. Tissue inhibitors of metalloproteinase (TIMP)-1 and TIMP-2 were known to inhibit MMP-9 and MMP-2, respectively. In the present study, we examined the expression of TIMP-1 and TIMP-2 in surgical specimen pairs of hepatocellular carcinoma and nontumoral liver and the correlation between their expression and clinicopathological characteristics.
Methods
The localization of both transcripts and protein of TIMP-1 and TIMP-2 was studied by using in situ hybridization and immunohistochemistry.
Results
TIMP-1 and TIMP-2 mRNA transcripts were found in tumor cells, hepatocyte, sinusoidal cells, endothelial cells and stromal cells. Signal intensity of TIMP-1 was stronger than that of TIMP-2. The results of immunohistochemical stainings were concordant with those obtained by in situ hybridization. Expression of TIMP-1 and TIMP-2 was observed in tumorous tissue, in nontumorous tissue and in the portions of the tumors adjacent to the capsules. However, a clear difference in TIMP-1 and TIMP-2 mRNA expression was not observed among the three tissue types. The intensity of TIMP-2 expression was generally weaker than that of TIMP-1, and the intensity of TIMP-1 and TIMP-2 mRNA expression did not correlate with variable clinicopathological characteristics.
Conclusion
TIMPs was expressed in tumor cells and many cell types of the nontumoral liver. Further investigations for TIMPs’ unknown functional role are needed.
Keywords: Tissue inhibitor of metalloproteinases, Carcinoma, Hepatocellular, In situ hybridization, Immunohistochemistry
INTRODUCTION
Recent studies have revealed a structurally related family of secreted metalloproteinases which degrade extracellular matrix (ECM) components, such as collagens, fibronectin, laminin and proteoglycans with different substrate specificities. Matrix metalloproteinases (MMPs), including collagenase, gelatinase, stromelysin and membrane-type MMP have been implicated in the remodeeing of ECM, including basement membrane1). ECM remodeling is associated with physiological and pathological processes, including wound healing, angiogenesis, hepatic fibrosis and, more notably, tumor invasion and metastasis2–6).
Hepatic fibrosis is not caused solely by an increase in connective tissue synthesis but is basically the result of an imbalance between enhanced ECM synthesis and diminished or altered ECM breakdown7–10). An essential step in the process of tumor invasion and metastasis involves the degradation of ECM, including basement membrane.
Most forms of chronic liver disease are associated with the development of hepatic fibrosis and cirrhosis. Hepatocellular carcinoma frequently occurs in hepatitis B or C virus-related chronic hepatitis or cirrhosis. In particular, cirrhosis has been regarded as a high-risk factor for developing hepatocellular carcinoma.
A large body of evidence indicates that MMPs play a crucial role in hepatic fibrosis, and enhanced MMPs activity has been associated with increases in invasive and metastatic potential in many types of human carcinoma, including hepatocellular carcinoma11–14).
In general, a number of control mechanisms exist to regulate MMPs activity in tissues. These mechanisms operate at different levels, including gene transcription, proenzyme activation and inhibition of activated enzymes. The latter mechanisms are regulated by tissue inhibitors of metalloproteinases (TIMPs), which bind to the catalytic site of the active enzyme as well as to the carboxyl terminus of the proenzyme, thereby inhibiting its activity and preventing its activation15–18).
TIMPs bind strongly and stoicheometrically to active form of MMPs and inactivate them. TIMP-1 and TIMP-2 also form tight binding complexes with proMMP-9 and proMMP-2, respectively, to inhibit their activation15–18). The imbalance of MMPs and TIMPs may contribute to the pathogenesis of hepatic fibrosis and tumor invasion and metastasis. Therefore, it is important to understand the role of TIMPs in hepatocellular carcinoma.
We examined localization of both transcripts and protein of TIMP-1 and TIMP-2 in surgical specimen pairs of hepatocellular carcinoma and nontumoral liver using in situ hybridization and immunohistochemistry, and compared it with clinicopathological characteristics.
MATERIALS AND METHODS
1. Tumor specimens
Formalin-fixed, paraffin-embedded tissue blocks were obtained from 25 patients who underwent surgery for hepatocellular carcinoma at Chonnam National University Hospital from January 1997 to December 1998. All pathologic slides were reviewed and adequate blocks that showed the junction between tumorous and adjacent nontumorous liver tissue were selected.
The study group consisted of 25 patients with hepatocellular carcinoma (19 men and 6 women; age range, 31–69 years; median, 58.0 years). Identified risk factors were as follows: HBV infection (15 patients), HCV infection (7 patients) and alcoholic cirrhosis (3 patients). Tumor size (range, 2–9 cm; median, 4.5 cm) was less than 4.5cm in 16 patients and >4.5cm in 9 patients.
The grade of tumor differentiation was as follows: well differentiated in 4 patients, moderately differentiated in 12 patients, and poorly differentiated in 9 patients. Tumors were infiltrative in 9 patients and encapsulated in 16 patients. Capsular invasion was present in 5 of the 16 patients with encapsulated tumors, and satellite nodules were present in 10 patients, with vascular invasion detected in 8 patients (Table 1).
Table 1.
Clinicopathological parameters of 25 patients with hepatocellular carcinoma
| Parameters | No. of cases(%) |
|---|---|
| Age(years) | |
| <58 | 12/25(48.0) |
| ≥58 | 13/25(52.0) |
| Sex | |
| Male | 19/25(76.0) |
| Female | 6/25(24.0) |
| Cause* | |
| HBV | 15/25(60.0) |
| HCV | 7/25(28.0) |
| Alcohol | 3/25(12.0) |
| Basal liver disease | |
| Cirrhosis | 15/25(60.0) |
| Chronic hepatitis | 10/25(40.0) |
| Tumor size (cm) | |
| <4.5 | 16/25(64.0) |
| ≥4.5 | 9/25(36.0) |
| Differentiation grade** | |
| WD | 4/25(16.0) |
| MD | 12/25(48.0) |
| PD | 9/25(36.0) |
| Pattern of growth | |
| Infiltrative | 9/25(36.0) |
| Encapsulated | 16/25(64.0) |
| Capsular invasion | |
| Absent | 11/16(68.7) |
| Present | 5/16(31.3) |
| Satellite nodules | |
| Absent | 10/25(40.0) |
| Present | 15/25(60.0) |
| Vascular invasion | |
| Absent | 17/25(68.0) |
| Present | 8/25(32.0) |
HBV; hepatitis B virus, HCV; hepatitis C virus
WD; well differentiated, MD; moderately differentiated, PD; poorly differentiated
2. In situ hybridization
For in situ hybridization, two biotinylated oligonucleotide probes complementary to TIMP-1 (TGTGGGTGGGGTGGGACACAGGTGC) and TIMP-2 (CCACTTCCTTCTCACTGACCGC) mRNA were synthesized. All in situ hybridization experiments were carried out using manual capillary action technology on the Micro-Probe staining system (Fisher Scientific, Pittsburgh, PA) with the modified one-hour method of Park et al19). Briefly, the slides were rapidly deparaffinized, cleared and rehydrated. The tissues were then digested with pepsin (Sigma, St. Louis, MO) at 2 mg/mL for 1 minute at 110°C. The probe was applied to the slides and the tissues were heated at 110°C for 10 minutes to denature any secondary mRNA structures. The hybridization of the probe and mRNA target was performed by exposing the slides to a microwave oven in which the temperature was gradually decreased from 110°C to 45°C (110°C for 1 min, 95°C for 7 min, 85°C for 7 min, 65°C for 5 min, 45°C for 20 minutes, respectively). The biotinylated hybrids were detected with streptavidin-horseradish peroxidase (Zymed, SanFrancisco, CA) for 7 minutes at 45°C. After preincubation in 3-amino-9-ethylcarbazole (AEC, Sigma, St. Louis, MO) for 7 minutes at 45°C, the tissues were washed with distilled water. Following the chromogen reaction, the tissues were counterstained with hematoxylin solution (Research Genetics, Huntsville, AL), washed with distilled water, air-dried and cover-slipped with Universal mount (Research Genetics, Huntsville, AL). In situ hybridization for negative control was performed with probe diluent in hybridization. To evaluate the degree of TIMP-1 and TIMP-2 mRNA expression in tumor tissue, the staining intensity was divided into the following three groups; 0, negative signal; 1, weak signal; 2, strong signal. Assessment of expression was evaluated by two independent observers without knowledge of the clinical outcomes. Consensus scores were assigned for each case by reviewing the slides with discrepancies in scoring. All sections on which the two observers disagreed were re-evaluated and after discussion there was total agreement on the classification.
3. Immunohistochemistry
All procedures for immunohistochemical staining were done by the Micro-Probe staining system (Fisher Scientific, Pittsburgh, PA) based on capillary action20). Paraffin sections of 4μm in thickness with mounted probe on slides were immunostained with anti-mouse monoclonal antibodies for TIMP-1 and TIMP-2 antigens (NeoMarkers, Union, CA) by the avidin-biotin peroxidase complex method20). Sections were dewaxed using absolute alcohol. Antigen retrieval was performed by microwave for 7 minutes in citrate buffer (2.1g/L monohydrate citric acid in distilled water, pH 6.0). After three washes in universal buffer, the slides were immersed in 0.6% hydrogen peroxide for 5 minutes to block the endogenous peroxidase activity. The primary antibodies, used at concentrations of 1:120, 1:100 respectively, were diluted in phosphate-buffered saline supplemented with 5% normal horse serum and 1% bovine serum albumin and then incubated with tissues overnight at room temperature. The slides were washed in universal buffer and incubated with biotinylated affinity-purified anti-mouse immunoglobulin G (Sigma, St. Louis, MO) for 7 minutes at 45°C. After three washes with universal buffer, streptavidin-alkaline phosphatase detection system (Biomeda, Foster, CA) was applied for 7 minutes. As the final step, the slides were developed for 15 minutes with the enzyme substrate, 3 amino-9-ethyl carbazole (AEC, Sigma, St. Louis, MO), and counterstained with hematoxylin solution for 1 minute (Research Genetics, Huntsville, AL). After dehydration, the tissue was sealed with a universal mount (Research Genetics, Huntsville, AL).
4. Statistical Analysis
Significant bivariate associations were tested for all study variables by χ2 test and Fisher’s exact test. The statistical software program used was Statistical Package for the Social Sciences (SPSS/PC+ 8.0, Chicago, IL, USA). A p-value of less than 0.05 was accepted as statistically significant.
RESULTS
1. Expression of TIMP-1 and TIMP-2 mRNA
TIMP-1 and TIMP-2 mRNA were detected in all hepatocellular carcinoma cases examined. Hybridization signals for TIMP-1 and TIMP-2 mRNA were detected in tumorous tissue, in nontumorous tissue and in the portions of the tumors adjacent to the capsules. However, a clear difference in TIMP-1 and TIMP-2 mRNA expression was not observed between tumorous tissue, nontumorous tissue and the portions of the tumors adjacent to the capsules. In nontumorous portion, hybridization signals for TIMP-1 and TIMP-2 mRNA were detected in hepatocytes, bile duct cells, sinusoidal lining cells and stromal cells (Figure 1 A, B). The expression of TIMP-1 and TIMP-2 mRNA in tumor cells was located in the cytoplasm and showed a diffuse or granular pattern (Figure 2 A, B). Intensity of expression varied among cases and in different areas of the same tumor. With regard to signal intensity of TIMP-1 mRNA, 12 of the cases were weak and 13 were strong (Table 2). With regard to signal intensity of TIMP-2 mRNA, 15 of the cases were weak and 10 were strong (Table 3). However, the intensity of TIMP-2 expression was generally weaker than TIMP-1.
Figure 1.
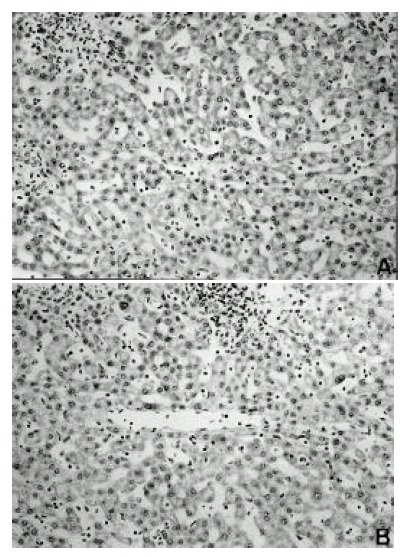
Detection of TIMP-1 and TIMP-2 mRNA by in situ hybridization in nontumorous portion surrounding hepatocellular carcinoma. Hybridization signals for TIMP-1 and TIMP-2 mRNA are detected in hepatocytes, bile duct cells, sinusoidal lining cells and stromal cells (× 200). (A) TIMP-1, (B) TIMP-2
Figure 2.
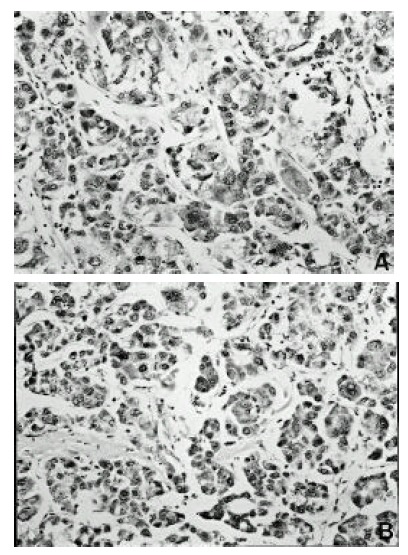
Detection of TIMP-1 and TIMP-2 mRNA by in situ hybridization in hepatocellular carcinoma. The expression of TIMP-1 and TIMP-2 m RNA in tumor cells is located in the cytoplasm and shows a diffuse or granular pattern (× 200). (A) TIMP-1, (B) TIMP-2
Table 2.
Correlation between expression of TIMP-1 mRNA and clinicopathological parameters of hepatocellular carcinoma
| Clinicopathological Parameters | n | Intensity of TIMP-1 mRNA expression
|
P-value | |
|---|---|---|---|---|
| Weak(n) | Strong(n) | |||
| Age(years) | ||||
| <58 | 12 | 7 | 5 | 0.320 |
| ≥58 | 13 | 5 | 8 | |
| Sex | ||||
| Male | 19 | 11 | 8 | 0.078 |
| Female | 6 | 1 | 5 | |
| Basal liver disease | ||||
| Cirrhosis | 15 | 8 | 7 | 0.513 |
| Chronic hepatitis | 10 | 4 | 6 | |
| Tumor size(cm) | ||||
| <4.5 | 16 | 9 | 7 | 0.303 |
| ≥4.5 | 17 | 7 | 10 | |
| Differentiation grade* | ||||
| WD | 4 | 2 | 2 | 0.817 |
| MD | 12 | 5 | 7 | |
| PD | 9 | 5 | 4 | |
| Pattern of growth | ||||
| Infiltrative | 9 | 4 | 6 | 0.973 |
| Encapsulated | 16 | 7 | 8 | |
| Capsular invasion | ||||
| Absent | 11 | 5 | 6 | 0.590 |
| Present | 5 | 3 | 2 | |
| Satellite nodules | ||||
| Absent | 10 | 4 | 6 | 0.742 |
| Present | 15 | 7 | 8 | |
| Vascular invasion | ||||
| Absent | 17 | 8 | 9 | 0.891 |
| Present | 8 | 4 | 9 | |
WD; well differentiated, MD; moderately differentiated, PD; poorly differentiated
Table 3.
Correlation between expression of TIMP-2 mRNA and clinicopathological parameters of hepatocellular carcinoma
| Clinicopathological Parameters | n | Intensity of TIMP-2 mRNA expression
|
p-value | |
|---|---|---|---|---|
| Weak(n) | Strong(n) | |||
| Age(years) | ||||
| <58 | 12 | 5 | 7 | 0.072 |
| ≥58 | 13 | 10 | 3 | |
| Sex | ||||
| Male | 19 | 13 | 6 | 0.126 |
| Female | 6 | 2 | 4 | |
| Basal liver disease | ||||
| Cirrhosis | 15 | 8 | 7 | 0.513 |
| Chronic hepatitis | 10 | 7 | 3 | |
| Tumor size(cm) | ||||
| <4.5 | 16 | 9 | 7 | 0.405 |
| ≥4.5 | 17 | 7 | 10 | |
| Differentiation grade* | ||||
| WD | 4 | 3 | 1 | 0.471 |
| MD | 12 | 8 | 4 | |
| PD | 9 | 4 | 5 | |
| Pattern of growth | ||||
| Infiltrative | 9 | 4 | 5 | 0.234 |
| Encapsulated | 16 | 11 | 5 | |
| Capsular invasion | ||||
| Absent | 11 | 8 | 3 | 0.611 |
| Present | 5 | 3 | 2 | |
| Satellite nodules | ||||
| Absent | 10 | 5 | 5 | 0.405 |
| Present | 15 | 10 | 5 | |
| Vascular invasion | ||||
| Absent | 17 | 9 | 8 | 0.294 |
| Present | 8 | 6 | 2 | |
WD; well differentiated, MD; moderately differentiated, PD; poorly differentiated
2. Expression of TIMP-1 and TIMP-2 protein
Immunohistochemical study was also performed for identifying the cellular localization of TIMP-1 and TIMP-2 proteins. In all hepatocellular carcinoma tissues, there was a clear immunoreactivity with TIMP-1 and TIMP-2 antibodies. Expression and location of TIMP-1 and TIMP-2 protein, as indicated by immunostaining, correlated well with in situ hybridization analysis of mRNA transcripts. TIMP-1 and TIMP-2 were expressed in tumor cells and tumor stroma. TIMP-1 and TIMP-2 immunoreactivities in tumor stroma were situated in fibroblasts, leukocytes, endothelial cells and ECM (Figure 3 A, B).
Figure 3.
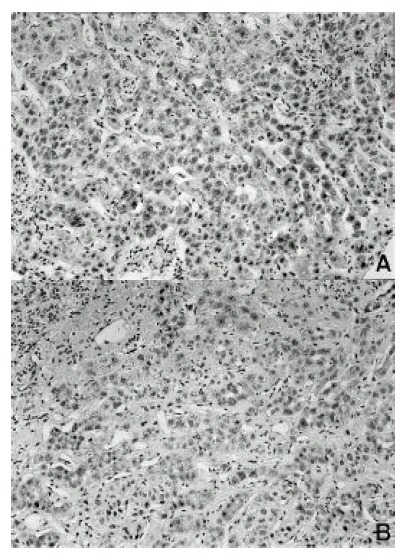
Immunoreactivity of TIMP-1 and TIMP-2 in hepatocellular carcinoma. The tumor cells express TIMP-1 and TIMP-2 in the cytoplasm. The stromal cells also express TIMP-1 and TIMP-2 (× 200). (A) TIMP-1, (B) TIMP-2
3. Correlation between TIMP-1 and TIMP-2 mRNA expression and clinicopathological parameters
We tested the relationships between intensity of TIMP-1 and TIMP-2 mRNA expression in tumorous tissue and the following clinicopathological parameters: tumor size, grade of differentiation, pattern of growth, capsule invasion, presence of satellite nodules, basal liver diseases and vascular invasion. There was no correlation between intensity of TIMP-1 and TIMP-2 mRNA expression in tumorous tissue and all clinicopathological parameters (Table 2, Table 3).
DISCUSSION
Hepatic fibrosis and cirrhosis are associated with excessive deposition of ECM in a distribution and composition different from the non-fibrotic organ9,10). These alterations relate particularly to an absolute and relative accumulation of collagen types I and III in fibrotic and cirrhotic livers21).
Disordered MMP activity could theoretically contribute to such pathological states as progressive hepatic fibrosis and cirrhosis. Advanced hepatic fibrosis and cirrhosis are generally considered to be irreversible conditions even after removal of the injurious agent. Moreover, the development of cirrhosis is characterized by a diminution of liver MMPs activity, implying that the capacity of the diseased liver to remodel the fibrotic matrix is cortically reduced. In studies undertaken to measure MMPs activity in hepatic fibrosis, a general pattern emerges. Initially there is a reversible phase with active ECM remodelling and detectable MMPs activity. In late disease, fibrosis becomes irreversible and, at this point, MMPs activity falls22–25).
These changes of MMPs activity operate at different levels and control the synthesis of the proenzyme, the secretion of the proenzyme that requires activation and the presence of the metalloproteinases inhibitors. First, all of them are secreted in latent forms (proenzyme) and must be activated for the express ion of their activities. Second, the metalloproteinase activities are regulated by common metalloproteinase inhibitors secreted from various types of cells, such as connective tissue cells, vascular endothelial cells and tumor cells.
Our study showed that, in nontumorous portion with either chronic hepatitis or cirrhosis, expression of TIMP-1 and TIMP-2 were detected in many cell types, including hepatocytes, bile duct cells, sinusoidal lining cells and stromal cells. These results suggest that TIMP-1 and TIMP-2 might be involved in hepatic fibrosis and cirrhosis. Previous reports demonstrated that expression of TIMP-1 and TIMP-2 was increased in human cirrhotic livers in comparison to normal liver26,27). Furthermore, Fukuda et al reported that the expression of TIMP-1 was observed in fibrous tissue, especially in the capsule of hepatocellular carcinoma28). These results may indicate a relationship between the increased expression of TIMP-1 and hepatic fibrosis, as well as a relationship between such increased expression and the hepatocellular carcinoma capsule formation.
Iredale et al reported that the cellular distribution of TIMP-1 and TIMP-2 appear to be similar, although only TIMP-1 is generally found in large amounts29). Our study also showed that a clear difference in TIMP-1 and TIMP-2 expression was not observed between tumorous tissue, nontumorous tissue and the portions of the tumors adjacent to the capsules, and the intensity of TIMP-2 expression was generally weaker than TIMP-1.
In general, an important step in tumor invasion and metastasis is the proteolytic breakdown of ECM in normal tissue surrounding the tumor. Among proteolytic enzymes, the MMPs are the most critical part of this step.
TIMPs concomitantly secreted into the cellular microenvironment interact with MMPs at several levels to stabilize latent enzymes, inhibit active enzymes and modulate the binding of soluble MMPs to surface-bound MMPs, and thereby control the net MMPs activity. Therefore, TIMPs are critical in regulating ECM degradation and tumor invasion and metastasis. Although some studies suggest a role of TIMPs in preventing tumor invasion, others show a complex relationship between MMPs and TIMPs in which the TIMPs were not shown to prevent tumor invasion30–34).
This paradoxical result indicates that TIMP-1 and TIMP-2 also exhibit other roles than the inhibition of MMPs, which may affect the malignant potential. Hayakawa et al35,36) reported that TIMP-1 and TIMP-2 molecules have growth promoting activity. However, it remains unclear whether TIMPs stimulate the growth of hepatocellular carcinoma cells. In our study, a clear difference in TIMP-1 and TIMP-2 expression was not observed between tumorous tissue, nontumorous tissue and the portions of the tumors adjacent to the capsules. Also, intensity of TIMP-1 and TIMP-2 mRNA expression did not correlate with variable clinicopathological characteristics. The effect of TIMPs on tumor invasion and metastasis are particularly complex. TIMPs synthesis has been known to be regulated by growth factors and cytokines such as transforming growth factor-β 1, interleukin and tumor necrosis factor-α37,38).
The influence of other genes, such as those coding for growth factors and metastasis suppressor and dominant metastasis genes, on the possible modulation of MMP/TIMP, together with possible repercussions for the invasive and metastatic properties of the cancer cell, also needs to be studied in depth.
In summary, expression of TIMP-1 and TIMP-2 was found in tumor cells, hepatocyte, sinusoidal cells, endothelial cells and stromal cells. Expression of TIMP-1 and TIMP-2 was observed in tumorous tissue, in nontumorous tissue and in the portions of the tumors adjacent to the capsules. Expression of TIMP-1 was stronger than that of TIMP-2 and intensity of TIMP-1 and TIMP-2 mRNA expression did not correlate with variable clinicopathological characteristics.
Further investigations for TIMPs’ unknown functional role are needed.
Acknowledgments
This study was supported by basic research funds from the Research Institute of Clinical Medicine, Chonnam National University Hospital.
REFERENCES
- 1.Emonard H, Grimaud JA. Matrix metalloproteinases: A review. Cell Mol Biol. 1990;36:131–153. [PubMed] [Google Scholar]
- 2.Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafdie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 3.Bissell DH, Friedman SL, Maher JJ, Roll FJ. Connective tissue biology and hepatic fibrosis: Report of a conference. Hepatology. 1990;11:488–498. doi: 10.1002/hep.1840110322. [DOI] [PubMed] [Google Scholar]
- 4.Tryggvason K, Hoyhtya M, Salo T. Proteolytic degradation of extracellular matrix in tumor invasion. Biochim Biophys Acta. 1987;907:191–217. doi: 10.1016/0304-419x(87)90006-0. [DOI] [PubMed] [Google Scholar]
- 5.Pauli BU, Knudson W. Tumor invasion: A consequence of destructive and compositional matrix alteration. Human Pathol. 1988;19:628–639. doi: 10.1016/s0046-8177(88)80168-0. [DOI] [PubMed] [Google Scholar]
- 6.Khokha R, Denhardt DT. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: A review of their role in tumorigenesis and tissue invasion. Invasion Metast. 1989;9:391–405. [PubMed] [Google Scholar]
- 7.Friedman SL. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 8.Friedman SL. Closing the signals of hepatic fibrosis. Gastroenterology. 1997;112:1406–1409. doi: 10.1016/s0016-5085(97)70158-6. [DOI] [PubMed] [Google Scholar]
- 9.Schuppan D, Herbst H, Milani S. Matrix, matrix synthesis and molecular networks in hepatic fibrosis. In: Zern MA, Reid LM, editors. Extracellular matrix: Chemistry, Biology and Pathobiology with emphasis on the liver. New York: Marcel Dekker; 1993. pp. 201–254. [Google Scholar]
- 10.Gressner AM, Bachem MG. Cellular sources of noncollagenous matrix proteins: role of fat storing cells in fibrogenesis. Semin Liver Dis. 1990;10:30–46. doi: 10.1055/s-2008-1040455. [DOI] [PubMed] [Google Scholar]
- 11.Davies B, Waxman J, Wasan H, Abel P, Williams G, Krausz T, Neal D, Thomas D, Hanby A, Balkwill FR. Levels of matrix metalloproteinases in bladder cancer correlate with tumor grade and invasion. Cancer Res. 1993;53:5365–5369. [PubMed] [Google Scholar]
- 12.D’Errico A, Garbisa S, Liotta LA, Castranovo V, Stetler-Stevenson WG, Grigioni WF. Augmentation of collagenase IV, lamin receptor and Ki 62 proliferation antigen associated with human colon, gastric and breast carcinoma progression. Mod Pathol. 1991;4:239–246. [PubMed] [Google Scholar]
- 13.Arii S, Mise M, Harada T, Furutani M, Ishigami S, Niwano M, Mizumoto M, Fukumoto M, Imamura M. Overexpression of matrix metalloproteinase 9 gene in hepatocellular carcinoma with invasive potential. Hepatology. 1996;24:316–22. doi: 10.1053/jhep.1996.v24.pm0008690399. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto H, Itoh F, Adachi Y, Sakamoto H, Adachi M, Hinoda Y, Imai K. Relation of enhanced secretion of active matrix metalloproteinases with tumor spread in human hepatocellular carcinoma. Gastroenterology. 1997;112:1290–1296. doi: 10.1016/s0016-5085(97)70143-4. [DOI] [PubMed] [Google Scholar]
- 15.Cawston TE. Proteinases and inhibitors. Br Med Bull. 1995;51:385–401. doi: 10.1093/oxfordjournals.bmb.a072968. [DOI] [PubMed] [Google Scholar]
- 16.Basbaum CB, Werb Z. Focalized proteolysis: spatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr Opin Cell Biol. 1996;8:731–738. doi: 10.1016/s0955-0674(96)80116-5. [DOI] [PubMed] [Google Scholar]
- 17.Birkedal-Hansen H. Proteolytic remodelling of extracellular matrix. Curr Opin Cell Biol. 1995;7:728–735. doi: 10.1016/0955-0674(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 18.Ries C, Petrides PE. Cytokine regulation of matrix metalloproteinase activity and its regulatory dysfunction in disease. Biol Chem H-S. 1995;376:345–355. [PubMed] [Google Scholar]
- 19.Park CS, Manahan LJ, Brigati DJ. Automated molecular pathology: one hour in situ hybridization. J Histotechnol. 1995;2:462–467. [Google Scholar]
- 20.Reed JA, Manahan LJ, Park CS, Brigati DJ. Complete one-hour immunohistochemistry based on capillary action. Biotechniques. 1992;13:434–443. [PubMed] [Google Scholar]
- 21.Rojkind M, Martinez-Palomo A. Increase in type I and III collagens in human alcoholic liver cirrhosis. Proc Natl Acad Sci USA. 1976;73:539–543. doi: 10.1073/pnas.73.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruyama K, Feinman L, Fainsilber Z, Nakano M, Okazaki I, Lieber CS. Mammalian collagenase increases in early alcoholic liver disease and decreases with cirrhosis. Life Sci. 1982;30:1379–1384. doi: 10.1016/0024-3205(82)90023-6. [DOI] [PubMed] [Google Scholar]
- 23.Montfort I, Perez-Tamayo R. Collagenase in experimental carbon tetrachloride cirrhosis of the liver. Am J Pathol. 1978;92:411–420. [PMC free article] [PubMed] [Google Scholar]
- 24.Murawaki Y, Yamada S, Koda M, Hirayama C. Collagenase and collagenolytic catthepsin in normal and fibrotic rat liver. J Biochem. 1990;108:241–244. doi: 10.1093/oxfordjournals.jbchem.a123187. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Tamayo R, Montfort I, Gonzalez E. Collagenolytic activity in experimental cirrhosis of liver. Exp Mol Pathol. 1987;47:300–308. doi: 10.1016/0014-4800(87)90015-3. [DOI] [PubMed] [Google Scholar]
- 26.Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arhtur MJP. Expression of tissue inhibitor of metalloproteinase-1 and -2 is increased in fibrotic human liver. Gastroenterology. 1996;110:821–831. doi: 10.1053/gast.1996.v110.pm8608892. [DOI] [PubMed] [Google Scholar]
- 27.Herbst H, Wege T, Milani S, Pellegrini G, Orzechowski HD, Bechstein WO, Neuhaus P, Gressner AM, Schuppan D. Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol. 1997;150:1647–1659. [PMC free article] [PubMed] [Google Scholar]
- 28.Fukuda Y, Imoto M, Koyama Y, Miyazawa Y, Nakano I, Hattori M, Urano F, Kodama S, Iwata K, Hayakawa T. Immunohistochemical study on tissue inhibitors of metalloproteinases in normal and pathological human livers. Gastroenterol Jpn. 1991;26:37–41. doi: 10.1007/BF02779506. [DOI] [PubMed] [Google Scholar]
- 29.Iredale JP. Tissue inhibitors of metalloproteinases in liver fibrosis. Int J Biochem Cell Biol. 1997;29:43–54. doi: 10.1016/s1357-2725(96)00118-5. [DOI] [PubMed] [Google Scholar]
- 30.DeClerk YA, Perez N, Shimada H, Boone TC, Langley KE, Taylor SM. Inhibition of invasion and metastasis in cells transfected with an inhibitor of metalloproteinases. Cancer Res. 1992;52:701–708. [PubMed] [Google Scholar]
- 31.Khokha R. Suppression of the tumorigenic and metastatic abilities of murine B16-F10 melaoma cells in vivo by the overexpression of the tissue inhibitor of metalloproteinase-1. J Natl Cancer Inst. 1994;86:299–234. doi: 10.1093/jnci/86.4.299. [DOI] [PubMed] [Google Scholar]
- 32.Martin DC, Ruther U, Sanchez-Sweatman OH, Orr FW, Khokha R. Inhibition of S V40 T antigen-induced hepatocellular carcinoma in TIMP-1 transgenic mice. Oncogene. 1996;13:569–576. [PubMed] [Google Scholar]
- 33.Visscher DW, Hoyhtya M, Ottosen SK, Liang CM, Sarkar FH, Crissman JD, Fridman R. Enhanced expression of tissue inhibitor of metalloproteinase-2(TIMP-2) in the stroma of breast carcinomas correlates with tumor recurrence. Int J Cancer. 1994;59:339–344. doi: 10.1002/ijc.2910590308. [DOI] [PubMed] [Google Scholar]
- 34.Yoshiji H, Gomez DE, Thorgeirsson UP. Enhanced RNA expression of tissue inhibitor of metalloproteinases-1 (TIMP-1) in human breast cancer. Int J Cancer. 1996;69:131–134. doi: 10.1002/(SICI)1097-0215(19960422)69:2<131::AID-IJC11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 35.Hayakawa T, Yamashita K, Tanzawa K, Uchijima JE, Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1(TIMP-1) for wide range of cells. FEBS Lett. 1992;298:29–32. doi: 10.1016/0014-5793(92)80015-9. [DOI] [PubMed] [Google Scholar]
- 36.Hayakawa T, Yamashita K, Ohuchi E, Shinagawa A. Cell growth-promoting activity of tissue inhibitor of metalloproteinases-2(TIMP-2) J Cell Sci. 1994;107:2373–2379. doi: 10.1242/jcs.107.9.2373. [DOI] [PubMed] [Google Scholar]
- 37.Lefebvre V, Peeters-Joris C, Vaes G. Modulation by interleukin 1 and tumor necrosis factr alpha of production of collagenase, tissue inhibitor of metalloproteinases and collagen types in differentiated and dedifferentiated articular chondrocytes. Biochem Biophys Acta. 1990;1052:366–378. doi: 10.1016/0167-4889(90)90145-4. [DOI] [PubMed] [Google Scholar]
- 38.McCune BK, Mullin BR, Flanders KC, Jaffurs WJ, Mullen LT, Sporn MB. Localization of transforming growth factor-β isotypes in lesions of the human breast. Hum Pathol. 1992;23:13–20. doi: 10.1016/0046-8177(92)90004-m. [DOI] [PubMed] [Google Scholar]


