Abstract
Background
About half of the world population is infected with H. pylori, but the transmission and the source of this infection are still unclear. Recently, dental plaque (DP) and saliva have been implicated as possible sources of H. pylori infection. This study was done to investigate the detection rates of H. pylori in the DP and saliva by use of PCR depending on H. pylori infection state of gastric mucosa.
Methods
In 46 subjects, gastric H. pylori colonization was evaluated with CLO test, microscopy of Gram stained mucosal smear, culture and histology after modified Giemsa staining in the antrum and body, respectively. A patient was regarded as H. pylori positive if one or more of the four aforementioned test methods demonstrated H. pylori colonization of the gastric mucosa. For detection of H. pylori in the DP and saliva, PCR assay was done with ET4-U and ET4-L primers. To estimate the sensitivity and specificity of this PCR, H. pylori positivity was evaluated in the antrum and body, separately.
Results
The sensitivity of mucosal PCR was 50.0% (27/54) and the specificity 86.8% (33/38). When a subject was regarded as H. pyloi positive, if either antrum or body mucosal H. pylori was is positive, the positive rate of mucosal PCR was 62.1% (18 subjects) in the 29 H. pylori-positive and 17.6% (3 subjects) in the 17 H. pylori-negative subjects. DP PCR was positive in 2 of 29 H. pylori-positive subjects (6.9%) and none in the 17 H. pylori-negative (0%). Saliva PCR was positive in 4 of 14 H. pylori-positive subjects (28.6%) and none of 6 H. pylori-negative (0%).
Conclusion
The detection rates of H. pylori in DP and saliva by PCR were rather low, 6.9% and 28.6%, respectively, and these rates might have been underestimated by low sensitivity of the PCR method used in this study. However, the results that H. pylori was found in the DP and saliva suggest that the oral cavity can perform a role as a reservoir of H. pylori in Korea.
Keywords: Helicobacter pylori, Dental plaque, Saliva, Polymerase chain reaction
INTRODUCTION
Helicobacter pylori (H. pylori), first isolated from a human gastric biopsy specimen in 1983, is now considered as a common worldwide gastric pathogen. It causes chronic type-B gastritis and is considered as a major cause of peptic ulcer disease and, presumably gastric malignancy1). About half of the world population is infected with H. pylori2) and oral-oral and fecal-oral modes of transmission have been postulated. However, the transmission and the source of this infection are still unclear. Recently, dental plaque (DP) and saliva have been implicated as possible sources of H. pylori infection3,4). In addition, the failure of triple therapy to clear H. pylori infection from DP, despite its clearance from gastric mucosa5), raised the possibility that DP is a potential source of reinfection of gastric mucosa. In our country, the reinfection rate of H. pylori was 12.8% per year6), higher than those of developed countries, 0.36–1.2% per year7–9), raising the necessity of investigating whether or not oral cavity such as DP or saliva performs does a role as a reservoir of H. pylori.
While H. pylori could have been isolated from the oral cavity in a few cases10–14), most attempts to culture it have failed15–18). Polymerase chain reaction (PCR)-based assays may be convenient tools for detecting H. pylori in saliva and DP because of their high sensitivity and specificity3,19). However, the detection rates of H. pylori by PCR ranged from 0% to 100%3,10,12,16,17,20–25), suggesting that these variations may reflect variable prevalence of H. pylori in the oral cavity but also that it can be originated from different specificity and sensitivity of the primers used. This study was done to investigate the detection rates of H. pylori in the DP and saliva by use of PCR depending on infection state of gastric mucosa. For this aim, we used a new nested PCR assay probe which has been proved to be very sensitive and specific for H. pylori26).
MATERIALS AND METHODS
A total of 46 subjects who visited the endoscopy room of Kangnam General Hospital from September 1997 to October 1998 participated in the study. They were selected in three kinds of ways: 32 subjects were by high suspicion of peptic ulcer disease by past ulcer history or upper GI series, and they were diagnosed as active duodenal ulcer (DU) (10 patients), benign gastric ulcer (BGU) (13 patients), DU scar (5 patients), active pyloric channel ulcer (3 patients), and both of DU and BGU (1 patient) after endoscopy; 11 were peptic ulcer patients undertaking follow-up gastroendoscopy at least 4 weeks after triple therapy (7 patients of BGU and 4 patients of DU); three subjects were spouses of peptic ulcer patients. Their mean age was 45.8±13.2 years old, and 36 (78.3%) were male. All subjects were informed of this study and consent was received.
DP and saliva specimens were always taken prior to the endoscopic procedure to exclude the possibility of contamination of the tooth surface and saliva with H. pylori during the withdrawal of the endoscope. After the frequency of dental visits during the previous 1 year was assessed, the subjects’ gingiva and plaque were assessed by using the gingival and plaque indices of Silness and Loe27).
The gingival index was as follows: 0, normal gingiva; 1, mild inflammation, slight change in color, slight edema, and no bleeding on probing; 2, moderate inflammation, redness, edema, and bleeding on probing; and 3, severe inflammation, marked redness and edema, ulceration, and tendency to spontaneous hemorrhage.
The plaque index was as follows: 0, no plaque; 1, film of plaque, visible only on removal on probe or by disclosing with color indicator system; 2, moderate accumulation of deposits within the pockets or on the margins which can be seen with the naked eye; and 3, heavy accumulation of material filling the niche between the gingival margin and the tooth surface, and the interdental region is filled with debris. DP was obtained from the incisor teeth with universal curette. The currette was immersed in 2% glutaraldehyde when not in use and was thoroughly rinsed first with glutaraldehyde and then with distilled water, before use in each subject. Supragingival plaques, collected by an upward scrape against the tooth surface, were immediately placed in sterile tube containing 0.1 mL of saline for PCR, and part of plaque was innoculated into CLO test gel. In 39 of 46 participating patients, about 0.1 mL of saliva was innoculated into CLO test gel, and in 20 subjects about 1 mL of saliva was collected in sterile tube for PCR. Both of DP and saliva for PCR were stored at −70°C until they were processed. After getting DP and saliva, the subjects undertook endoscopy. Six biopsy specimens were taken within 3 cm of the pyloric ring and in the middle body, respectively. These biopsy specimens were analyzed with CLO test (one specimen), microscopy of Gram stained mucosal smear (one specimen), culture (one specimen), and histology after modified Giemsa staining (2 specimens). Remaining one specimen from antrum and body, respectively, was immediately placed in sterile tube containing 0.3 mL of saline for PCR, and stored at −70°C until they were processed. A patient was regarded as H. pylori positive if one or more of the four aforementioned test methods demonstrated H. pylori colonization of the gastric mucosa6).
Extraction of DP was done as follows28): after thawing of frozen plaque samples, DP was suspended in 100 μL of TE containing Mutanolysin (final concentration, 0.1 μg/μL) and lysozyme (final concentration, 5 μg/μL) (both enzymes were purchased from Sigma Chemical Co., St. Louis, MO. USA). After 1 hour at 37°C, 900μL of a lysis buffer (which contained following per 100 mL of 0.1 M Tris [pH 6.4]: 120 g of guanidine isothiocyanate, 22 mL of a 0.2 M EDTA solution adjusted to pH 8.0 with sodium hydroxide, and 2.6 mL of Triton X-100) was added together with 40 μL of diatomaceous earth (Celite; Sigma Chemical Co.). The samples were mixed and then incubated at room temperature for 10 min. The samples were again mixed and centrifugated at 12,000 xg for 20 sec. The DNA containing pellet was washed twice with 1 mL of guanidine isothiocyanate in Tris, twice with 1 mL of 70% ethanol, and once with 1 mL of 100% acetone. The pellets were dried by incubation at 56°C for 10 min, and then the DNA pellet was eluted in 300 μL of TE by incubation at 56°C for 10 min. The supernatant was extracted with 300 μL of phenol:isoamylalcohol:chloroform (25:1:24) two times. The upper phase was mixed with two volumes of cold 100% ethanol and 1/10 volume of 3 M sodium acetate (pH 4.8), and stored at −70°C for 20 min. The pellet was washed with 1 mL of 70% ethanol and dried at 56°C, and resuspended with 55 μL of TE. To prepare the genomic DNA for PCR from saliva and gastric biopsy, gastric biopsy samples were transferred to 0.5 mL of digestion buffer [20 mM Tris-HCl (pH 8.0), 10 mM EDTA (pH 8.0), 0.5% SDS] containing protease K (final concentration, 100 μg/mL), and for saliva 0.5 mL of this digestion buffer was added. After incubation at 52°C for 3 hours, DNA was extracted with 0.5 mL of phenol, 0.5 mL of chloroform, and 0.5 mL of phenol:isoamylalcohol:chloroform (25:1:24), sequentially. The upper phase (about 0.4 mL) was mixed with 2 volumes of cold 100% ethanol and 1/10 volume of 3 M sodium acetate (pH 5.2). After centrifugation, the DNA pellet was washed with 70% ethanol, and finally resuspended with 100 μL of TE. The primers (ET4), developed and sequenced by Dr. Eapen Thomas (East Tennessee State University)26) were synthesized at GenoTech (Daejeon, Korea). The sequences of the primers were: ET4-U (24 bp) 5′ AAA ATC AGG CCT ATC GCT TTG TAT 3′; and ET4-L (21 bp) 5′ GCC CCC ATA AAC ACC AAG AGT 3′. This pair of primers had a span of 203 bp. The buffer of the PCR contained 1.5 mM MgCl2, PCR buffer, 0.4 mM dNTP, 0.25 μM ET4-U and ET4-L primers each, and 0.25 units of Taq polymerase. The total volume of the PCR was 20 μL. One microliter of 200 ng DNA specimens of gastric mucosa and saliva, and as much as possible up to 113 ng of DP specimen were used as templates. For each pair of primers, the following conditions were the same: initial denaturation at 95°C for 10 min: denaturation, annealing, and extension by 40 cycles, with each cycle consisting of 95°C/45 sec, 60°C/30 sec, and 72°C/45 sec. There was another longer extension of 6 min at 72°C. Positive and negative controls were performed for each batch of amplifications. The DNA extracted from H. pylori ATCC 43629 served as a positive control, and water as a negative control. The amplified products were analyzed by 1% agarose gel electrophoresis with ethidium bromide, and observed under ultraviolet light.
RESULTS
Among 46 participating subjects, gastric H. pylori was positive in 25 (54.3%) in the antrum, and 29 subjects (63.0%) in the body by CLO test, touch print of Gram stain, Giemsa stain and/or culture. In 4 subjects, H. pylori was found only in the body. When mucosal PCR was done in these 92 mucosal specimens (antrum and body in each subject), 203 bp fragment was found in the 32 specimens (Figure 1). To estimate the sensitivity and specificity of the PCR, H. pylori positivity was evaluated in the antrum and body, separately. In the antrum, the PCR was positive in 12 of 25 H. pyloi-positive subjects (sensitivity: 48.0%), and negative in 18 of 21 H. pyloi-negative subjects (specificity: 85.7%). In the body, the PCR was positive in 15 of 29 H. pyloi-positive subjects (sensitivity: 51.7%), and negative in 15 of 17 H. pyloi-negative subjects (specificity: 88.2%). Taken together, the sensitivity of mucosal PC R was 50.0% (27/54) and the specificity 86.8% (33/38) (Table 1).
Figure 1.
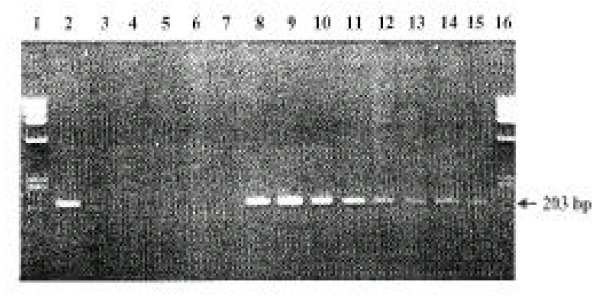
Representative PCR products from the gastric mucosal DNA preparations. The amplified DNA fragments were 203 bp in the electrophoresis on 1% agarose gel. Lane 1 and 16: Ladder DNA markers (Phix174 DNA Hae Three Marker, Promega Co.).
Lane 2: positive control.
Lane 3: negative control.
Lane 4–7: negative PCR.
Lane 8–15: positive PCR.
Table 1.
The sensitivity and specificity of gastric mucosal PCR
| PCR | True positive | True negative |
|---|---|---|
| Positive | 27 | 5 |
| Negative | 27 | 33 |
| Total | 54 | 38 |
sensitivity: 50.0%(27/54), specificity: 86.8%(33/38)
When a subject was regarded as H. pyloi positive if either antrum or body mucosal H. pylori was positive, the positivity rate of mucosal PCR was 62.1% (18 subjects) in the 29 H. pylori-positive subjects, and 17.6% (3 subjects) in the 17 H. pyloi-negative subjects (Table 2). Among these 18 mucosal PCR positive subjects, 9 subjects showed PCR positive in both antrum and body, 4 in the antrum only and 5 in the body only. All three mucosal PCR positive subjects in the 17 H. pyloi-negative were followed up cases of two DU and one BGU patients after triple therapy, and one showed PCR positive in both antrum and body, one in the antrum and another in the body only. The dental visits in the previous one year was 0.13±0.30, and the mean indexes of gingiva and plaque were 1.54±0.70, and 2.02±0.70, respectively. When gastric mucosa and DP were compared, DP CLO test was positive in 29 gastric H. pylori-positive subjects and in 16 of 17 H. pylori-negative subjects (94.1%)(Table 2). However, DP PCR was positive in only two of 29 gastric H. pylori-positive subjects (6.9%) and none in the 17 H. pylori-negative subjects (Table 2, Figure 2A). Two with DP PCR positive were active BGU and active pyloric channel ulcer patient, respectively. These two patients showed gastric mucosal H. pylori test positive in both the antrum and body, but mucosal PCR was all negative.
Table 2.
Comparison of H. pylori tests in the gastric mucosa and dental plaque
| Gastric Mucosal | H. Pylori | |
|---|---|---|
| Positive | Negative | |
| No. | 29 | 17 |
| Mucosal PCR positive | 18(62.1%) | 3(17.6%) |
| Dental plaque CLO test positive | 29(100%) | 16(94.1%) |
| Dental Plaque PCR positive | 2(6.9%) | 0(0%) |
Figure 2.
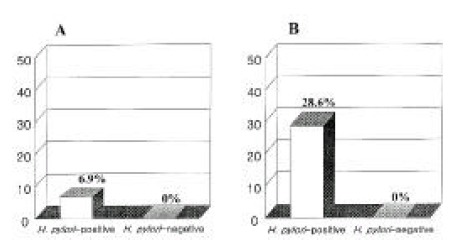
The positive rates of H. pylori PCR in the dental plaque (A) and saliva (B) depending on H. pylori positivity of gastric mucosa. PCR of dental plaque was positive in 6.9%, and that of saliva 28.6% in gastric H. pylori-positive subjects. In contrast, there was none in gastric H. pylori-negative subjects.
When gastric mucosa and saliva were compared, saliva CLO test was positive in 21 of 25 H. pylori-positive subjects (84.0%) and positive in 11 of 15 H. pylori-negative subjects (73.3%) (Table 3). However, saliva PCR was positive in 4 of 14 H. pylori-positive subjects (28.6%) and none of 6 H. pylori-negative subjects (Table 3, Figure 2B). Four saliva PCR positive subjects were all positive in saliva CLO test. They were diagnosed as DU in two, BGU in one and pyloric channel ulcer in one, and all of them showed active ulcers. Three of them were mucosal PCR positive in both antrum and body, but was remaining one was negative in both areas. The patient with negative mucosal PCR showed DP PCR positive, and, in the remaining three, all DP PCR were negative.
Table 3.
Comparison of H. pylori tests in the gastric mucosa and saliva
| Gastric Mucosal | H. Pylori | |
|---|---|---|
| Positive | Negative | |
| No. | 25 | 15 |
| Saliva CLO test positive | 21(84.0%) | 11(73.3%) |
| Saliva PCR positive | 4/14(28.6%) | 0/6(0%) |
DISCUSSION
It has been hypothesized that H. pylori infection is acquired by either oral-oral or fecal-oral transmission, or by common source exposure. Although H. pylori has been isolated from feces by culture29), detected in fecal samples30) and in drinking water by PCR assay31,32) and also isolated from cats33,34), to date no environmental source of H. pylori has been recognized with certainty. However, many studies have provided evidence that there is a significant correlation of H. pylori in the stomach and the mouth, and it is thought to be possible that H. pylori in the mouth plays an important role in transmission and recurrence after eradication therapy. In our country, the reinfection rate of H. pylori was rather high, 12.8% per year, suggesting that oral cavity might have some role as a reservoir of H. pylori.
H. pylori infection in the stomach is easily detected by the rapid urease test, histology, urea breath test and serology2), but detection of this bug in the oral cavity seems to be complicated. Several reports showed high positivity rate of urease test in the DP, saliva and gingival pockets, such as 84%–100%4,5,12). Similarly, our study showed the positivity rates of CLO test in the DP and saliva as 100% and 84.0%, respectively, from subjects with gastric H. pylori infection, but they were also high, 94.1% and 73.3% from subjects without gastric H. pylori infection (Table 2, 3). However, the PCR positivity rates in the DP and saliva were so low, 6.9% and 28,6%, respectively, even in gastric H. pylori-positive subjects, that CLO test in the DP and saliva looked like not reflecting the real H. pylori in each area. It is known that a positive urease test on a specimen obtained from the oral cavity should be interpreted with caution.
One study22) showed that a patient had H. pylori-like organisms in samples collected from tongue and palate. Both strains were urease, catalse and oxidase positive and grew microaerophilically, but they were negative on H. pylori-specific PCR analysis, demonstrating the possibility of false identification22). There are other urease-producing bacteria in the oral cavity, such as Actinomyces Viscosus and Strecptococcus Vestibularis, which may cause false-positive results35). Culture of H. pylori is recognized as the “gold standard” for the diagnosis of the infection. However, we could not succeed in H. pylori culture from DP and saliva specimens in any of these 46 participating subjects (data not shown) mainly due to overgrowth of other bacteria. However, recently there have been reports of successful culture from samples of oral cavity, 11% from saliva11), 19% from DP36), and 13% from oral cavity22). It is speculated that nonculturable coccoid forms of the organisms may survive in the mouth, and more specific and sensitive culture methods are required for the detection of H. pylori in the oral cavity37).
To date, many PCR assays have been developed for detecting H. pylori in the oral cavity. Most of them have been based on the sequence of urease genes and 16S ribosomal RNA genes. However, reports of high prevalence of H. pylori in the oral cavity as detected by urease gene-based PCR assay10,21,22) have been questioned because urease-positive organisms are commonly present in cultures from the oral mucosa38). It is suggested that to confirm the presence of H. pylori DNA in DP with PCR assays, a sequence that is not part of the urease gene should be used. In addition, results from the oral cavity have shown a great variation of PCR-positive rates: from 0 to 92.9%3,21,23–25). It is possible that sets of primers designed from different sequences may have different sensitivities and specificities and cause conflicting data. In our study, we used a new nested PCR assay which was developed from H. pylori genomic DNA with no homologous sequences in GenBank26,37). With this nested PCR assay with ET4-U and ET4-L primers, a 203 bp DNA fragment was amplified in 33 of 45 saliva samples collected from patients with gastric H. pylori infection. However, in our study, the sensitivity of the PCR in the gastric mucosa was rather low, 50.0%, and the specificity was relatively high 86.8%, based on H. pylori tests such as CLO test, touch print of Gram stain, Giemsa stain and culture. There is the possibility that this low sensitivity of the PCR might have caused an unerestimation of the detection rates of H. pylori in DP and saliva like 6.9% and 28.6%, respectively (Table 2, 3, Figure 2). In one study using three pairs of primers, the detection rates of H. pylori DNA in DP samples were 26.5%(9/34) for HPD1/HPD2, 78.9%(30/38) for HP1/ HP2, and 100%(40/40) for EHC-U/EHC-L, showing that PCR primers are very important39). Because the primers, ET4-L and ET4-U, were very sensitive and specific for Western H. pylori26), there is the possibility that H. pylori harbored in Koreans may be different from that in Westerners, especially in the amplifed 203 bp DNA sequence by these primers. In addition, several other factors are known to cause decrease of detection rates of H. pylori in DP and saliva. First, proper sampling is important: that is, saliva samples collected in the morning before teeth were brushed gave a higher rate of detection of H. pylori37). Second, collecting saliva samples directly into digestion buffer was important37). Third, the number of PCR cycles, the amount of template DNA and optimal buffers might have affected the detection rates of PCR. For example, we used 200 ng of DNA as templates for gastric mucosa and saliva, but below 50 ng for most a cases of DP, because collected DP usually showed very scanty amount of DNA. Therefore, there is the possibility that the lower detection rate of H. pylori in DP, 6.9%(2/29) than in saliva, 28.6%(4/14) might be caused by less amount of DNA in DP than in saliva, especially with low sensitivity rate of our PCR method. In addition, it is speculated that H. pylori in saliva is derived from DP because the positive rate of DP H. pylori was higher (100%) than that of saliva (59–70%)3,20).
In conclusion, the detection rates of H. pylori in DP and saliva by PCR were rather low, 6.9% and 28.6%, respectively, and these rates might have been underestimated by low sensitivity of the PCR method used in this study. However, the results that H. pylori was found in the DP and saliva suggest that oral cavity can preform a role as a reservoir of H. pylori in Korea.
REFERENCES
- 1.Lambert JR, Lin SK, Aranda-Michel J. Helicobacter pylori. Scand J Gastroenterol. 1995;30(Suppl 208):33–46. doi: 10.3109/00365529509107760. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin CS, Mendall MM, Northfield TC. Helicobacter pylori infection. Lancet. 1997;349:265–269. doi: 10.1016/S0140-6736(96)07023-7. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Musich PR, Ha T, Ferguson DA, Jr, Patel NR, Chi DS, Thomas E. High prevalence of Helicobacter pylori in saliva demonstrated by a novel PCR assay. J Clin Pathol. 1995;48:662–666. doi: 10.1136/jcp.48.7.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majmudar P, Shah SM, Dhunjibhoy KR, Desai HG. Isolation of Helicobacter pylori from dental plaques in healthy volunteers. Indian J Gastroenterol. 1990;9:271–272. [PubMed] [Google Scholar]
- 5.Desai HG, Gill HH, Shankaran K, Mehta PR, Prabhu SR. Dental plaque: a permanent reservoir of Helicobacter pylori? Scan J Gastroenterol. 1991;26:1205–1208. doi: 10.3109/00365529108998615. [DOI] [PubMed] [Google Scholar]
- 6.Kim N, Lim SH, Lee KH, Jung HC, Song IS, Kim CY. Helicobacter pylori reinfection rate and duodenal ulcer recurrence in Korea. J Clin Gastroenterol. 1998;27:321–326. doi: 10.1097/00004836-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Forbes GM, Glaser ME, Cullen DJ, Warren JR, Christiansen KJ, Marshall BJ, Collins BJ. Duodenal ulcer treated with Helicobacter pylori eradication: seven-year follow-up. Lancet. 1994;343:258–260. doi: 10.1016/s0140-6736(94)91111-8. [DOI] [PubMed] [Google Scholar]
- 8.Borody TJ, Andrews P, Mancuso N, McCauley D, Jankiewicz E, Ferch N, Shortis NP, Brandl S. Helicobacter pylori reinfection rate in patients with cured duodenal ulcer. Am J Gastroenterol. 1994;89:529–532. [PubMed] [Google Scholar]
- 9.Bell GD, Powell KU, Burridge SM, Harrison G, Rameh B, Weil J, Gant PW, Jones PH, Trowell JE. Reinfection or recrudescence after apparently successful eradication of Helicobacter pylori infection: implications for treatment of patients with duodenal ulcer disease. Q J Med. 1993;86:375–382. [PubMed] [Google Scholar]
- 10.Banatvala N, Lopez CR, Owen R, Abdi Y, Davis G, Hardie J, Feldman R. Helicobacter pylori in dental plaque. Lancet. 341(380):1993. doi: 10.1016/0140-6736(93)90191-i. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson DA, Jr, Li C, Patel NR, Mayberry WR, Chi DS, Thomas E. Isolation of Helicobacter pylori from saliva. J Clin Microbiol. 1993;31:2802–2804. doi: 10.1128/jcm.31.10.2802-2804.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pytko-Polonczyk J, Konturek SJ, Karczewska E, Bielanski W, Kaczmarczyk-Stachowska A. Oral cavity as permanent reservoir of Helicobacter pylori and potential source of reinfection. J Physiol Pharmacol. 1996;47:121–129. [PubMed] [Google Scholar]
- 13.Shames B, Krajden S, Fuksa M, Babida C, Penner JL. Evidence for the occurrence of the same strain of Campylobacter pylori in the stomach and dental plaque. J Clin Micorbiol. 1989;27:2849–2850. doi: 10.1128/jcm.27.12.2849-2850.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song QS, Zheng ZT, Yu H. Helicobacter pylori in dental plaque. Chung Hua Nei Ko Chih. 1994;33:459–461. [PubMed] [Google Scholar]
- 15.Bernander S, Dalen J, Gastrin B, Hedenborg L, Lamke LO, Obrn R. Absence of Helicobacter pylori in dental plaques in Helicobacter pylori positive dyspeptic patients. Eur J Clin Microbiol Infect Dis. 1993;12:282–285. doi: 10.1007/BF01967259. [DOI] [PubMed] [Google Scholar]
- 16.Bickley J, Owen RJ, Fraser AG, Pounder RE. Evaluation of the polymerase chain reaction for detecting the urease C gene of Helicobacter pylori in gastric biopsy samples and dental plaque. J Med Microbiol. 1993;39:338–344. doi: 10.1099/00222615-39-5-338. [DOI] [PubMed] [Google Scholar]
- 17.Hardo PG, Tugnait A, Hassan F, Lynch DAF, West AP, Mapstone NP, Quirke P, Chalmers DM, Kowolik MJ, Axon ATR. Helicobacter pylori infection and dental care. Gut. 1995;37:44–46. doi: 10.1136/gut.37.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luman W, Alkout AM, Blackwell CC, Weir DM, Palmer KR. Helicobacter pylori in the mouth-negative isolation from dental plaque and saliva. Eur J Gastroenterol Hepatol. 1996;8:11–14. doi: 10.1097/00042737-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Clayton CL, Kleanthous H, Coates PJ, Morgan DD, Tabaqchali S. Sensitive detection of Helicobacter pylori by using polymerase chain reaction. J Clin Microbiol. 1992;30:192–200. doi: 10.1128/jcm.30.1.192-200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Ha T, Ferguson DA, Jr, Chi DS, Zhao R, Patel NR, Krishnaswamy G, Thomas E. A newly developed PCR assay of H. pylori in gastric biopsy, saliva and feces. Evidence of high prevalence of H. pylori in saliva supports oral transmission. Dig Dis Sci. 1996;41:2142–2149. doi: 10.1007/BF02071393. [DOI] [PubMed] [Google Scholar]
- 21.Mapstone NP, Lynch DAF, Lewis FA, Axon ATR, Tompkins DS, Dixon MF, Quirke P. Identification of Helicobacter pylori DNA in the mouths and stomachs of patients with gastritis using PCR. J Clin Pathol. 1993;46:540–543. doi: 10.1136/jcp.46.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Namavar F, Roosendaal R, Kuipers EJ, de Groot P, Bijl MW, Pena AS, de Graff J. Presence of Helicobacter pylori in the oral cavity, oesophagus, stomach and faeces of patients with gastritis. Eur J Clin Microbiol Infect Dis. 1995;14:234–237. doi: 10.1007/BF02310363. [DOI] [PubMed] [Google Scholar]
- 23.Olsson K, Wadstrom T, Tyszkiewicz T. H. pylori in dental plaque. Lancet. 1993;341:956–957. doi: 10.1016/0140-6736(93)91245-h. [DOI] [PubMed] [Google Scholar]
- 24.Wahlfors J, Meurman JH, Toskala J, Korhonen A, Alakuijala P, Janatuien E, Karkkainen UM, Nuutinen P, Janne J. Development of a rapid PCR method for identification of Helicobacter pylori in dental plaque and gastric biopsy specimens. Eur J Clin Microbiol Infect Dis. 1995;14:780–786. doi: 10.1007/BF01690993. [DOI] [PubMed] [Google Scholar]
- 25.Hammar M, Tyszkeiwicz T, Wadstrom T, O’Toole PW. Rapid detection of Helicobacter pylori in gastric biopsy material by polymerase chain reaction. J Clin Microbiol. 1992;30:54–58. doi: 10.1128/jcm.30.1.54-58.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang C, Li C, Ha T, Ferguson DA, Jr, Chi DS, Laffan JJ, Thomas E. Identification of H. pylori in saliva by a nested PCR assay derived from a newly cloned DNA probe. Dig Dis Sci. 1998;43:1211–1218. doi: 10.1023/a:1018847522200. [DOI] [PubMed] [Google Scholar]
- 27.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 28.Parrish KD, Greenberg EP. A rapid method for extraction and purification of DNA from dental plaque. Appl Environ Microbiol. 1995;61:4120–4123. doi: 10.1128/aem.61.11.4120-4123.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas JE, Gibson GR, Darboe MK, Dale A, Weaver LT. Isolation of Helicobacter pylori from human faeces. Lancet. 1992;340:1194–1195. doi: 10.1016/0140-6736(92)92894-l. [DOI] [PubMed] [Google Scholar]
- 30.Mapstone NP, Lynch DA, Lewis FA, Axon AT, Tompkins DS, Dixon MF, Quirke P. PCR identification of Helicobacter pylori in faeces from gastritis patients. Lancet. 341(447):1993. doi: 10.1016/0140-6736(93)93053-4. [DOI] [PubMed] [Google Scholar]
- 31.Hulten K, Han SW, Enroth H, Klein PD, Opekun AR, Gilman RH, Evans DG, Engstrand L, Graham DY, El-Zaatari FA. Helicobacter pylori in the drinking water in Peru. Gastroenterology. 1996;110:1031–1035. doi: 10.1053/gast.1996.v110.pm8612990. [DOI] [PubMed] [Google Scholar]
- 32.Shahamat M, Mai U, Paszko-Kolva C, Kessel M, Colwell RR. Use of autoradiography to assess viability of Helicobacter pylori in water. Appl Environ Microbiol. 1993;59:1231–1235. doi: 10.1128/aem.59.4.1231-1235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox JG, Perkins S, Yan L, Shen Z, Attardo L, Pappo J. Local immune response in Helicobacter pylori-infected cats and identification of H. pylori in saliva, gastric fluid and faeces. Immunology. 1996;88:400–406. doi: 10.1046/j.1365-2567.1996.d01-677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Handt LK, Fox JG, Dewhirst FE, Fraser GJ, Paster BJ, Yan LL, Rozmiarek H, Rufo R, Stalis IH. Helicobacter pylori isolated from the domestic cat; public health implications. Infect Immunol. 1994;62:2367–2374. doi: 10.1128/iai.62.6.2367-2374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng LHH, Webberley M, Evans M, Hanson N, Brown R. Helicobacter pylori in dental plaque and gastric mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:421–423. doi: 10.1016/s1079-2104(96)80017-5. [DOI] [PubMed] [Google Scholar]
- 36.Khandaker K, Palmer KR, Eastwood MA, Scott AC, Desai M, Owen RJ. DNA fingerprints of Helicobacter pylori from mouth and antrum of patients with chronic ulcer dyspepsia. Lancet. 342(751):1993. doi: 10.1016/0140-6736(93)91747-a. [DOI] [PubMed] [Google Scholar]
- 37.Thomas E, Jiang C, Chi DS, Li C, Ferguson DA., Jr The role of the oral cavity in Helicobacter pylori infection. Am J Gastroenterol. 1997;92:2148–2154. [PubMed] [Google Scholar]
- 38.Lambert I, Clyne M, Drumm B. H. pylori in dental plaques. Lancet. 1993;341:956–957. [PubMed] [Google Scholar]
- 39.Song Q, Haller B, Schmid RM, Adler G, Bode G. Helicobacter pylori in dental plaque. A comparison of different PCR primer sets. Dig Dis Sci. 1999;44:479–484. doi: 10.1023/a:1026680618122. [DOI] [PubMed] [Google Scholar]


