Abstract
Primary intestinal T-cell lymphoma is a rare disease entity, which is approximately 10% to 25% of intestinal lymphomas, and most of the lymphomas occur in the small intestine. We report here a case of a 56-year-old woman who has been suffering from chronic diarrhea and weight loss for 6 months. Abdominal CT scan and small bowel series showed diffuse wall thickening of the small bowel. Gastroscopic examination showed diffuse erythematous lesions on the esophagus and small gastric ulcerations on the antrum of the stomach, and colonoscopic examination also showed multiple punched-out ulcerations and erosions on the entire colon, including the sigmoid colon to the terminal ileum. Diffuse infiltration of CD 3 positive lymphoma cells was found on biopsy. The patient was diagnosed as primary intestinal T-cell lymphoma with diffuse involvement of the entire gastrointestinal tracts from the esophagus to the rectum. Although the patient received systemic combination chemotherapy and achieved partial response initially, the lymphoma relapsed repeatedly.
Keywords: Intestinal, Lymphoma, T-lymphocytes, Gastrointestinal system, Malabsorption syndromes
INTRODUCTION
Approximately 10% to 25% of primary intestinal lymphomas have a T-cell immunophenotype. In contrast to B-cell lymphomas, which tend to form annular to polypoid masses in the distal or terminal ileum, intestinal T-cell lymphomas appear as ulcerated plaques or strictures in the proximal small intestine1). Although it has been estimated that 8–21% of intestinal lymphomas develop multifocally, diffuse and extensive gastrointestinal involvement of malignant lymphoma is very rare2,3). We report a case of primary intestinal T-cell lymphoma involving the entire gastrointestinal tract from the esophagus to the rectum in a 56-year-old woman.
CASE4
A 56-year-old woman was referred to our hospital with complaints of chronic diarrhea, weight loss, indigestion and abdominal pain lasting for 11 months. Before admission, the patient visited a private clinic and underwent gastroscopic and colonoscopic examinations. Gastroscopic examination showed small gastric ulcerations on the antrum and colonoscopic examination showed multiple punched-out ulcerations and erosions on the entire colon, including the sigmoid colon to the terminal ileum. Biopsy results were not diagnostic at that time. Under the clinical impression of intestinal tuberculosis, she received 2 months of antituberculosis medication without improvement of symptoms. The follow-up biopsy result of colonoscopic examination showed intestinal lymphoma of T-cell type. The patient was then referred for further evaluation. On admission, the patient looked pale, emaciated and dehydrated. An eleven kg weight loss was noted during the last 6 months (47kg--> 36kg). She denied fever or night sweating except complained of profuse watery diarrhea (5–6/day) and indigestion. She denied aggravation of diarrhea symptoms after wheat ingestion. No improvement of diarrhea was found by withdrawal of wheat intake. There was no palpable lymphadenopathy or hepatosplenomegaly. Diffuse abdominal tenderness on the whole abdomen was noted on abdominal physical examination. Initial laboratory findings were as follows: WBC 8,300/mm3, Hb 10.7 g/dL, Hct 32.6%, platelet 409,000/mm3, urine analysis was normal, stool occult blood was negative, serum total protein 5.2 mg/dL, albumin 2.3 mg/dL, serum LDH 128 IU, calcium 7.9 mg/dL, phosphorous 4.4 mg/dL, ALT/AST 12/13 U, cholesterol 101 mg/dL, BUN 13 mg/dL and creatinine 1.0 mg/dL. Chest X-ray was normal. Gastroscopic examination showed diffuse irregular nodular and depressed lesions on the angle and the lower body and multiple red spots on the antrum, suggesting erosive gastritis. But esophagus and stomach biopsies showed diffuse infiltration of lymphoma cells with epitheliotropic features (Figure 1, 2). Duodenal biopsy also revealed lymphoma cell infiltration, and villous atrophy and blunting were found. Colonoscopic findings were the same as noted previously (Figure 3) and the biopsy showed diffuse infiltration of small to medium-sized lymphoma cells (Figure 4). The infiltrated lymphoma cells were immunostained for UCHL-1 (+), CD 3 (+), MT-1 (+), L-26 (−) and MB-2 (−) (Figure 1.2.4). Small bowel series also showed chronic granulomatous lesions on the cecum and the distal ileum. Abdominal CT scan revealed diffuse wall thickening of the bowels, including the esophagus, the stomach and parts of the small bowel and colonic loop. There was no evidence of involvement of lymphoma on bilateral bone marrow biopsies. Finally, the patient was diagnosed as primary intestinal T-cell lymphoma involving the entire gastrointestinal tract (Ann Arbor Stage IVB) with malabsorption. She was treated with combination chemotherapy including cyclophosphamide, adriamycin, vincristine and prednisone (CHOP). Although after two cycles of chemotherapy the initially observed bowel wall thickening disappeared on follow-up abdominal CT scan and no definitive nodular or ulcerative lesions were noted, on follow-up gastro-and colonoscopic examinations lymphoma cells remained on the biopsy. Despite further treatment with 4 cycles of systemic chemotherapy, only a partial response was achieved.
Figure 1.
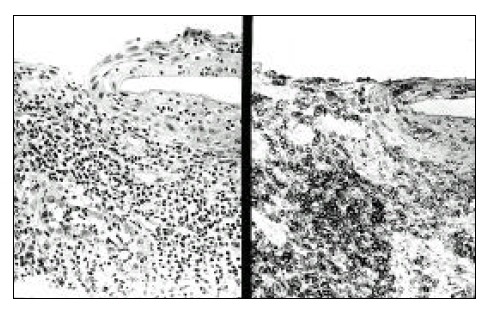
On esophageal biopsy, the mucosal architecture is replaced by small to medium-sized lymphoid cells (left) which react strongly positive to CD-3 (right).
Figure 2.
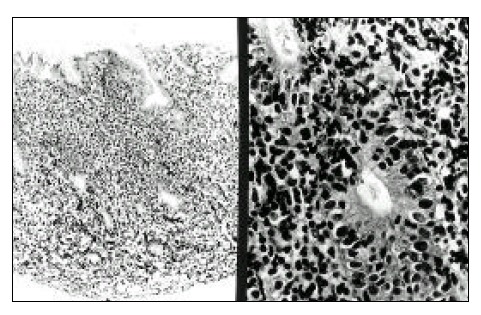
Gastric biopsy shows diffuse infiltration of lymphoma cells (left) and intraepithelial lymphocytosis are also noted (right).
Figure 3.
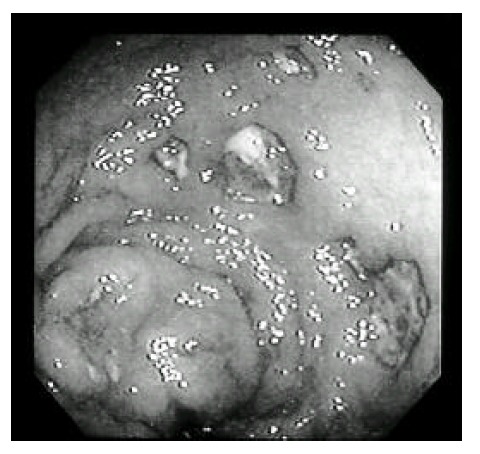
Colonoscopic exam shows diffuse hyperemia, ulceration and nodularity.
Figure 4.
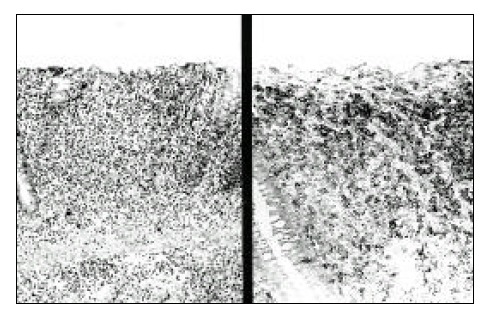
The mucosal architecture of the colon is mostly replaced by medium-sized lymphoma cells (left) that are reactive to CD-3 (right).
The patient was readmitted to our hospital with complaints of hematochezia. Colonoscopic examination showed internal hemorrhoid and diffuse hyperemic mucosa and multiple ulcerations were noted throughout the rectum to cecal area, suggesting relapse of lymphoma. After two cycles of salvage chemotherapy with etoposide, dexamethasone, adriamycin and cisplatin (EDAP), the bowel lesions were slightly improved but lymphoma cells were still found, indicating refractory to chemotherapeutic agents, agten then, the patient was treated without a specific treatment except supportive care. Eventually, the patient died of spontaneous bowel perforation.
DISCUSSION
The gastrointestinal tract is the most common site for primary extranodal lymphomas. The stomach is the most common site of primary gastrointestinal lymphoma, followed by the small intestine. Esophageal and colorectal lymphomas are rare. The small intestine accounts for 37% of these tumors and the ileum is more frequently involved than the jejunum4,5). Domizioet al6) collected over a period of four decades, 66% were B-cell and 34% T-cell tumors. Eighty-three percent of the T-cell tumors were high-grade malignancies and 49% were enteropathy-associated. Enteropathy-associated T-cell lymphoma occurs commonly in the jejunum, either alone or in combination with multiple other sites in the gastrointestinal tract1). Although several cases of primary intestinal T-cell lymphomas were reported7,8), it has not been reported that the patient had extensive lymphoma lesions confirmed by biopsy throughout the entire gastrointestinal tract which is very rare in Korea.
According to the cell size and nuclear morphologic features, intestinal T-cell lymphomas are subdivided into pleomorphic small cell, pleomorphic medium and/or large cell, immunoblastic, unclassifiable9), large cell anaplastic6) and rare other types, as well as a statement about whether the lymphoma is associated with enteropathy and eosinophilia10). Another classification, as well as the revised European-American classification of lymphoid neoplasm, does not stratify intestinal lymphomas into morphologic subtypes but recognizes them as a clinicopathologic entity that may or may not be associated with enteropathy11,12). Recently, Chott et al reported that intestinal T-cell lymphoma can be divided into two main categories: (1) small to medium-sized cell, comprising pleomorphic small and monomorphic medium-sized cell lymphomas; and (2) large cell, comprising the remaining subtypes. Because enteropathy may be associated with any subtype of intestinal T-cell lymphoma, presence vs absence should be reported13). In our case, most of the infiltrating lymphoma cells were composed of small to medium-sized cells immunostained for CD 3.
Several clinical spectrums may be explained by the association of malabsorption and lymphoma: patients with frank celiac disease; patients with intestinal mucosal pathology without evidence of malabsorption; or patients with latent celiac disease, in whom there is abnormal intestinal mucosal immunity without histologic evidence of villous flattening14,15). Although jejunal biopsy was not performed in our case, villous atrophy and blunting on duodenal biopsy indicate that malabsorption might be derived from the coexistence of celiac disease. Enteropathy-associated T-cell lymphoma is a specific subtype of primary intestinal T-cell lymphoma that 16,17). Most patients may have suffered from abdominal pain, weight loss, malabsorption and nonspecific symptoms for a period of years. However, presentation of this disease as an acute illness with perforation, obstruction or hemorrhage is not uncommon. A small proportion of patients has a history of celiac disease dating back to childhood. On the other hand, in many cases of enteropathy associated T-cell lymphoma, there is no history of celiac disease or biopsy evidence of gluten sensitivity and even the resected jejunal mucosa may appear normal18). The definition of enteropathy associated with lymphoma was based on the microscopic appearance of the uninvolved jejunal mucosa characterized by villous atrophy and increase of intraepithelial infiltration of the lymphoid cells (epitheliotropism)19). We noted that intraepithelial lymphocytosis (epitheliotropism), which is known to be a characteristic finding of enteropathy-associated T-cell lymphoma, was found on biopsy in this patient. In that sense, despite the fact that there was no clinical evidence of gluten-sensitive celiac disease, the microscopic findings could not completely exclude the presence of enteropathy.
Although the management of patients with primary intestinal T-cell lymphoma is the same as for those with other histologic type of aggressive lymphoma, the prognosis of intestinal T-cell lymphoma, especially enteropathy-associated T-cell lymphoma, is very poor compared with B-cell intestinal lymphoma6,20). In this patient, although rapid clinical and radiological responses were achieved after the initial two cycles of systemic chemotherapy, the biopsy result showed remaining lymphoma cells. Early relapse occurred and eventually the disease became refractory to systemic chemotherapy.
In conclusion, primary intestinal T-cell lymphoma involving the entire gastrointestinal tract is a very rare disease and is frequently associated with malabsorption. Since this disease has a poor response to systemic chemotherapy, a new treatment approach such as immunotherapy, may be needed to manage the disease.
REFERENCES
- 1.Isaacson PG. Gastrointestinal lymphomas of T-and B-cell types. Mod Pathol. 1999;12:151–158. [PubMed] [Google Scholar]
- 2.Lewin KJ, Ranchod M, Dorfman RF. Lymphomas of the gastrointestinal tract: a study of 117 cases presenting with gastrointestinal disease. Cancer. 1978;42:693–707. doi: 10.1002/1097-0142(197808)42:2<693::aid-cncr2820420241>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Mori S, Yamaguchi K. Primary malignant lymphomas of the intestine: a clinicopathological study of 116 cases. Recent Advances in Research. 1981;20:126–133. [Google Scholar]
- 4.d’Amore F, Christensen BE, Brincker H, Pedersen NT, Thorling K, Hastrup J, Pedersen M, Jensen MK, Johansen P, Andersen E. Clinicopathological features and prognostic factors in extranodal non-Hodgkin’s lymphomas. Eur J Cancer. 1991;27:1201–1208. doi: 10.1016/0277-5379(91)90081-n. [DOI] [PubMed] [Google Scholar]
- 5.Crump M, Gospodarowicz M, Shepherd FA. Lymphoma of the gastrointestinal tract. Sem Oncol. 1999;26:324–337. [PubMed] [Google Scholar]
- 6.Domizio P, Owen RA, Shepherd NA, Talbot IC, Norton AJ. Primary lymphoma of the small intestine: A clinicopathologic study of 119 cases. Am J SurgPathol. 1993;17:429–442. doi: 10.1097/00000478-199305000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Kim YH, Song TJ, Ryu HS, Hyun JH. A case of primary T-cell lymphoma of the duodenum. Korean J Intern Med. 1991;6:44–50. doi: 10.3904/kjim.1991.6.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min KR, Park KN, Lee JD. Histological and immunohistochemical studies on primary gastrointestinal lymphomas. J Hanyang Med Coll. 1992;12:164–178. doi: 10.3346/jkms.1993.8.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall PA, Jass JR, Levison DA, Morson BC, Shepherd NA, Sobin L, Stansfeld AG. Classification of primary gut lymphomas. Lancet. 1988;ii:958. doi: 10.1016/s0140-6736(88)92620-7. [DOI] [PubMed] [Google Scholar]
- 10.Hall PA, Levison DA. Malignant lymphoma in the gastrointestinal tract. Semin Diagn Pathol. 1991;8:163–177. [PubMed] [Google Scholar]
- 11.Issacson PG, Spencer J, Wright DH. Classifying primary gut lymphomas. Lancet. 1988;ii:1148–1149. doi: 10.1016/s0140-6736(88)90574-0. [DOI] [PubMed] [Google Scholar]
- 12.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, DeWolf-Peeters C, Falini B, Gatter KC. A revised European-American classification of lymphoid neoplasm: a proposal from the international lymphoma study group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 13.Chott A, Vesely M, Simonitsch I, Mosberger I, Hanak H. Classification of intestinal T-cell neoplasms and their differential diagnosis. Am J Clin Pathol. 1999;111(Suppl.1):S68–74. [PubMed] [Google Scholar]
- 14.Swinson CM, Slavin G, Coles EC, Booth CC. Celiac disease and malignancy. Lancet. 1983;1:111–115. doi: 10.1016/s0140-6736(83)91754-3. [DOI] [PubMed] [Google Scholar]
- 15.O’Mahony S, Vesty JP, Ferguson A. Similarities in intestinal humoral immunity in dermatitis herpetiformis without enteropathy and in celiac disease. Lancet. 1990;335:1487–1490. doi: 10.1016/0140-6736(90)93029-o. [DOI] [PubMed] [Google Scholar]
- 16.Isaacson PG, Wright DH. Intestinal lymphoma associated with malabsorption. Lancet. 1978;1:67–70. doi: 10.1016/s0140-6736(78)90004-1. [DOI] [PubMed] [Google Scholar]
- 17.Egan LJ, Walsh SV, Stevens FM, Connolly CE, Egan EL, McCarthy CF. Celiac-associated lymphoma. A single institution experience of 30 cases in the combination chemotherapy era. J Clin Gastroenterol. 1995;21:123–129. [PubMed] [Google Scholar]
- 18.Loberant N, Cohen I, Noi I, Herskovits M, Szvalb S. Enteropathy -associated T cell lymphoma: a case report with radiographic and computed tomography appearance. J Surg Oncol. 1997;65:50–54. doi: 10.1002/(sici)1096-9098(199705)65:1<50::aid-jso10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.O’Farrelly C, Feighery C, O’Brian DS. Humoral response to wheat protein in patients with celiac disease and enteropathy associated T-cell lymphoma. Br Med J. 1986;293:908–910. doi: 10.1136/bmj.293.6552.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaacson PG. Gastrointestinal lymphoma. Hum Pathol. 1994;25:1020–1029. doi: 10.1016/0046-8177(94)90060-4. [DOI] [PubMed] [Google Scholar]


