Abstract
Oxidative stress appears to have an important role in glucocorticoid insensitivity, as a crucial problem in asthma therapy. We studied the preventive effect of antioxidant N-acetylcysteine (NAC) on the airway hyper-responsiveness (AHR) and the accumulation of inflammatory cells in the airways in an animal model of steroid resistant acute exacerbation of asthma. Systemically sensitized Balb/C mice were exposed to Ovalbumin aerosol on days 13, 14, 15 and 16, followed by intratracheal lipopolysaccharide (LPS) to induce acute exacerbation. NAC (intraperitoneal, 320 mg/kg 30 min before and 12 hours after each challenge) reduced hyper-responsiveness with/out dexamethasone. LPS application caused neutrophilia in bronchoalveolar lavage fluid (BALF) and eosinophil count was higher than respective control in BALF as well as neutrophils after dexamethasone treatment. NAC significantly decreased neutrophil and eosinophil count in BALF as well as inflammatory cytokines (IL-13 and IL-5).We concluded that addition of NAC to asthma therapy has beneficial preventive effects in an animal model of steroid resistant acute exacerbation of asthma.
Keywords: steroid resistant asthma, acute exacerbation, N-acetylcysteine, eosinophil, neutrophil
Introduction
Asthma is a chronic disorder which is associated with inflammation and immune cell infiltration to the airways (Song et al., 2009[25]). Constant presence of inflammatory mediators and immune cells in airways result in airway hyper-responsiveness (AHR) which is clinically pictured as breathlessness, coughing and wheezing (Li et al., 2010[18]). Among asthma medications glucocorticoids are the most effective and first line therapeutics in reducing inflammation. However, some patients with severe asthma experience resistance to steroids even at high doses of glucocorticoids. Steroid insensitivity is a crucial problem in asthma therapy and adds cost burden to the health system as well as patient discomfort (Barnes, 2010[3]; Marwick et al., 2010[21]). In contrast to mild/moderate asthma, the major characteristic of severe asthma is neutrophilia and an elevation in oxidative stress in the lung (Marwick et al., 2010[21]).
The oxidative stress may have both exogenous and endogenous sources and its increase in the lung is a key element in development of glucocorticoid resistance in respiratory diseases (Chung and Adcock, 2008[9]; Ciencewicki et al., 2008[10]). As an endogenous source, pro-inflammatory cells such as macrophages and neutrophils can produce reactive oxygen/nitrogen species (Marwick et al., 2010[21]). Comparing to other cell types, neutrophils and their resulting inflammation is irresponsive to glucocorticoids (Marwick et al., 2009[22]). As oxidative stress plays an important role in glucocorticoid insensitivity, targeting it with antioxidants seems to be a good choice.
N-acetylcysteine (NAC) has shown a therapeutic effect in oxidative stress decrease in some respiratory diseases (Cai et al., 2009[8]). Therefore, there is an open window for NAC's likely effect on glucocorticoid sensitivity in severe neutrophilic asthma. In this study we investigated the predictive effect of NAC, as an antioxidant, in an animal model of steroid resistant acute exacerbation of asthma.
Materials and Methods
Animals
Male specific pathogen free Balb/C mice, 6-8 weeks, were purchased from animal services unit, University of Newcastle, NSW. Animals were housed in individual cages. Animals were kept in an approved condition under controlled temperature and humidity and received food and water ad libitum with 12 hour light/dark cycle. All protocols for experiments and procedures were approved by the Animal Care and Use Committee of the University of Newcastle.
Experimental model
Animals were sensitized intra-peritoneally (i.p.) by injection of 50 μg of chicken egg ovalbumin (OVA) (Grade V, ≥ 98 % pure; Sigma, St Louis, MO) adsorbed to 1 mg of Alhydrogel (Reheis Inc., Berkley Heights, NJ, USA) suspended in total volume of 200 µL of 0.9 % sterile saline on day 0 before the commencement of aerosol challenge. Non-sensitized animals were injected the same suspension without OVA. All groups were exposed to aerosolized OVA (10 mg/ml OVA dissolved in 0.9 % sterile saline for 30 min) on days 13, 14, 15 and 16 using an ultrasonic nebulizer in a whole-body inhalation exposure chamber. Intratracheal (i.t.) lipopolysaccharide (LPS) (100 ng/mouse) was applied to sensitized group and saline to non-sensitized group on day 22.
Drug treatment
N-acetylcysteine (NAC) (Sigma-Aldrich) was dissolved in phosphate buffer saline (PBS) and was neutralized to the pH 7.4 with bicarbonate (1 mol/L) to the final volume of 200 µL (Máximo Cardoso et al., 2006[23]). Freshly made NAC solution was injected i.p. (320 mg/kg) every 12 hours during aerosol days starting 30 min before the first challenge and ending 12 hours after the last challenge. At 24 and 2 hours before the LPS challenge, animals received either dexamethasone (Dex) (1 mg/kg; Sigma-Aldrich), or vehicle alone by i.p. injection. Moreover, NAC was injected to the animals 30 min before and 12 hours after LPS challenge.
Experimental groups
There were 8 groups in this study and groups included 8 animals each. Groups were as follow: 1) PBS/OVA/Veh/Veh (non-sensitized, NAC vehicle, Dex vehicle), 2) PBS/OVA/NAC/Veh (non-sensitized, NAC, Dex vehicle), 3) PBS/OVA/ Veh/Dex (non-sensitized, NAC vehicle, Dex), 4) PBS/OVA/NAC/Dex (non-sensitized, NAC, Dex), 5) OVA/OVA/Veh/ Veh (sensitized, NAC vehicle, Dex vehicle), 6) OVA/OVA/NAC/Veh (sensitized, NAC, Dex vehicle), 7) OVA/OVA/Veh/ Dex (sensitized, NAC vehicle, Dex), 8) OVA/ OVA/NAC/Dex (sensitized, NAC, Dex).
Animals were sacrificed on day 23 for AHR and inflammatory cell assessment in broncho-alveolar lavage fluid (BALF).
Measurement of airway hyper-responsiveness
Airway responses to increasing doses of β-methacholine (Mch) (Sigma-Aldrich) were determined 24 hours after LPS challenge by means of Flexivent apparatus (Scireq; Montreal, Quebec, Canada). Mice were anesthetized with a combination of Ketamine-Xylazine. Concentrations for Ketamine (Parnell, Alexandria, New South Wales, Australia) and Xylazine (Troy Laboratories, Smithfield, New South Wales, Australia) were 40 mg/ml and 2 mg/ml, respectively. Animals were tracheostomized and a tracheal cannula was inserted in trachea and mice attached to the machine. A supplementary dose of anesthetic mixture was injected after ventilation started at the rate of 450 breaths/min with tidal volume of 8 ml/kg and positive end-expiratory pressure of 2 cm H2O. After mice were settled and baseline was recorded, Mch challenge was performed starting with aerosolized saline as a first dose followed by rising concentrations of freshly prepared Mch (0.1, 0.3, 1, 3, 10 and 30 mg/ml; Sigma-Aldrich). Each dose was nebulized through the ultrasonic nebulizer provided by Scireq to be installed on the inspiration pathway of the machine. If coefficient of determination (COD) was less than 95 %, the record was excluded. Rn corresponds to the resistance of central airways. AHR was described as a significant change in Rn comparing to control group. Data is shown as percentage increase in Rn over saline control (Li et al., 2010[18]; Ito et al., 2008[14]).
Broncho-alveolar lavage (BAL)
After performing Flexivent, mice were sacrificed with sodium pentobarbitone (100 mg/kg) (Lethabarb, Virbac, Australia) and the broncho-alveolar lavage was performed with 2 × 0.9 ml Hank's Buffered Salt Solution (HBSS). BALF from each animal was collected in a plastic tube separately and kept on ice. All tubes then centrifuged (3000 g) at 4 °C for 5 min. Cell pellets were re-suspended in 50 µL of RBC lysis buffer and centrifuged again after 5 min. Pellets re-suspended in 200 µL HBSSC and cell viability was carried out with Trypan blue. Then cell suspensions were cytospined (Cytospin 4, Thermo Shandon, NC, USA). After 24 hours, slides were stained with May-Grünwald/Giemsa and cover slipped; then counted on at least 300 cells (Ito et al., 2008[14]). Differential cell counts were compared to the respective control group.
RT-QPCR
Total RNA extracted from the whole lung samples, stored in -80 °C, and reverse transcription were carried out for samples using TRIzol RNA isolation buffer following the manufacturer's instructions (Invitrogen). cDNA synthesis was performed using oligo(dT)-primed reverse transcriptase reactions consuming 0.5 mg RNA from each sample. Transcription levels of IL-13 and IL-5 genes, and HPRT, as a housekeeping gene, were quantified. IL-13 forward: "5'-AGCTGCAACATCACACAAGACC" and reverse "5'-TGGGCTACTTCGATTTTGGTATCG", IL-5 forward "5'-TGTTGACAAGCAATGAGACGATGA" and reverse "5'-AATAGCATTTCCACAGTACCCCCA" and HPRT forward "AGGCCAGACTTTGTTGGATTTGAA" and reverse "CAACTTGCGCTCATCTTAGGCTTT" primers (Sigma, UK) were used for real-time PCR. Mastercycler ep realplex and cyber green were used to quantify gene transcripts and to detect changes in amplicon levels with each sequential amplification cycle. The levels of mRNA from groups were normalized to HPRT.
Statistical analysis
Data are presented as mean ± SD. Statistical analysis were performed by one way analysis of variance (ANOVA) and two way ANOVA followed by Bonferroni test for post-hoc analysis using GraphPad Prism 5 (GraphPad Software Inc, San Diego, CA, USA). Significance was defined at the level of p<0.05.
Results
NAC reduces hyper-responsiveness with/out dexamethasone
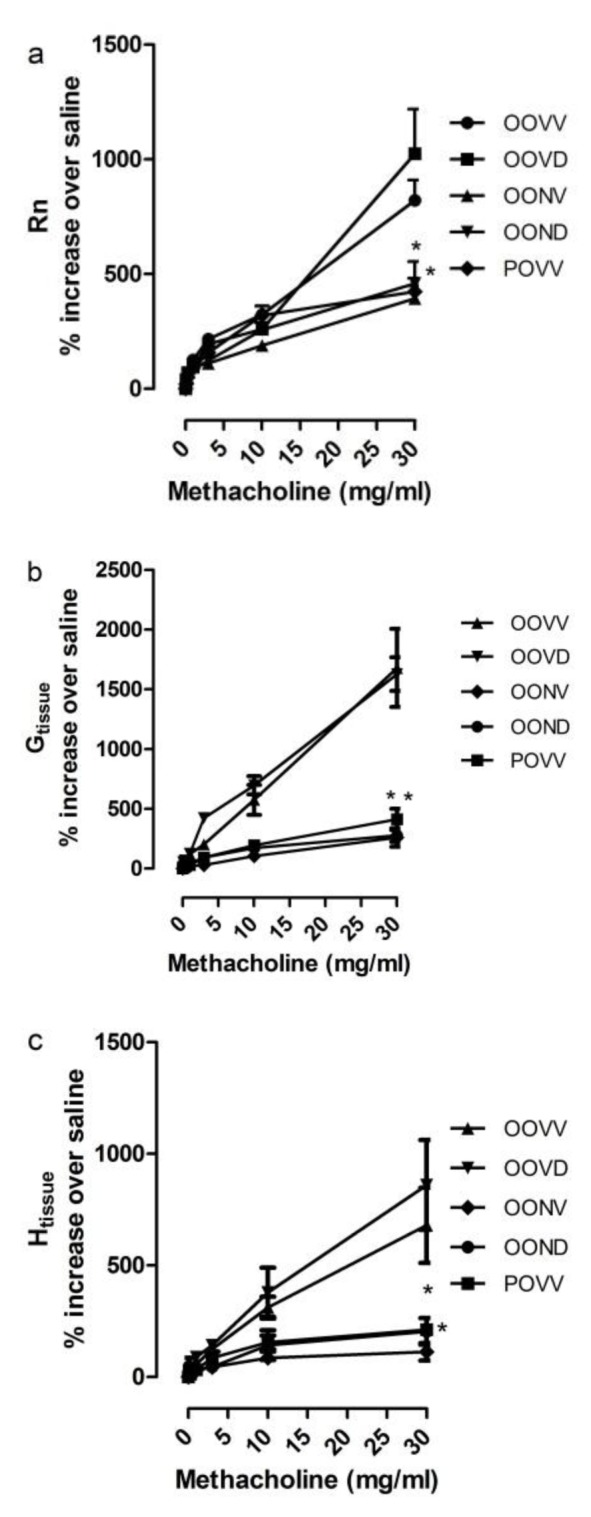
OVA sensitized, OVA aerosolized mice showed AHR (Figure 1(Fig. 1)) and increase in airway inflammation comparing to respective control group. Significant increase in airway resistance (Rn), tissue damping (G) and tissue elastance (H) were observed after LPS challenge (Figure 1(Fig. 1)). OVA aerosol treated mice exhibited higher AHR rate in dose 30 mg/ml Mch comparing to respective control group which was persistent after dexamethasone application (Figure 1(Fig. 1)). However, NAC could decrease Rn, G and H with or without application of Dex in OVA treated mice (Figure 2(Fig. 2)).
Figure 1. Asthma induction contributes in AHR which is resistant to Dex. AHR was studied using Flexivent apparatus and increasing doses of Mch. Airway resistance (Rn) (a), Tissue damping (b) and tissue elastance (c) were significantly increased in asthma exacerbation. Dex application did not reduce the AHR. *p<0.05, as compared to respective controls.
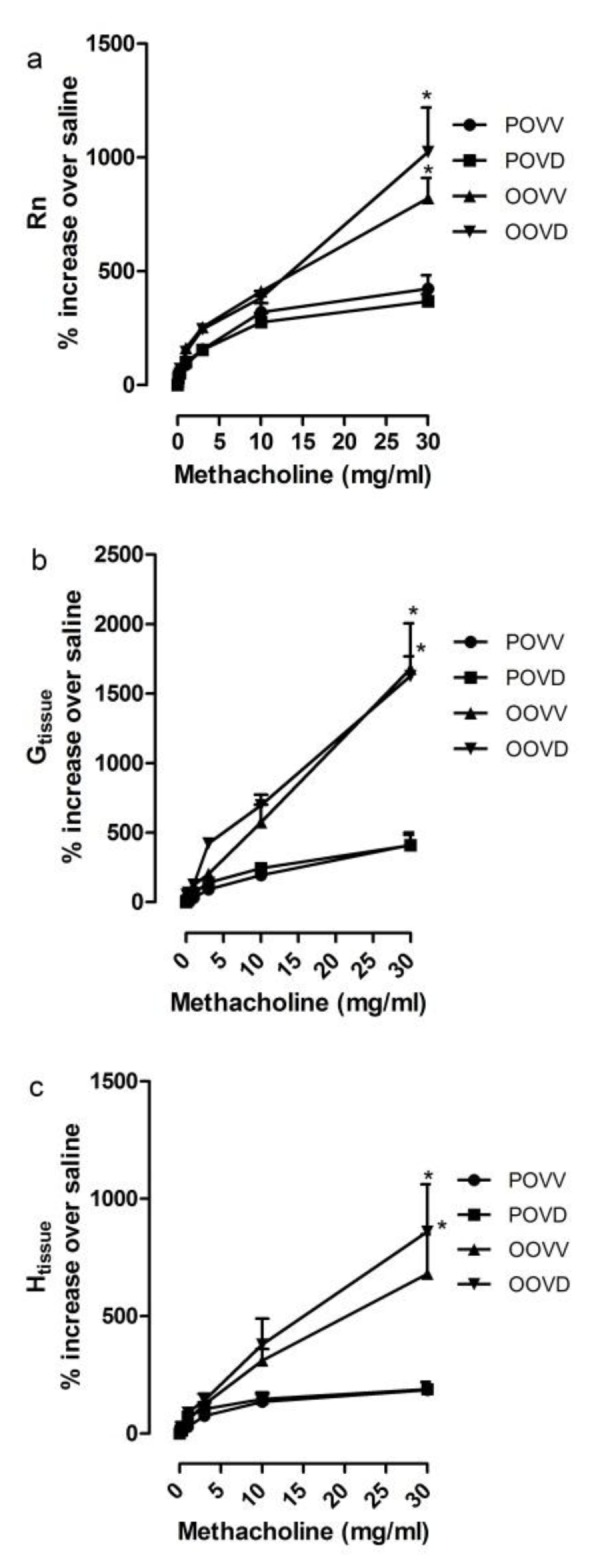
Figure 2. NAC reduces AHR in asthma exacerbation with/out Dex. AHR was studied using Flexivent apparatus and increasing doses of Mch. Airway resistance (Rn) (a), Tissue damping (b) and tissue elastance (c) were decreased to the control levels in asthma exacerbation with NAC application (320 mg/kg every 12 hours). *p<0.05, as compared to respective controls.
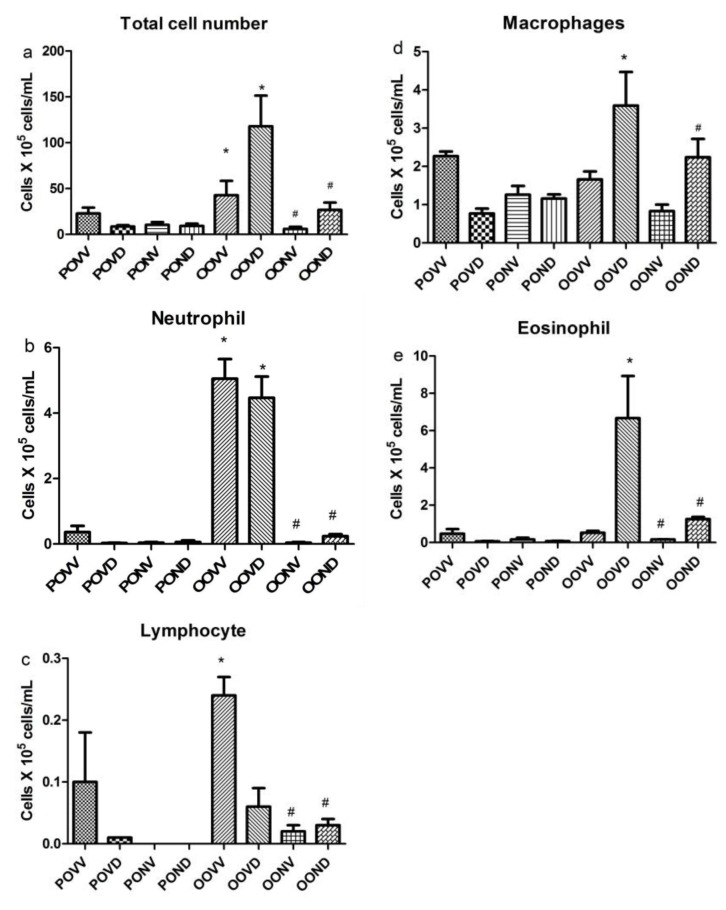
NAC decreases airway inflammation by affecting cell recruitment
Asthma exacerbation resulted in total cell number increase in airways which was decreased significantly by NAC application (Figure 3a(Fig. 3)). LPS treatment caused neutrophilia in airways which was resistant to dexamethasone. NAC significantly decreased neutrophil count in BALF (p<0.05) (Figure 3b(Fig. 3)). Total number of lymphocytes was increased in BALF after LPS induction. However, this increase was not resistant to Dex. NAC decreased the lymphocyte recruitment to the lung without Dex presence (Figure 3c(Fig. 3)). Macrophages were recruited to the lung after LPS challenge followed by Dex injection which was decreased significantly to respective control levels by NAC application (Figure 3d(Fig. 3)). Eosinophil count was higher than respective control in BALF after LPS induction and Dex treatment. However, NAC decreased eosinophilia in this group (Figure 3e(Fig. 3)).
Figure 3. Inducing LPS contributes to neutrophilia in airways which is resistant to Dex. NAC application prevented neutrophilia (a). Number of lymphocytes was increased with asthma induction which was not resistant to Dex. NAC prevented lymphocyte accumulation in airways (b). The number of macrophages and eosinophils increased with asthma induction and Dex application but not with asthma induction alone (c-e). *p<0.05, as compared to respective control; #p<0.05, as compared to respective asthma.
NAC decreases inflammation by regulating gene expression
Gene expression was quantified using Cyber green and real time PCR machine and the level of amplicons were normalized to HPRT gene expression. Asthma exacerbation induction increased the IL-13 and IL-5 gene expression levels in the lung which was resistant to Dex. As it is shown in Figure 4(Fig. 4), IL-13 gene expression increased dramatically after asthma exacerbation and Dex application did not affect the increase in IL-13 gene expression. However, NAC decreased IL-13 expression level to control levels even in absence of Dex. IL-5 gene expression surged in asthma exacerbation. This surge was intensely higher in Dex treated asthma group. NAC application diminished the IL-5 level with or without Dex use (Figure 5(Fig. 5)).
Figure 4. IL-13 expression is fed by asthma induction. Induction of asthma resulted in a dramatic increase in IL-13 gene expression which was resistant to steroid therapy. Application of NAC (320 mg/kg) every 12 hours starting 30 min before the first OVA challenge and finishing 12 hours after the last challenge prevented the expression of this chemokine and kept it to the control levels. Data are presented as means ± S.E. (error bars), n = 8; *p<0.05 for the comparisons to the respective control and #p<0.05 for the comparisons to the respective asthma group.
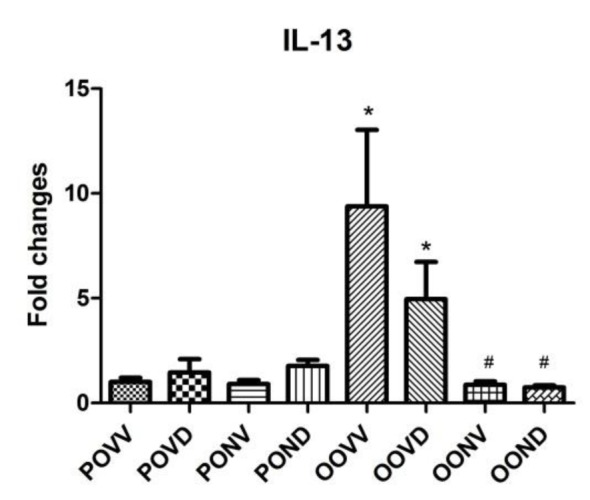
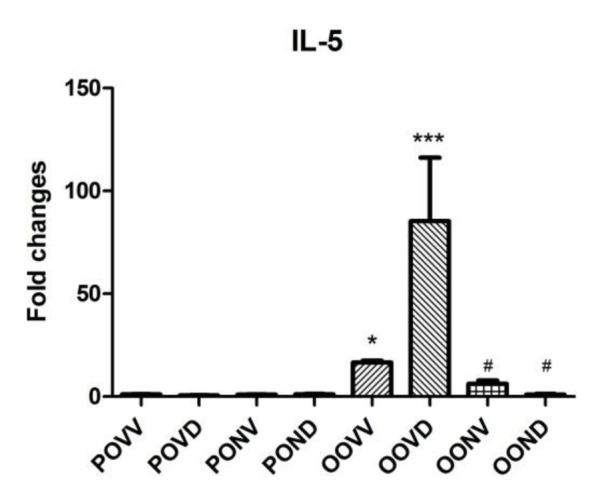
Figure 5. IL-5 expression is increased by asthma induction. Induction of asthma resulted in an increase in IL-5 gene expression in asthma group while this rise was drastic after Dex application. Application of NAC (320 mg/kg) every 12 hours starting 30 min before the first OVA challenge and finishing 12 hours after the last challenge prevented the expression of this chemokine and kept it to the control levels. Data are presented as means ± S.E. (error bars), n = 8; *p<0.05 and ***p<0.001 for the comparisons to the respective control and #p<0.05 for the comparisons to the respective asthma group.
Discussion
Applied model led to steroid resistant acute exacerbation of asthma in mice which resulted in AHR, increased airway resistance, tissue damping and tissue elastance. Also, total cell number, especially neutrophils, elevated in airways after LPS induction. NAC application decreased AHR and cell recruitment to the lung.
Intraperitoneal injection of OVA sensitizes mice and activates immune cells in airways (Ito et al., 2008[14]). OVA aerosol increases the cell activation, migration and cytokine secretion. IL-13 and IL-5, released from Th2 cells, increase eosinophils by stimulating bone marrow to produce more progenitor cells and alter their differentiation to eosinophils and affect their migration to the lung (Larché, 2006[16]). Moreover, these cytokines increase airway hyper-responsiveness which is the clinical picture of asthma. Also, LPS presentation to the immune cells recruits neutrophils to the lung (Li et al., 2010[18]). The results of this study show that intratracheal administration of LPS lead in neutrophilia in BALF (Figure 3b(Fig. 3)). Interestingly, Dex application increased the number of eosinophils in BALF in steroid resistant asthmatic mice. Previous studies show excessive IL-5 secretion in presence of Dex helps in survival of eosinophils in the lung which is in accordance with our results (Bloom et al., 2004[7]).
High levels of oxidative stress are seen in patients with asthma and have been implicated as a driving force behind the inflammatory response and corticosteroid insensitivity in severe asthma (Kirkham and Rahman, 2006[15]). Therefore, antioxidants that neutralize this oxidative stress in the airways might be of benefit and may restore corticosteroid sensitivity in asthmatic patients (Barnes, 2004[2]). NAC is one of the most widely investigated antioxidants and reduces oxidant induced lung damage in a variety of experimental models (Cortijo et al., 2001[12]; Leff et al., 1993[17]; Bernareggi et al., 1999[5]). However, the influence of NAC in experimental models of asthma remains uncertain. Blesa et al. reported that oral treatment with NAC produce anti-hyperreactivity and anti-inflammatory effects in an animal model of allergic asthma (Blesa et al., 2002[6]). On the other hand, Aliyali et al. demonstrated that addition of NAC to usual asthma medication has no significant effect in treatment of asthma exacerbation (Aliyali et al., 2010[1]). According to our knowledge, there is no study to investigate the impact of NAC in steroid resistant asthma. We found that NAC was effective to reduce the AHR as well as the eosinophil and neutrophil counts in bronchoalveolar lavage fluid in animal model of steroid resistant asthma.
Oxidative stress activates p38 Mitogen Activated Protein Kinase (MAPK) in airway smooth muscle (Wuyts et al., 2003[26]). p38 MAPK could lead to glucocorticoid insensitivity suggestively by interfering with the nuclear localization of glucocorticoid receptors (Irusen et al., 2002[13]). In addition, p38 MAPK pathway provides cell proliferation and differentiation in airway smooth muscle which is responsible for AHR. Beside antioxidant properties, NAC inhibits p38 MAPK pathway (Wuyts et al., 2003[26]). Hence, NAC could be of benefit in restoring steroid sensitivity and reducing the AHR in steroid resistant asthma.
NAC increases GSH levels in cells and provides antioxidant defense to the cells. NAC affects immune responses and cytokine production (Bengtsson et al., 2001[4]). It is shown that oxidative burst of eosinophils is decreased by application of NAC on human eosinophils in vitro. Additionally, reactive oxidative species production by neutrophils is reduced by NAC which is the result of GSH level elevation in these cells (Martinez-Losa et al., 2007[20]). As it is shown in Figure 3(Fig. 3), NAC reduces the eosinophil and neutrophil count in asthma groups comparing to the control groups which is in accordance with the results of other studies. The suggested mechanism is NAC inhibits NADPH oxidase translocation to neutrophils' and eosinophils' membrane (Martinez-Losa et al., 2007[20]). Besides its direct effect on eosinophils, NAC has effect on immune cell attractive chemokines including IL-5 (Bengtsson et al., 2001[4]). As explained before, IL-5 is an eosinophil recruiting cytokine (Shannon et al., 2008[24]) and (Corren, 2012[11]). Reducing IL-5 by NAC (Figure 5(Fig. 5)) decreases the number of eosinophils in lung (Figure 3e(Fig. 3)). IL-13 is another cytokine released from Th-2 cells and is a marker of asthma (Liang et al., 2012[19]). NAC application reduced IL-13 (Figure 4(Fig. 4)) and AHR consequently. Studies have shown that NAC affected cytokine release from Th-2 cells which confirms our results (Bengtsson et al., 2001[4]).
In conclusion the results of this study indicated that addition of NAC to asthma therapy could be effective in increasing steroid sensitivity, reducing AHR and decreasing inflammatory cells in airways in an animal model of steroid resistant acute exacerbation of asthma.
References
- 1.Aliyali M, Poorhasan Amiri A, Sharifpoor A, Zalli F. Effects of N-acetylcysteine on asthma exacerbation. Iran J Allergy Asthma Immunol. 2010;9:103–109. [PubMed] [Google Scholar]
- 2.Barnes PJ. Corticosteroid resistance in airway diseases. Proc Am Thorac Soc. 2004;1:264–268. doi: 10.1513/pats.200402-014MS. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ. Mechanisms and resistance in glucocorticoid control of inflammation. J Steroid Biochem Mol Biol. 2010;120:76–85. doi: 10.1016/j.jsbmb.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Bengtsson A, Lundberg M, Avila-Cariño J, Jacobsson G, Holmgren A, Scheynius A. Thiols decrease cytokine levels and down-regulate the expression of CD30 on human allergen-specific T helper (Th) 0 and Th2 cells. Clin Exp Immunol. 2001;123:350–360. doi: 10.1046/j.1365-2249.2001.01453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernareggi M, Radice S, Rossoni G, Oriani G, Chiesara E, Berti F. Hyperbaric oxygen increases plasma exudation in rat trachea: involvement of nitric oxide. Br J Pharmacol. 1999;126:794–800. doi: 10.1038/sj.bjp.0702354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blesa S, Cortijo J, Martinez-Losa M, Mata M, Seda E, Santangelo F, et al. Effectiveness of oral N -acetylcysteine in a rat experimental model of asthma. Pharmacol Res. 2002;45:135–140. doi: 10.1006/phrs.2001.0917. [DOI] [PubMed] [Google Scholar]
- 7.Bloom JW, Chacko J, Lohman IC, Halonen M, Martinez FD, Miesfeld RL. Differential control of eosinophil survival by glucocorticoids. Apoptosis. 2004;9:97–104. doi: 10.1023/B:APPT.0000012126.06126.c4. [DOI] [PubMed] [Google Scholar]
- 8.Cai S, Chen P, Zhang C, Chen JB, Wu J. Oral N-acetylcysteine attenuates pulmonary emphysema and alveolar septal cell apoptosis in smoking-induced COPD in rats. Respirology. 2009;14:354–359. doi: 10.1111/j.1440-1843.2009.01511.x. [DOI] [PubMed] [Google Scholar]
- 9.Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31:1334–1356. doi: 10.1183/09031936.00018908. [DOI] [PubMed] [Google Scholar]
- 10.Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008;122:456–468. doi: 10.1016/j.jaci.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corren J. Inhibition of interleukin-5 for the treatment of eosinophilic diseases. Discov Med. 2012;13:305–312. [PubMed] [Google Scholar]
- 12.Cortijo J, Cerdá-Nicolás M, Serrano A, Bioque G, Estrela JM, Santangelo F, et al. Attenuation by oral N-acetylcysteine of bleomycin-induced lung injury in rats. Eur Respir J. 2001;17:1228–1235. doi: 10.1183/09031936.01.00049701. [DOI] [PubMed] [Google Scholar]
- 13.Irusen E, Matthews JG, Takahashi A, Barnes PJ, Chung KF, Adcock IM. p38 Mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma. J Allergy Clin Immunol. 2002;109:649–657. doi: 10.1067/mai.2002.122465. [DOI] [PubMed] [Google Scholar]
- 14.Ito K, Herbert C, Siegle JS, Vuppusetty C, Hansbro N, Thomas PS, et al. Steroid-resistant neutrophilic inflammation in a mouse model of an acute exacerbation of asthma. Am J Respir Cell Mol Biol. 2008;39:543–550. doi: 10.1165/rcmb.2008-0028OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther. 2006;111:476–494. doi: 10.1016/j.pharmthera.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Larché M. Immunoregulation by targeting T cells in the treatment of allergy and asthma. Curr Opin Immunol. 2006;18:745–750. doi: 10.1016/j.coi.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Leff JA, Wilke CP, Hybertson BM, Shanley PF, Beehler CJ, Repine JE. Postinsult treatment with N-acetyl-L-cysteine decreases IL-1-induced neutrophil influx and lung leak in rats. Am J Physiol Lung Cell Mol Physiol. 1993;265:L501–L506. doi: 10.1152/ajplung.1993.265.5.L501. [DOI] [PubMed] [Google Scholar]
- 18.Li JJ, Wang W, Baines KJ, Bowden NA, Hansbro PM, Gibson PG, et al. IL-27/IFN-γ induce MyD88-dependent steroid-resistant airway hyperresponsiveness by inhibiting glucocorticoid signaling in macrophages. J Immunol. 2010;185:4401–4409. doi: 10.4049/jimmunol.1001039. [DOI] [PubMed] [Google Scholar]
- 19.Liang RY, Wu W, Huang J, Jiang SP, Lin Y. Magnesium affects the cytokine secretion of CD4(+) T lymphocytes in acute asthma. J Asthma. 2012 Nov 8;epub ahead of print. doi: 10.3109/02770903.2012.739240. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Losa M, Cortijo J, Juan G, O'Connor JE, Sanz MJ, Santangelo F, et al. Inhibitory effects of N-acetylcysteine on the functional responses of human eosinophils in vitro. Clin Exp Allergy. 2007;37:714–722. doi: 10.1111/j.1365-2222.2007.02694.x. [DOI] [PubMed] [Google Scholar]
- 21.Marwick JA, Adcock IM, Chung KF. Overcoming reduced glucocorticoid sensitivity in airway disease molecular mechanisms and therapeutic approaches. Drugs. 2010;70:929–948. doi: 10.2165/10898520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Marwick JA, Caramori G, Stevenson CS, Casolari P, Jazrawi E, Barnes PJ, et al. Inhibition of PI3Kdelta restores glucocorticoid function in smoking-induced airway inflammation in mice. Am J Respir Crit Care Med. 2009;179:542–548. doi: 10.1164/rccm.200810-1570OC. [DOI] [PubMed] [Google Scholar]
- 23.Máximo Cardoso L, de Almeida Colombari DS, Vanderlei Menani J, Alves Chianca D, Jr, Colombari E. Cardiovascular responses produced by central injection of hydrogen peroxide in conscious rats. Brain Res Bull. 2006;71:37–44. doi: 10.1016/j.brainresbull.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Shannon J, Ernst P, Yamauchi Y, Olivenstein R, Lemiere C, Foley S, et al. Differences in airway cytokine profile in severe asthma compared to moderate asthma. Chest. 2008;133:420–426. doi: 10.1378/chest.07-1881. [DOI] [PubMed] [Google Scholar]
- 25.Song DJ, Min MG, Miller M, Cho JY, Broide DH. Environmental tobacco smoke exposure does not prevent corticosteroids reducing inflammation, remodeling, and airway hyperreactivity in mice exposed to allergen. Am J Physiol Lung Cell Mol Physiol. 2009;297:L380–L387. doi: 10.1152/ajplung.90588.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wuyts WA, Vanaudenaerde BM, Dupont LJ, Demedts MG, Verleden GM. N-acetylcysteine reduces chemokine release via inhibition of p38 MAPK in human airway smooth muscle cells. Eur Respir J. 2003;22:43–49. doi: 10.1183/09031936.03.00064803. [DOI] [PubMed] [Google Scholar]