Abstract
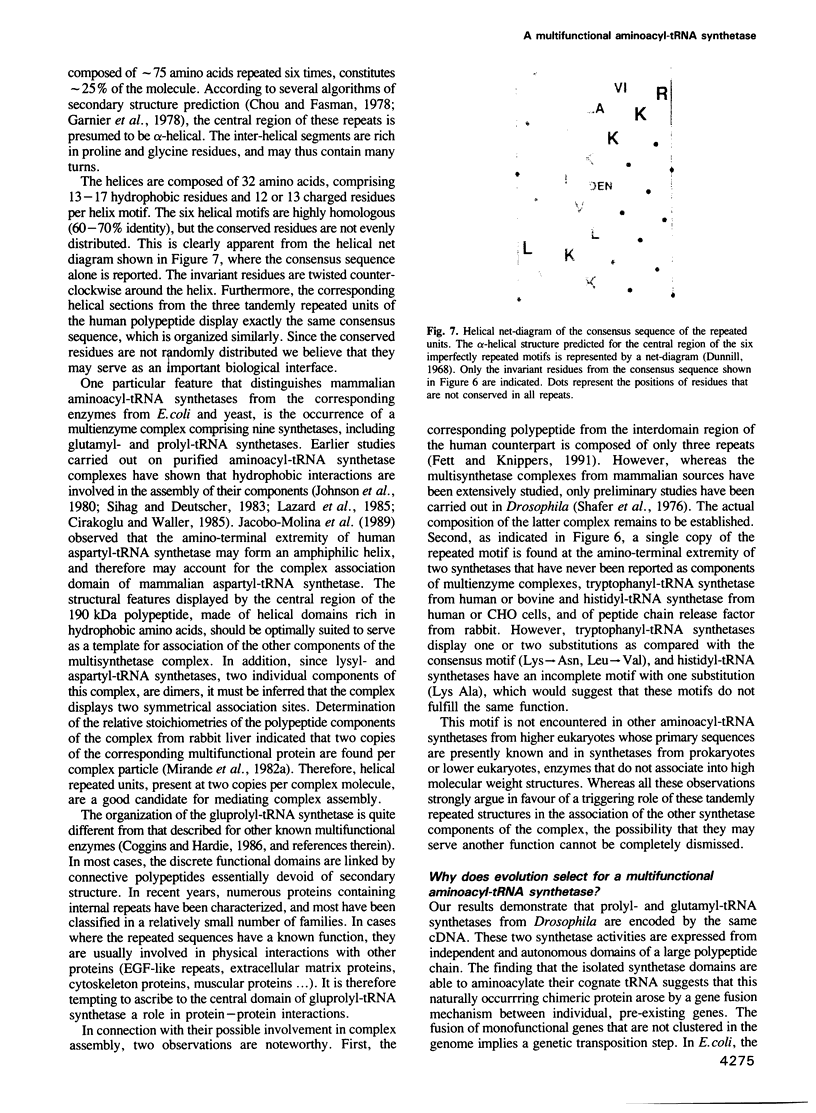
In higher eukaryotes, nine aminoacyl-tRNA synthetases are associated within a multienzyme complex which is composed of 11 polypeptides with molecular masses ranging from 18 to 150 kDa. We have cloned and sequenced a cDNA from Drosophila encoding the largest polypeptide of this complex. We demonstrate here that the corresponding protein is a multifunctional aminoacyl-tRNA synthetase. It is composed of three major domains, two of them specifying distinct synthetase activities. The amino and carboxy-terminal domains were expressed separately in Escherichia coli, and were found to catalyse the aminoacylation of glutamic acid and proline tRNA species, respectively. The central domain is made of six 46 amino acid repeats. In prokaryotes, these two aminoacyl-tRNA synthetases are encoded by distinct genes. The emergence of a multifunctional synthetase by a gene fusion event seems to be a specific, but general attribute of all higher eukaryotic cells. This type of structural organization, in relation to the occurrence of multisynthetase complexes, could be a mechanism to integrate several catalytic domains within the same particle. The involvement of the internal repeats in mediating complex assembly is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bec G., Kerjan P., Zha X. D., Waller J. P. Valyl-tRNA synthetase from rabbit liver. I. Purification as a heterotypic complex in association with elongation factor 1. J Biol Chem. 1989 Dec 15;264(35):21131–21137. [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
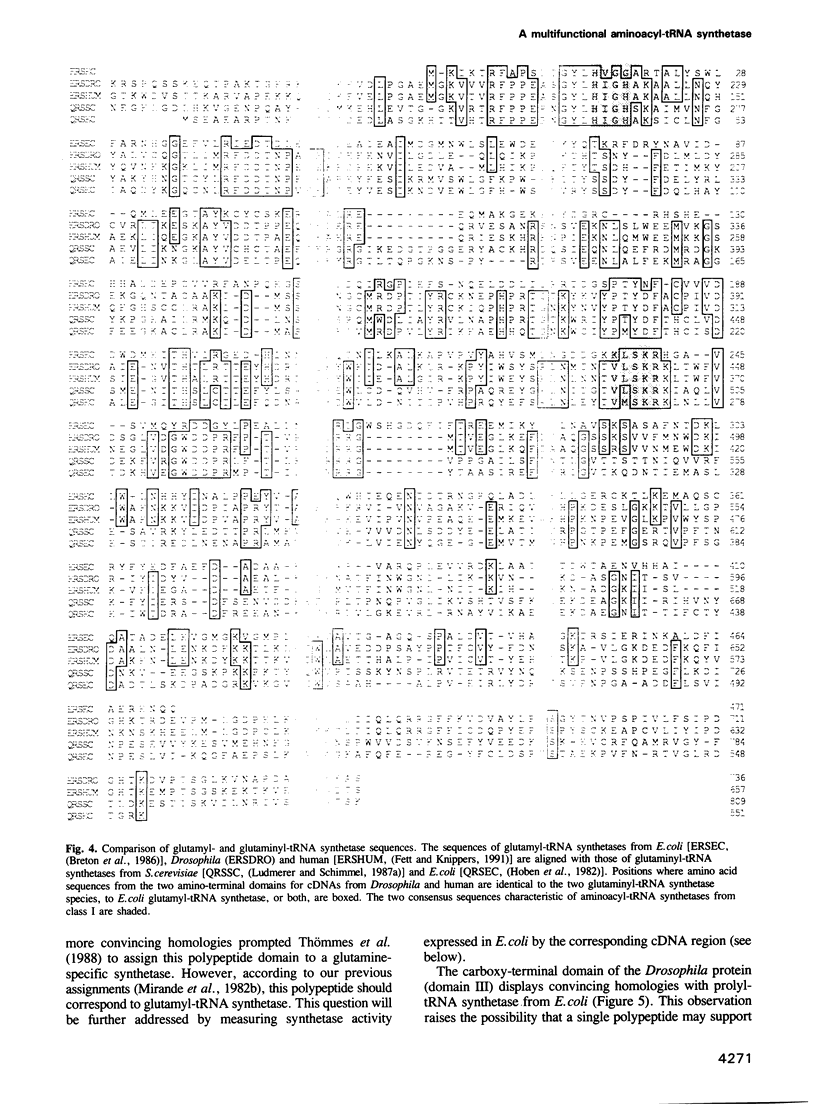
- Breton R., Sanfaçon H., Papayannopoulos I., Biemann K., Lapointe J. Glutamyl-tRNA synthetase of Escherichia coli. Isolation and primary structure of the gltX gene and homology with other aminoacyl-tRNA synthetases. J Biol Chem. 1986 Aug 15;261(23):10610–10617. [PubMed] [Google Scholar]
- Breton R., Watson D., Yaguchi M., Lapointe J. Glutamyl-tRNA synthetases of Bacillus subtilis 168T and of Bacillus stearothermophilus. Cloning and sequencing of the gltX genes and comparison with other aminoacyl-tRNA synthetases. J Biol Chem. 1990 Oct 25;265(30):18248–18255. [PubMed] [Google Scholar]
- Brick P., Bhat T. N., Blow D. M. Structure of tyrosyl-tRNA synthetase refined at 2.3 A resolution. Interaction of the enzyme with the tyrosyl adenylate intermediate. J Mol Biol. 1989 Jul 5;208(1):83–98. doi: 10.1016/0022-2836(89)90090-9. [DOI] [PubMed] [Google Scholar]
- Brunie S., Zelwer C., Risler J. L. Crystallographic study at 2.5 A resolution of the interaction of methionyl-tRNA synthetase from Escherichia coli with ATP. J Mol Biol. 1990 Nov 20;216(2):411–424. doi: 10.1016/S0022-2836(05)80331-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cirakoglu B., Waller J. P. Leucyl-tRNA and lysyl-tRNA synthetases, derived from the high-Mr complex of sheep liver, are hydrophobic proteins. Eur J Biochem. 1985 Aug 15;151(1):101–110. doi: 10.1111/j.1432-1033.1985.tb09074.x. [DOI] [PubMed] [Google Scholar]
- Cusack S., Berthet-Colominas C., Härtlein M., Nassar N., Leberman R. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 A. Nature. 1990 Sep 20;347(6290):249–255. doi: 10.1038/347249a0. [DOI] [PubMed] [Google Scholar]
- Dessen P., Fondrat C., Valencien C., Mugnier C. BISANCE: a French service for access to biomolecular sequence databases. Comput Appl Biosci. 1990 Oct;6(4):355–356. doi: 10.1093/bioinformatics/6.4.355. [DOI] [PubMed] [Google Scholar]
- Dunnill P. The use of helical net-diagrams to represent protein structures. Biophys J. 1968 Jul;8(7):865–875. doi: 10.1016/S0006-3495(68)86525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriani G., Delarue M., Poch O., Gangloff J., Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990 Sep 13;347(6289):203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- Eriani G., Dirheimer G., Gangloff J. Aspartyl-tRNA synthetase from Escherichia coli: cloning and characterisation of the gene, homologies of its translated amino acid sequence with asparaginyl- and lysyl-tRNA synthetases. Nucleic Acids Res. 1990 Dec 11;18(23):7109–7118. doi: 10.1093/nar/18.23.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriani G., Dirheimer G., Gangloff J. Cysteinyl-tRNA synthetase: determination of the last E. coli aminoacyl-tRNA synthetase primary structure. Nucleic Acids Res. 1991 Jan 25;19(2):265–269. doi: 10.1093/nar/19.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett R., Knippers R. The primary structure of human glutaminyl-tRNA synthetase. A highly conserved core, amino acid repeat regions, and homologies with translation elongation factors. J Biol Chem. 1991 Jan 25;266(3):1448–1455. [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hoben P., Royal N., Cheung A., Yamao F., Biemann K., Söll D. Escherichia coli glutaminyl-tRNA synthetase. II. Characterization of the glnS gene product. J Biol Chem. 1982 Oct 10;257(19):11644–11650. [PubMed] [Google Scholar]
- Hou Y. M., Shiba K., Mottes C., Schimmel P. Sequence determination and modeling of structural motifs for the smallest monomeric aminoacyl-tRNA synthetase. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):976–980. doi: 10.1073/pnas.88.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hountondji C., Dessen P., Blanquet S. Sequence similarities among the family of aminoacyl-tRNA synthetases. Biochimie. 1986 Sep;68(9):1071–1078. doi: 10.1016/s0300-9084(86)80181-x. [DOI] [PubMed] [Google Scholar]
- Jacobo-Molina A., Peterson R., Yang D. C. cDNA sequence, predicted primary structure, and evolving amphiphilic helix of human aspartyl-tRNA synthetase. J Biol Chem. 1989 Oct 5;264(28):16608–16612. [PubMed] [Google Scholar]
- Johnson D. L., Van Dang C., Yang D. C. Purification and characterization of lysyl-tRNA synthetase after dissociation of the particulate aminoacyl-tRNA synthetases from rat liver. J Biol Chem. 1980 May 10;255(9):4362–4366. [PubMed] [Google Scholar]
- Katinka M., Cossart P., Sibilli L., Saint-Girons I., Chalvignac M. A., Le Bras G., Cohen G. N., Yaniv M. Nucleotide sequence of the thrA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5730–5733. doi: 10.1073/pnas.77.10.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazard M., Mirande M., Waller J. P. Purification and characterization of the isoleucyl-tRNA synthetase component from the high molecular weight complex of sheep liver: a hydrophobic metalloprotein. Biochemistry. 1985 Sep 10;24(19):5099–5106. doi: 10.1021/bi00340a021. [DOI] [PubMed] [Google Scholar]
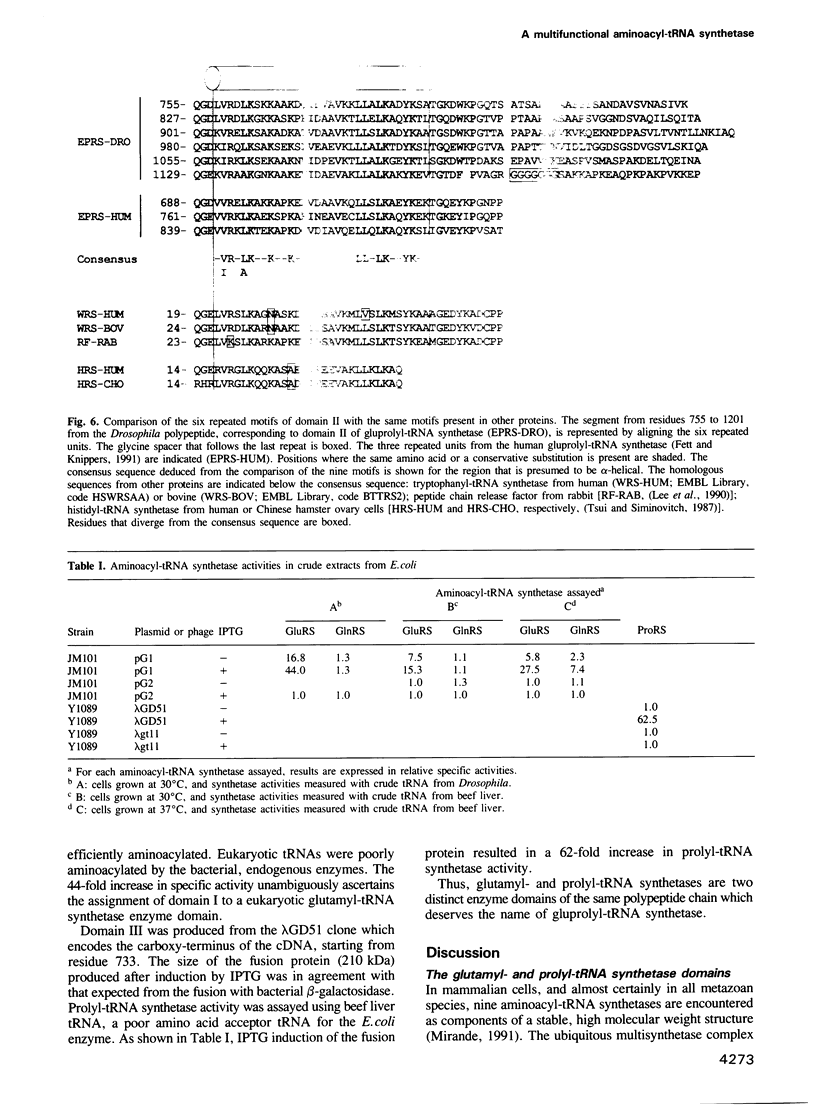
- Lee C. C., Craigen W. J., Muzny D. M., Harlow E., Caskey C. T. Cloning and expression of a mammalian peptide chain release factor with sequence similarity to tryptophanyl-tRNA synthetases. Proc Natl Acad Sci U S A. 1990 May;87(9):3508–3512. doi: 10.1073/pnas.87.9.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludmerer S. W., Schimmel P. Construction and analysis of deletions in the amino-terminal extension of glutamine tRNA synthetase of Saccharomyces cerevisiae. J Biol Chem. 1987 Aug 5;262(22):10807–10813. [PubMed] [Google Scholar]
- Ludmerer S. W., Schimmel P. Gene for yeast glutamine tRNA synthetase encodes a large amino-terminal extension and provides a strong confirmation of the signature sequence for a group of the aminoacyl-tRNA synthetases. J Biol Chem. 1987 Aug 5;262(22):10801–10806. [PubMed] [Google Scholar]
- Mirande M. Aminoacyl-tRNA synthetase family from prokaryotes and eukaryotes: structural domains and their implications. Prog Nucleic Acid Res Mol Biol. 1991;40:95–142. doi: 10.1016/s0079-6603(08)60840-5. [DOI] [PubMed] [Google Scholar]
- Mirande M., Cirakoğlu B., Waller J. P. Macromolecular complexes from sheep and rabbit containing seven aminoacyl-tRNA synthetases. III. Assignment of aminoacyl-tRNA synthetase activities to the polypeptide components of the complexes. J Biol Chem. 1982 Sep 25;257(18):11056–11063. [PubMed] [Google Scholar]
- Mirande M., Gache Y., Le Corre D., Waller J. P. Seven mammalian aminoacyl-tRNA synthetases co-purified as high molecular weight entities are associated within the same complex. EMBO J. 1982;1(6):733–736. doi: 10.1002/j.1460-2075.1982.tb01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirande M., Kellermann O., Waller J. P. Macromolecular complexes from sheep and rabbit containing seven aminoacyl-tRNA synthetases. II. Structural characterization of the polypeptide components and immunological identification of the methionyl-tRNA synthetase subunit. J Biol Chem. 1982 Sep 25;257(18):11049–11055. [PubMed] [Google Scholar]
- Mirande M., Le Corre D., Waller J. P. A complex from cultured Chinese hamster ovary cells containing nine aminoacyl-tRNA synthetases. Thermolabile leucyl-tRNA synthetase from the tsH1 mutant cell line is an integral component of this complex. Eur J Biochem. 1985 Mar 1;147(2):281–289. doi: 10.1111/j.1432-1033.1985.tb08748.x. [DOI] [PubMed] [Google Scholar]
- Mirande M., Waller J. P. Molecular cloning and primary structure of cDNA encoding the catalytic domain of rat liver aspartyl-tRNA synthetase. J Biol Chem. 1989 Jan 15;264(2):842–847. [PubMed] [Google Scholar]
- Motorin YuA, Wolfson A. D., Orlovsky A. F., Gladilin K. L. Mammalian valyl-tRNA synthetase forms a complex with the first elongation factor. FEBS Lett. 1988 Oct 10;238(2):262–264. doi: 10.1016/0014-5793(88)80492-7. [DOI] [PubMed] [Google Scholar]
- Nolan J. M., Lee M. P., Wyckoff E., Hsieh T. S. Isolation and characterization of the gene encoding Drosophila DNA topoisomerase II. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3664–3668. doi: 10.1073/pnas.83.11.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney S. D., Royal N. J., Neuman de Vegvar H., Herlihy W. C., Biemann K., Schimmel P. Primary structure of a large aminoacyl-tRNA synthetase. Science. 1981 Sep 25;213(4515):1497–1501. doi: 10.1126/science.7025207. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Söll D., Steitz T. A. Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 A resolution. Science. 1989 Dec 1;246(4934):1135–1142. doi: 10.1126/science.2479982. [DOI] [PubMed] [Google Scholar]
- Ruff M., Krishnaswamy S., Boeglin M., Poterszman A., Mitschler A., Podjarny A., Rees B., Thierry J. C., Moras D. Class II aminoacyl transfer RNA synthetases: crystal structure of yeast aspartyl-tRNA synthetase complexed with tRNA(Asp). Science. 1991 Jun 21;252(5013):1682–1689. doi: 10.1126/science.2047877. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P. Aminoacyl tRNA synthetases: general scheme of structure-function relationships in the polypeptides and recognition of transfer RNAs. Annu Rev Biochem. 1987;56:125–158. doi: 10.1146/annurev.bi.56.070187.001013. [DOI] [PubMed] [Google Scholar]
- Schray B., Thömmes P., Knippers R. Glutaminyl-tRNA synthetase as a component of the high-molecular weight complex of human aminoacyl-tRNA synthetases. An immunological study. Biochim Biophys Acta. 1990 Oct 23;1087(2):226–234. doi: 10.1016/0167-4781(90)90209-k. [DOI] [PubMed] [Google Scholar]
- Schön A., Kannangara C. G., Gough S., Söll D. Protein biosynthesis in organelles requires misaminoacylation of tRNA. Nature. 1988 Jan 14;331(6152):187–190. doi: 10.1038/331187a0. [DOI] [PubMed] [Google Scholar]
- Sellami M., Fasiolo F., Dirheimer G., Ebel J. P., Gangloff J. Nucleotide sequence of the gene coding for yeast cytoplasmic aspartyl-tRNA synthetase (APS); mapping of the 5' and 3' termini of AspRS mRNA. Nucleic Acids Res. 1986 Feb 25;14(4):1657–1666. doi: 10.1093/nar/14.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihag R. K., Deutscher M. P. Perturbation of the aminoacyl-tRNA synthetase complex by salts and detergents. Importance of hydrophobic interactions and possible involvement of lipids. J Biol Chem. 1983 Oct 10;258(19):11846–11850. [PubMed] [Google Scholar]
- Thömmes P., Fett R., Schray B., Kunze N., Knippers R. The core region of human glutaminyl-tRNA synthetase homologies with the Escherichia coli and yeast enzymes. Nucleic Acids Res. 1988 Jun 24;16(12):5391–5406. doi: 10.1093/nar/16.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui F. W., Siminovitch L. Isolation, structure and expression of mammalian genes for histidyl-tRNA synthetase. Nucleic Acids Res. 1987 Apr 24;15(8):3349–3367. doi: 10.1093/nar/15.8.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. N., Tener G. M. Chromatography of Drosophila tRNA on BD-cellulose. Can J Biochem. 1973 Jun;51(6):896–902. doi: 10.1139/o73-111. [DOI] [PubMed] [Google Scholar]