Abstract
Eremosparton songoricum (Litv.) Vass. is an endemic and extremely drought-resistant desert plant with populations that are gradually declining due to the failure of sexual recruitment. The effects of drought stress on the seed germination and physiological characteristics of seeds and seedlings were investigated. The results showed that the germination percentage decreased with an increase of polyethylene glycol 6000 (PEG) concentration: -0.3 MPa (5 % PEG) had a promoting effect on seed germination, -0.9 MPa (15 % PEG) dramatically reduced germination, and -1.8 MPa (30 % PEG) was the threshold for E. songoricum germination. However, the contents of proline and soluble sugars and the activity of CAT increased with increasing PEG concentrations. At the young seedling stage, the proline content and CAT, SOD and POD activities all increased at 2 h and then decreased; except for a decrease at 2 h, the MDA content also increased compared to the control (0 h). These results indicated that 2 h may be a key response time point for E. songoricum to resist drought stress. The above results demonstrate that drought stress can suppress and delay the germination of E. songoricum and that the seeds accumulate osmolytes and augment the activity of antioxidative enzymes to cope with drought injury. E. songoricum seedlings are sensitive to water stress and can quickly respond to drought but cannot tolerate drought for an extended period. Although such physiological and biochemical changes are important strategies for E. songoricum to adapt to a drought-prone environment, they may be, at least partially, responsible for the failure of sexual reproduction under natural conditions.
Keywords: drought stress, seed germination, seedling growth, physiological characteristics, Eremosparton songoricum
Introduction
Drought is a severe limitation of plant growth, development and productivity, particularly in arid and semi-arid regions (Galle et al., 2007[12]), and the response characters of plants exposed to drought stress have become a crucial environmental research topic in drought-prone regions. Seed germination and early seedling growth are potentially the most critical stages for water stress (Ahmad et al., 2009[2]). Many studies on plant responses to drought stress with regard to seed germination and seedling growth have been recently reported, including studies of agricultural crops, Oryza sativa, Avena sativa, Spinacia oleracea, Amaranthus mangostanus and helianthus annuus (Ahmad et al., 2009[2]; Chen and Arora, 2011[9]; Gao et al., 2008[13]; Mut et al., 2010[26]; Sun et al., 2011[31]), and grasses, Anthephora pubescens, Heteropogon contortus, Themeda triandra and Medicago sativa (den Berg and Zeng, 2006[11]; Wang et al., 2009[35]). In contrast, few reports have focused on desert plants, even though adaptation to drought stress in desert plants, particularly for some dominant species, is critical to the conservation and restoration of these plants.
Eremosparton songoricum (Litv.) Vass. is a leafless perennial semi-shrub belonging to the genus Eremosparton, subtribe Coluteinae, tribe Galegeae in Papilionoideae (Shi et al., 2010[27]). This leguminous desert shrub is a rare species and is a unique species of Eremosparton in China (Yin et al., 2006[37]). With strong wind resistance and sand-fixing characteristics, E. songoricum is considered to be a pioneer plant of sand dunes and plays an important role in maintaining the stability of desert ecosystems (Shi et al., 2010[28]). However, this species has been gradually declining over the past decades and is listed as rare due to both internal (heritability, reproductivity, adaptability) and external (environmental pressure, human disturbance) factors (Yin et al., 2006[37]).
E. songoricum grows in mobile and semi-mobile sand dunes and tolerates frequent water scarcity. Therefore, as a pioneer desert plant, drought stress may be the most primary and crucial environmental factor for the survival and reproduction of E. songoricum. This shrub has two reproductive strategies: asexual reproduction via underground rhizomes and sexual propagation by seeds (Liu et al., 2010[20]; Wang et al., 2011[33]). However, in natural populations, sexual propagation often fails due to low seed production (<16 %) and poor seedling recruitment: the proportion of non-dormant seeds that develop into seedlings and survive to the end of the growing season is only 0.2 % (Zhang et al., 2008[39], 2011[38]). Recently, the mechanisms responsible for the failure of E. songoricum sexual reproduction have been studied with regard to aspects of pollination ecology (Shi et al., 2010[28]), fruit limitation (Shi et al., 2010[27]), soil seed bank distribution (Liu et al., 2011[19]; Shi et al., 2011[29]), seed recruitment (Ma et al., 2007[22]), population dynamics, genetic diversity and differentiation (Liu et al., 2010[20][21]; Wang et al., 2011[34]). However, the physiological responses and consequences of the effects of drought stress on seed germination and early seedling growth as a means to clarify the observed reproductive failure have not been reported to date.
To evaluate the physiological responses to PEG-induced drought stress, seeds were germinated under various PEG concentrations (0, 5, 10, 15, 20, 25 and 30 %) to determine the germination and recovery percentages. In addition, the malondialdehyde (MDA) content, free proline and soluble sugar concentrations and activities of superoxide dismutase (SOD), catalase (CAT) and peroxidase enzyme (POD) were measured. Furthermore, the above parameters and the chlorophyll content were also monitored in two-week-old seedlings under 20 % PEG treatment at different time points (i.e., 0, 0.5, 2, 6, 12, 24 and 48 h). The objectives of this research were (1) to understand the physiological and biochemical mechanisms of E. songoricum adaptation to drought stress during the seed germination and early seedling stages and (2) to explore whether drought stress on E. songoricum seed germination and early seedling growth accounts for the failure of sexual recruitment. Suggestions for the ecological restoration of this rare and precious species are also presented.
Materials and Methods
Seed materials and storage
Mature E. songoricum seeds were collected from two populations located in the northeastern region of Gurbantunggut Desert in late September 2011. The seeds were stored dry in paper bags in the laboratory prior to use, as follow: temperature of approximately 10-20 °C and relative humidity of approximately 20-30 % in opaque paper bags.
Seed germination
Germination assays were performed by evenly distributing the seeds in a 10-cm-diameter sterile Petri dish with two layers of filter paper saturated with 8 ml of a treatment solution to mimic drought stress. PEG at different concentration was used to establish an osmotic potential: we dissolved 0, 5, 10, 15, 20, 25 and 30 g of PEG per 100 ml distilled water to generate six osmotic stress levels (0, -0.3, -0.6, -0.9, -1.2, -1.5 and -1.8 MPa, respectively); 0 % PEG served as the control. The seeds were soaked in a 98 % sulfuric acid solution for 10-15 minutes to break physical dormancy, followed by washing with ddH2O. Five replicates of 50 seeds of each osmotic potential were used to assess the germination percentage. The dishes were placed in a germinator at 25 °C/10 °C with a 12 h light/dark cycle (100 mol m-2 s-1) and a relative humidity of 60 %. The germinated seeds were counted and removed daily for seven days; a seed with an emerged radicle was considered to have germinated. The germinated seeds collected from all treatments were stored at -80 °C until use.
With continuous observation, the un-germinated seeds were transferred to distilled water to calculate the recovery percentage. The seeds were checked and recorded daily for seven days. At the end of the experiment, the germination percentage, daily germination percentage and recovery germination percentage were calculated.
Seedling cultivation
Five 50-seed replicates of E. songoricum were germinated and cultivated in distilled water prior to the stress treatment in which the solution and filter paper were changed every 2 days. After two weeks of growth under normal conditions, the drought stress treatment was initiated by applying 20 % PEG solution, and the seedlings were harvested after 0, 0.5, 2, 6, 12, 24 and 48 h of drought treatment and stored at -80 °C until analyses.
Physiological parameters of seeds and seedlings
The absorbance measurements of all the samples in this study were measured using a Lambda 35 spectrophotometer (Perkin Elmer) with three repeated measurements. Unless otherwise stated, the assays were performed at 0 °C in an ice bath.
Measurement of proline, soluble sugars and MDA
The free proline content in germinated seeds was determined using the measurement method described by Bates et al. (1973[5]). 0.15 g ground material was added to 5.0 ml aliquot of 3 % (w/v) sulfosalicylic acid and boiled in a water bath at 100 °C for 10 min. After centrifugation at 2000 g for 5 min, 200 μl aliquot of the supernatant was mixed with 400 μl glacial acetic acid and 600 μl 2.5 % ninhydrin, and boiled at 100 °C for 40 min. After cooling, the mixture was thoroughly mixed with 800 μl toluene. The toluene layer was carefully removed and read at 520 nm. Toluene was used as the blank. The proline concentration (μg g-1 FW) was calculated using L-proline for the standard curve. The soluble sugar content was determined using the anthrone method (Khan et al., 2000[14]); the change in absorbance was measured at 630 nm, and the content of soluble sugars was expressed as μg g-1 FW. Lipid peroxidation was tested by measuring the MDA content according to Cakmak and Horst (1991[8]). Briefly, 0.12 g germinated seeds were ground in 1.2 ml 0.1 % (w/v) trichloroacetic acid (TCA), then centrifuged at 12000 g for 10 min. 0.3 ml 0.5 % (w/v) thiobarbituric acid (TBA) was added to 0.3 ml of the supernatant. The mixture was boiled at 100 °C for 20 min. The reaction was stopped by placing the reaction tubes in an ice bucket. The A532, A600 and A450 values of the supernatant were recorded. The interference of soluble sugars in the samples at A532 and A450 was corrected by subtraction. The MDA content (µmol g-1 FW) was calculated according to the formula:
[MDA] = 6.45 × (A532 − A600) − 0.56 × A450
where A532, A600 and A450 represent the absorbance of the mixture at 532, 600, and 450 nm, respectively.
Enzyme assays
To evaluate the activity of antioxidant enzymes, 0.2 g samples were homogenized with 4 ml of a cold solution containing 1 % (w/v) polyvinylpolypyrrolidone, 50 mM phosphate buffer (pH 7.8), 1 mM ascorbic acid and 10 % glycerol, as described by Beauchamp and Fridovich (1971[6]). The homogenate was centrifuged at 12000 x g for 15 min, and the supernatants were immediately stored at -80 °C until the subsequent assays. The total SOD activity was measured by evaluating the ability to reduce nitro blue tetrazolium (NBT), as described by Stewert and Bewley (1980[30]). One unit of SOD activity was defined as the amount of enzyme that caused 50 % inhibition of the initial rate of the reaction in the absence of enzyme and was expressed as U g-1 FW. The CAT activity was determined by the decomposition of H2O2 using the method of Aebi (1984[1]). One unit of CAT activity was defined as an increase in 0.01 units at A240 and was expressed as U g-1 FW. The POD activity was determined as described by Kochba et al. (1977[16]). One unit of POD activity was defined as the amount of enzyme that resulted in a 0.01 increase at A470 per min and was expressed as U g-1 FW.
Photosynthetic pigment content
Seedling material (0.2 g) was ground in 10 ml 96 % ethanol at room temperature to extract the photosynthetic pigments. The Chl a, Chl b, and Chl a + b were measured at 470, 646 and 663 nm, respectively, and were calculated according to Lichtenthaler (1987[18]). The pigment content was calculated on a fresh weight basis and expressed as mg g-1 FW.
Data analysis
All of the statistical analyses were performed using SPSS software (Standard released version 11.5 for Windows, SPSS Inc., IL, USA), and the figures were generated using Origin 8.0 software. All the data were analyzed using a one-way analysis of variance (ANOVA) at the 95 % level. An LSD multiple comparison test was then used to compare significant differences among the different PEG concentrations and different time points.
Results
Effects of drought stress on germination
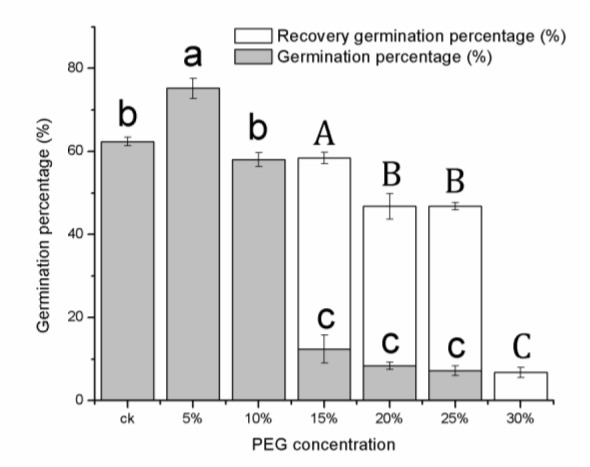
Germination was significantly affected by the osmotic potential. As shown in Figure 1(Fig. 1), an increase in PEG stress markedly decreased the germination percentage of E. songoricum. The highest germination percentage was observed at the 5 % PEG concentration (79.6 %), and the difference from the control (64.8 %) was significant (P < 0.05). The seeds germinated more often and vigorously under mild stress (0, 5 and 10 % PEG concentrations) than heavy stress (15, 20 and 25 %). A significant decline in the germination percentage was recorded at 15 % PEG, indicating that 15 % PEG (-0.9 MPa) is a threshold value for the good germination of E. songoricum seeds. The germination percentage at the 15, 20 and 25 % PEG concentrations was 20.4, 15.6 and 8.4 %, respectively. Conversely, no seeds germinated at the 30 % PEG concentration, which indicated that 30 % PEG (-1.8 MPa) is the lowest osmotic potential for E. songoricum germination. The recovery experiment showed that the seeds exposed to a heavy drought stress (more than 15 %) germinated well after being transferred to distilled water, with recovery germination percentages at 46, 38.4, 39.6 and 6.8 % at 15, 20, 25 and 30 % PEG, respectively.
Figure 1. Germination percentage of E. songoricum seeds under different PEG treatment. The E. songoricum seeds with different PEG treatment were placed in a germinator at 25 °C/10 °C with a 12 h light/dark cycle and a relative humidity of 60 % for seven days. The un-germinated seeds were transferred to distilled water to calculate the recovery percentage. Different lowercase letters indicate significant differences in germination percentage and capital letters indicate significant differences in recovery percentage (P < 0.05).
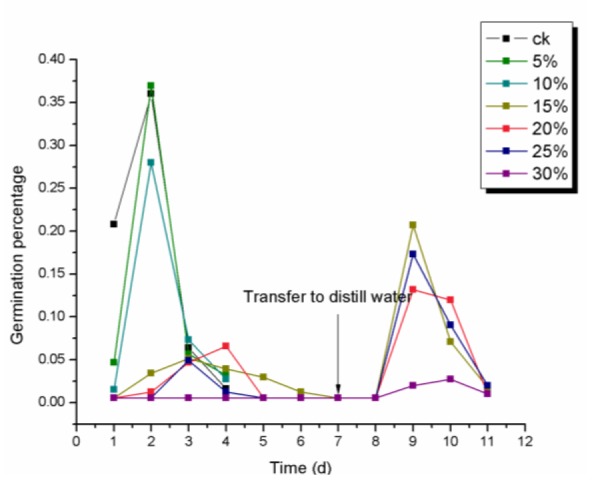
The daily germination percentage data showed that the seeds germinated more quickly in the first two days under a mild drought stress (lower than 15 %) (Figure 2(Fig. 2)). However, the maximal germination was delayed (3rd day) with gradually increasing stress intensity (15, 20, 25 and 30 %). The recovery experiment showed the same tendency. The germination climax was the second day after the transfer to distilled water.
Figure 2. Daily germination percentage of E. songoricum seeds under different PEG treatment. The E. songoricum seeds with different PEG treatment were placed in a germinator at 25 °C/10 °C with a 12 h light/dark cycle and a relative humidity of 60 % for seven days, then the ungerminated seeds were transferred to distilled water for five days to calculate the recovery percentage. The lines with different color represent different PEG concentration.
Physiological response of seeds under drought stress
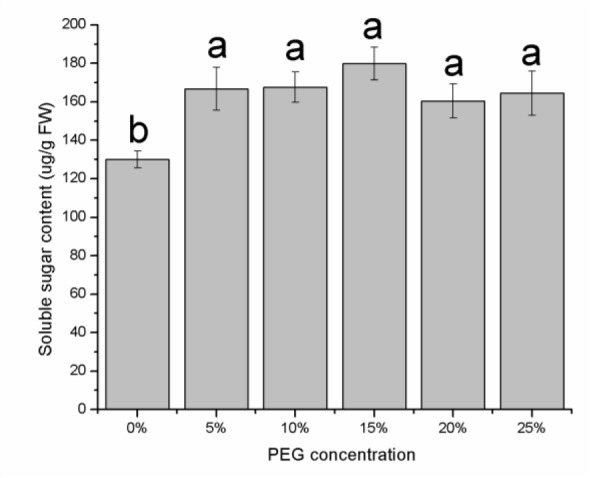
The proline content increased significantly in relation to the drought stress caused by PEG (Figure 3(Fig. 3)), and the differences in the proline content between the 0, 5, 10, 15 and 20 % PEG samples were significant (P < 0.05). The increase in proline content at 20 % and 15 % PEG was 3.2 and 2.8 times, respectively, compared to the control. There was a slight increase in soluble sugar concentration with an increase in the osmotic potential (Figure 4(Fig. 4)). The soluble sugar content was not significant different among the 5, 10, 15, 20 and 25 % PEG treatments (P > 0.05), even though soluble sugar content was greater than in the control (P < 0.05).
Figure 3. Proline content in E. songoricum seeds under different PEG treatment. Values are mean ± S.E (n = 3). Values with different alphabetical superscripts are significantly different at P<0.05.
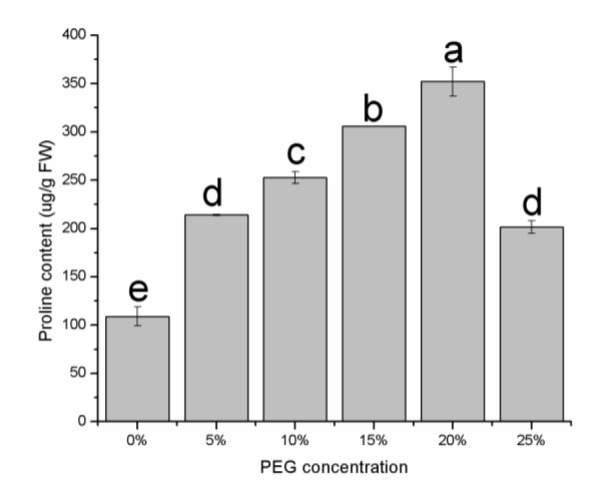
Figure 4. Soluble sugar content in E. songoricum seeds under different PEG treatment. Values are mean ± S.E (n = 3). Values with different alphabetical superscripts are significantly different at P<0.05.
The MDA levels in the seeds were determined to evaluate lipid peroxidation. As shown in Figure 5(Fig. 5), the MDA content was slightly altered at 0, 5 and 10 % PEG, yet increased sharply at 15, 20 and 25 % PEG; the maximum increase was observed at 15 % and 20 % PEG. This result indicated that the plant suffered only minor damage with the mild drought stress but severe lipid peroxidation of the cell membranes occurred with the increased stress. The SODand POD activities showed a general declining trend with increases in the PEG concentration (Figure 6(Fig. 6)), whereas the CAT activity showed an increasing trend and rose significantly at the concentration of 25 % PEG (Figure 6(Fig. 6)). These results showed that CAT may be the main antioxidant enzyme that acts in the prevention of oxidative damage at the E. songoricum seed germination stage.
Figure 5. MDA content in E. songoricum seeds under different PEG treatment. Values are mean ± S.E (n = 3). Values with different alphabetical superscripts are significantly different at P<0.05.
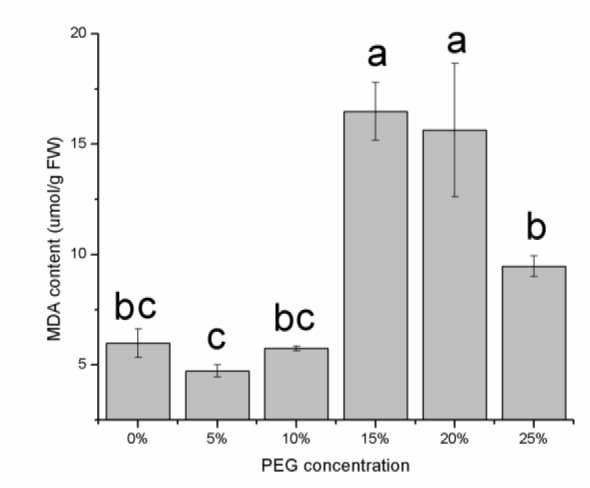
Figure 6. Antioxidative enzyme activities in E. songoricum seeds under different PEG treatment. Activities of superoxide dismutase (SOD) (A), catalase (CAT) (B) and peroxidases (POD) (C) were measured after treatment. Values are mean ± S.E (n = 3) . Values with different alphabetical superscripts are significantly different at P<0.05.
Physiological response of seedlings under drought stress
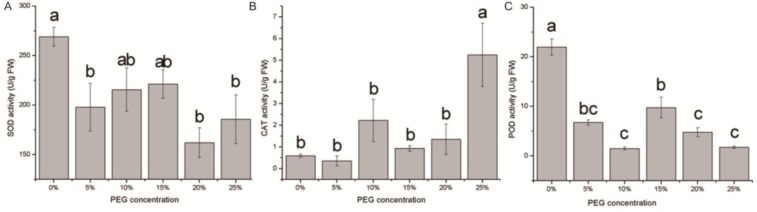
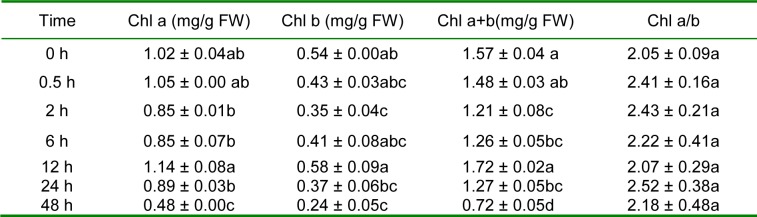
The physiological parameters of the seedlings were measured at 0, 0.5, 2, 6, 12, 24 and 48 h after the imposition of the drought treatment (20 % PEG). The proline content in the seedlings could be described as an unimodal curve, reaching its overall peak value at 2 h (Figure 7(Fig. 7)). The difference in the proline content between 2 h and the other time points was significant. The MDA levels showed an increasing tendency within 48 h (Figure 8(Fig. 8)); a disturbance was observed within the first 0.5 h, reflecting the rapid damage caused by drought stress. At 2 h, the MDA levels decreased to the level at 0 h (P > 0.05), suggesting that the lipid peroxidation of membranes was not serious at 2 h. Combined with the proline data, 2 h may be the response time point of E. songoricum seedlings under drought stress. The photosynthetic pigment content of the seedlings at the different time points is shown in Table 1(Tab. 1). During the 48 h treatment, a minor decrease was observed during the continuous PEG stress but was not significant. The Chl a and Chl b levels showed the same tendency, and the highest total pigment content was observed at 12 h.
Figure 7. Proline content in E. songoricum seedlings among different time point after drought stress (20 % PEG). Values are mean ± S.E (n = 3). Values with different alphabetical superscripts are significantly different at P<0.05.
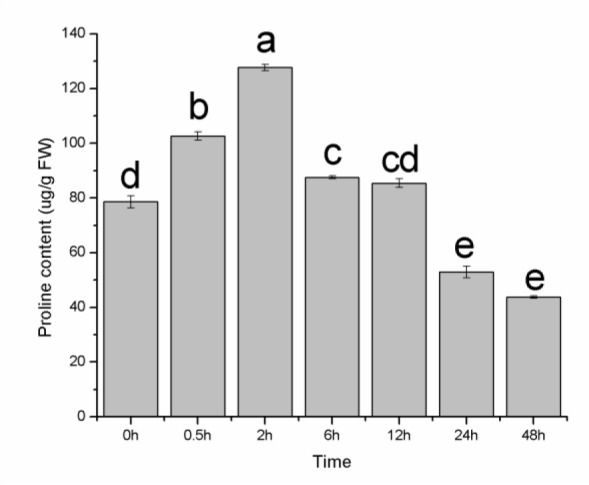
Figure 8. MDA content in E. songoricum seedlings among different time point after drought stress (20 % PEG). Values are mean ± S.E (n = 3). Values with different alphabetical superscripts are significantly different at P<0.05.
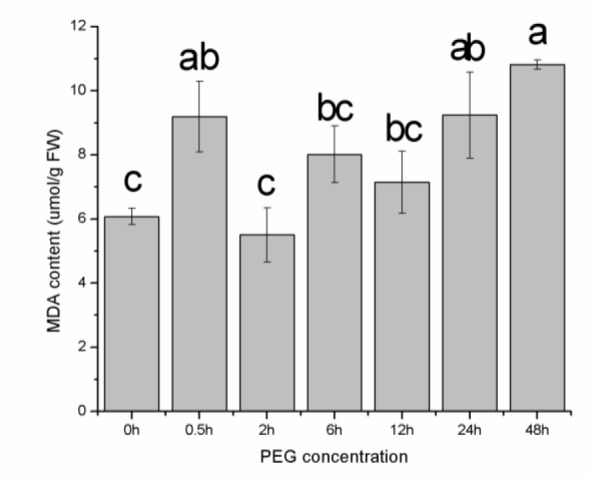
Table 1. Photosynthetic pigment content in E. songoricum seedlings among different time point after drought stress (20 % PEG). Values represent the mean ± S.E (n = 3). Different letters within a column indicate significant differences across seven time points (P < 0.05).
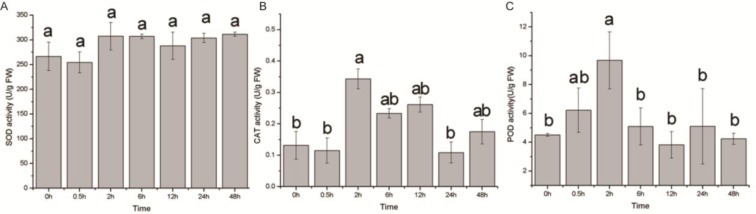
The changes in the seedlings SOD activity during the 48 h of 20 % drought stress are shown in Figure 9(Fig. 9). An increase was observed after 2 h of treatment, and the levels were maintained up to 48 h. Similarly, the POD activity was markedly increased at 2 h and declined thereafter (Figure 9(Fig. 9)); no significant changes among 6, 12, 24 and 48 h were observed. The activities of CAT in the E. songoricum seedlings fluctuated (two peaks curve can be used to describe the activities), and the maximum value was also found at 2 h (Figure 9(Fig. 9)). At 0.5 h, the above antioxidant enzyme activities were not significantly different from the initial value but had increased by 2 h.
Figure 9. Antioxidative enzyme activities in E. songoricum seedlings among different time points after drought stress (20 % PEG). Activities of superoxide dismutase (SOD) (A), catalase (CAT) (B) and peroxidases (POD) (C) were measured after treatment. Values are mean ± S.E (n = 3). Values with different alphabetical superscripts are significantly different at P<0.05.
Discussion
Seed germination and early seedling growth are critical stages for plant establishment (Li et al., 2011[17]), and plants are more sensitive to drought stress during these stages. Research on physiological and biochemical responses under drought stress in the phases of seed germination and early seedling growth is important in fully understanding the traits in early life stage and, to a certain extent, in understanding the interior reasons for low seedling recruitment under natural conditions.
Effect of drought stress on seed germination
Drought stress greatly affects seed germination, but the response intensity and harmful effects of stress depend on the species. In our study, drought stress had a negative effect on the seed germination of E. songoricum. Specifically, the germination percentage decreased with an increase in the PEG concentration. Our results indicated that -1.8 MPa (30 % PEG) is the threshold for E. songoricum to germinate and that -0.9 MPa (15 % PEG) is the threshold for E. songoricum to germinate well. Compared to some other desert plants, the thresholds of Periploca sepium, Elaeagnus angustifolia, and Caragana korshinskii to germinate well were -1.2 MPa (Sun et al., 2011[31]), which was lower than our results for E. songoricum. The results implied that the seeds of E. songoricum have a high water demand for germination well, as the seeds did not germinate under heavy drought stress. This higher moisture requirement reflects the limited ability of E. songoricum to resist drought stress during the germination stage.
The recovery experiments showed that the seeds can still germinate successfully after drought treatment. E. songoricum germination was still observed after the PEG treatment, and the maximum value of the recovery germination percentage reached 39.6 %. Even after treatment with 30 % PEG, approximately 8 % of the seeds still germinated. This response pattern may demonstrate that the seeds can retain their germination ability until sufficient moisture is available for optimal germination. This germination strategy is important for E. songoricum to adapt to the unpredictability of precipitation events in a desert environment. In the Gurbantunggut Desert (the habitat of E. songoricum), the mean annual precipitation is approximately 79.5 mm, but the mean potential annual evaporation is 2607 mm (Zhang et al., 2006[40]). Thus, the climate there is extremely arid, with the seeds only able to germinate from late April to early May when the snow melts and the comparatively frequent precipitation leads to a soil water content that promotes germination. This germination trait reflects an important survival mechanism to adapt the environment. Indeed, many desert plants exhibit the same strategy to survive such a harsh environment (An et al., 2011[3]; Den Berg and Zeng, 2006[11]; Maraghni et al., 2010[23]; Tobe et al., 2001[32]).
Physiological response of seeds under drought stress
Several strategies have evolved in plants to cope with the challenges caused by abiotic stresses. One of the osmotic stress defense mechanisms is to accumulate organic osmolytes (such as proline, soluble sugars, glycine betaine, and organic acids) in the cytoplasm to maintain the plant water potential during drought stress. In our study, the proline content in the seeds increased with the progressively increasing PEG concentration, indicating that the E. songoricum seeds can accumulate proline to lower the osmotic potential to maintain cellular structures under drought stress. This may partly explain why E. songoricum seeds can tolerate mild drought stress. Soluble sugars are a type of osmolyte that maintains the water potential of plants. As observed in Kochia sieversiana (Yang et al., 2007[36]) and in Zea mays (Mei and Song, 2008[25]), the soluble sugar content increased with increasing stress; consistent with these results, the soluble sugar content of E. songoricum increased significantly compared to the control. However, among 5, 10, 15, 20 and 25 % PEG the difference was not significant. This revealed that soluble sugars may make a minor contribution to resisting drought stress in seeds of E. songoricum.
Severe water stress causes perturbations in the metabolic processes of the mitochondria and chloroplasts, leading to an overproduction of reactive oxygen species (ROS) (Apel and Hirt, 2004[4]), which cause damage to macromolecules and subcellular components and are the most damaging consequence of abiotic stress (Berjak, 2006[7]). Plants have evolved a complex antioxidative defense system (including POD, SOD and CAT) to alleviate the damage caused by ROS (Khan and Panda, 2008[15]). Our results revealed reductions in the activity of SOD and POD in seeds under drought stress, whereas the CAT activity increased significantly at the concentration of 25 % PEG. This pattern partly agrees with the results in Medicago sativa (with SOD and CAT increasing and POD decreasing with increased PEG) (Wang et al., 2009[35]). Our findings indicate that CAT may be the main enzyme contributing to the drought stress response in E. songoricum seeds. The protection against drought stress conferred by SOD and POD is limited, and progressive water stress causes the activity of POD and SOD decline.
MDA is often used to evaluate membrane lipid peroxidation. In our study, slight drought stress had little impact on the MDA content, whereas a higher PEG concentration (15, 20 and 25 %) significantly increased the MDA content. These results suggested that the membrane stability had been destroyed and that lipid peroxidation had occurred only at the high PEG concentration. In other words, the seeds of E. songoricum are protected from membrane damage under mild drought stress but cannot tolerate the progressed water stress.
Physiological response of seedlings under drought stress
A drought environment has an influence on the seed germination percentage and also seriously affects early seedling growth: seedlings are highly sensitive to water stress and require high moisture for continued growth (Markesteijn and Poorter, 2009[24]). At a young seedling stage, the proline content increased in the initial two hours after the plants were subjected to the drought stress and rapidly reached a maximum value, a result that indicates that the seedlings can accumulate proline rapidly to resist drought. However, the declining trend after 2 h demonstrates that this ability to balance the osmotic potential via proline accumulation is limited during the seedling stage; furthermore, the seedlings cannot endure drought stress for a long period, which can also be demonstrated by the changing pattern of the MDA content and activity of antioxidative enzymes. At 2 h, the MDA content decreased significantly and then increased. The activity of SOD, CAT and POD all increased in the first two hours and then decreased thereafter, with a maximum value at 2 h. Based on these results, 2 h may be a key point for a response to the drought stress during the seedling stage. Prior to this point, the drought response system was triggered, and damage was ameliorated by the antioxidative enzymes and osmolytes. However, after this point, proline content and the activity of antioxidative enzymes were decreased, and the MDA content was increased accordingly. These findings demonstrate that E. songoricum seedlings can rapidly respond to drought stress but cannot endure drought for a long period of time. In addition, the photosynthetic pigment content was decreased during the experiment, which is in contrast to Campylotropis polyantha (Li et al., 2011[17]) and Cakile maritima (Debez et al., 2008[10]) in which the photosynthetic pigment content increased with an increase in the level of stress.
As a result, successful seedling growth requires a sustainable water supply in nature. However, according to the field observations of Liu (Liu et al., 2011[19]), rainfall during the seedling growth season is unpredictable; most importantly, the root elongation rate of early seedlings (1.61 cm/d) was less than the rate of retreat of the moist soil layer in the desert (2.78 cm/d) after an occasional rainfall. Therefore, the rate of seedling root growth cannot keep up with the downward movement of the plant-available moisture, creating a permanent drought environment in the vicinity of the seedlings until the next rainfall. Our study demonstrates that E. songoricum seedlings cannot endure drought for an extended period, which may provide evidence at the physiological level that water stress is the reason for the low seedling survival rate of E. songoricum in the wild and why this species has become rare and endangered.
Conclusion
Drought has a suppression effect on the seed germination of E. songoricum, whereas a slight stress (5 % PEG) has a promoting effect on seed germination. E. songoricum seeds appear to tolerate mild drought stress (≤ 15 % PEG) better than heavy stress (> 15 % PEG) and can maintain their germination ability in a drought environment and germinate with seasonal water supply. These characteristics are all adaptation strategies for E. songoricum to reproduce in a harsh environment.
During the stress period, seeds can accumulate proline and soluble sugars to cope with drought stress, whereas the contribution of antioxidative enzymes is limited (only CAT increased). This result reveals that the drought physiological response mechanism of E. songoricum at the seed germination stage is mainly dependent on the accumulation of osmolytes, such as proline and soluble sugars.
The seedlings of E. songoricum can respond rapidly to drought stress but cannot endure a long-term continuous drought stress. The physiological mechanism of E. songoricum seedlings to drought stress is due to both the accumulation of osmolytes (proline) and the augmentation of the activity of antioxidative enzymes.
According to the above findings, we propose that a sufficient water supply should ensure the regeneration of endangered E. songoricum populations in the field. Specifically, heavy drought stress should be avoided during the germination stage, and the seedlings should not be exposed to long-term drought stress during the seedling establishment stage.
Notes
Haiyan Li and Xiaoshuang Li contributed to the work equally and should be regarded as co-first authors. Tel: +86 991 7823109; fax: +86 991 7823109
Acknowledgements
Funds for this study were provided by Project of Xinjiang Committee of Science and Technology (No. 200933122) and the National Natural Science Foundation (31070472, 31100399) of P. R. China.
References
- 1.Aebi H. Catalase in vitro. Meth Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad S, Ahmad R, Ashraf MY, Ashraf M, Waraich EA. Sunflower (Helianthus Annuus L.) response to drought stress at germination and seedling growth stages. Pak J Bot. 2009;41:647–654. [Google Scholar]
- 3.An YY, Liang ZS, Zhang Y. Seed germination responses of Periploca sepium Bunge, a dominant shrub in the Loess hilly regions of China. J Arid Environ. 2011;75:504–508. [Google Scholar]
- 4.Apel K, Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 5.Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- 6.Beauchamp C, Fridovich I. Superoxide dismutase improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 7.Berjak P. The challenge of recalcitrant germplasm cryopreservation. J Hortic Sci Biotech. 2006;81:781–782. [Google Scholar]
- 8.Cakmak I, Horst WJ. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max) Physiol Plantarum. 1991;83:463–468. [Google Scholar]
- 9.Chen K, Arora R. Dynamics of the antioxidant system during seed osmopriming, post-priming germination, and seedling establishment in Spinach (Spinacia oleracea) Plant Sci. 2011;180:212–220. doi: 10.1016/j.plantsci.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Debez A, Koyro HW, Grignon C, Abdelly C, Huchzermeyer B. Relationship between the photosynthetic activity and the performance of Cakile maritima after long-term salt treatment. Physiol Plant. 2008;133:373–385. doi: 10.1111/j.1399-3054.2008.01086.x. [DOI] [PubMed] [Google Scholar]
- 11.Den Berg L, Zeng YJ. Response of South African indigenous grass species to drought stress induced by polyethylene glycol (PEG) 6000. S Afr J Bot. 2006;72:284–286. [Google Scholar]
- 12.Galle A, Haldimann P, Feller U. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytol. 2007;174:799–810. doi: 10.1111/j.1469-8137.2007.02047.x. [DOI] [PubMed] [Google Scholar]
- 13.Gao JM, Xiao Q, Ding LP, Chen MJ, Yin L, Li JZ, et al. Differential responses of lipid peroxidation and antioxidants in Alternanthera philoxeroides and Oryza sativa subjected to drought stress. Plant Growth Regul. 2008;56:89–95. [Google Scholar]
- 14.Khan AA, McNeilly T, Collins JC. Accumulation of amino acids, proline, and carbohydrates in response to aluminum and manganese stress in maize. J Plant Nutr. 2000;23:1303–1314. [Google Scholar]
- 15.Khan MH, Panda SK. Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-Salinity stress. Acta Physiol Plant. 2008;30:81–89. [Google Scholar]
- 16.Kochba J, Lavee S, Spiegel PR. Differences in peroxidase activity and isoenzymes in embryogenic and nonembryogenic “Shamouti” orange ovular callus lines. Plant Cell Physiol. 1977;18:463–467. [Google Scholar]
- 17.Li FL, Bao WK, Wu N. Morphological, anatomical and physiological responses of Campylotropis polyantha (Franch.) Schindl. seedlings to progressive water stress. Sci Hortic-Amsterdam. 2011;127:436–443. [Google Scholar]
- 18.Lichtenthaler HK. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth Enzymol. 1987;148:350–382. [Google Scholar]
- 19.Liu HL, Shi X, Wang JC, Yin LK, Huang ZY, Zhang DY. Effects of sand burial, soil water content and distribution pattern of seeds in sand on seed germination and seedling survival of Eremosparton songoricum (Fabaceae), a rare species inhabiting the moving sand dunes of the Gurbantunggut Desert of China. Plant Soil. 2011;345:69–87. [Google Scholar]
- 20.Liu Y, Zhang DY, Yang HL. Spatial genetic structure in five natural populations of Eremosparton songoricum as revealed by ISSR analysis. Biodiv Sci. 2010;18:129–136. [Google Scholar]
- 21.Liu Y, Zhang DY, Yang HL, Liu MY, Shi X. Fine-scale genetic structure of Eremosparton songoricum and implication for conservation. J Arid Land. 2010;2:26–32. [Google Scholar]
- 22.Ma WB, Zhang DY, Yin LK. Variation of seed sizes of Eremosparton songoricum geographic populations and germination characteristics (in Chinese) Arid Land Geogr. 2007;30:674–679. [Google Scholar]
- 23.Maraghni M, Gorai M, Neffati M. Seed germination at different temperatures and water stress levels, and seedling emergence from different depths of Ziziphus lotus. S Afr J Bot. 2010;76:453–459. [Google Scholar]
- 24.Markesteijn L, Poorter L. Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought- and shade-tolerance. J Ecol. 2009;97:311–325. [Google Scholar]
- 25.Mei YQ, Song SQ. Early morphological and physiological events occurring during germination of maize seeds. Agr Sci China. 2008;7:950–957. [Google Scholar]
- 26.Mut Z, Akay H, Aydin N. Effects of seed size and drought stress on germination and seedling growth of some oat genotypes (Avena sativa L.) Afr J Agr Res. 2010;5:1101–1107. [Google Scholar]
- 27.Shi X, Wang JC, Zhang DY, Gaskin J, Pan BR. Pollen source and resource limitation to fruit production in the rare species Eremosparton songoricum (Fabaceae) Nord J Bot. 2010;28:438–444. [Google Scholar]
- 28.Shi X, Wang JC, Zhang DY, Gaskin J, Pan BR. Pollination ecology of the rare desert species Eremosparton songoricum (Fabaceae) Aust J Bot. 2010;58:35–41. [Google Scholar]
- 29.Shi X, Zhang DY, Wang JC, Liu HL, Pan BR. Characteristics of soil seed bank of dersert plant E.songoricum and their effects on seed germination (in Chinese) J Desert Res. 2011;31:968–973. [Google Scholar]
- 30.Stewart RRC, Bewley JD. Lipid-peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980;65:245–248. doi: 10.1104/pp.65.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun YD, Du XH, Zhang WJ, Sun L, Li R. Seed germination and physiological characteristics of Amaranthus mangostanus L. under drought stress. Adv Mater Res-Switz. 2011;183-5:1071–1074. [Google Scholar]
- 32.Tobe K, Zhang LP, Qiu GYY, Shimizu H, Omasa K. Characteristics of seed germination in five non-halophytic Chinese desert shrub species. J Arid Environ. 2001;47:191–201. [Google Scholar]
- 33.Wang JC, Shi X, Yin LK, Zhang DY. Role of clonal integration in life strategy of sandy dune plant, Eremosparton Songoricum (litv) vass (fabaceae): experimental approach. Pol J Ecol. 2011;59:455–461. [Google Scholar]
- 34.Wang JC, Shi X, Zhang DY, Yin LK. Phenotypic plasticity in response to soil moisture availability in the clonal plant Eremosparton songoricum (Litv.) Vass. J Arid Land. 2011;3:34–39. [Google Scholar]
- 35.Wang WB, Kim YH, Lee HS, Kim KY, Deng XP, Kwak SS. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol Bioch. 2009;47:570–577. doi: 10.1016/j.plaphy.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Yang CW, Chong JN, Li CY, Kim CM, Shi DC, Wang DL. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil. 2007;294:263–276. [Google Scholar]
- 37.Yin LK, Tan LX, Wing B. Rare and endangered higher plants endemic in Xinjiang of China. Urumchi: Xinjiang Sci Tech Publ; 2006. pp. 74–75. [Google Scholar]
- 38.Zhang DY, Liu HL, Shi X, Wang J, Zhang Y. Limitations on the recruitment of the rare sand shrubby legume Eremosparton songoricum (Fabaceae) in Gurbantunggut Desert, China (in Chinese) J Arid Land. 2011;3:75–84. [Google Scholar]
- 39.Zhang DY, Ma WB, Shi X, Wang JC, Wang XY. Distribution and bio-ecological characteristics of Eremosparton songoricum, a rare plant in Gurbantunggut desert (in Chinese) J Desert Res. 2008;28:430–436. [Google Scholar]
- 40.Zhang YM, Wang HL, Wang XQ, Yang WK, Zhang DY. The microstructure of microbiotic crust and its influence on wind erosion for a sandy soil surface in the Gurbantunggut Desert of Northwestern China. Geoderma. 2006;132:441–449. [Google Scholar]


