Abstract
Purpose
The study aims to elucidate the changes in testicular spermatogenic function in high-fat diet (HFD)-induced obese rats and to evaluate the protective effects of metformin intervention.
Methods
Male Sprague–Dawley rats (n = 18) were randomly divided into a control group (standard diet), an HFD group, and a metformin group (HFD + metformin at 100 mg/kg, once daily by oral gavage). After 8 weeks, rats were euthanized, and the weights of body and testes were measured. Testis and epididymis were dissected and hematoxylin-eosin-stained for histopathological examination and semen parameter analysis. Blood samples were collected for assessment of sex hormones and metabolic parameters (serum glucose, insulin, and leptin). Spermatogenic cell apoptosis was accessed by TUNEL.
Results
Compared with the control group, the final body weight and weight gain were significantly higher in HFD rats, while the testicle weight and coefficients were lower. In HFD rats, metformin treatment induced weight loss and increased testicle weight (P < 0.05). In HFD rats, obvious pathological changes in the testicular tissue were characterized by small, atrophic, and distorted seminiferous tubules and destroyed basement membrane. Metformin treatment protected against the HFD-induced decrease in the number of spermatogonia, Sertoli cells, and Leydig cells (P < 0.05); ameliorated the HFD-induced increases in serum glucose, insulin, leptin, and estrogen; and decreased serum testosterone (P < 0.05) and reduced the rate of testicular cell apoptosis in obese male rats. Finally, metformin significantly improved semen parameters (including concentration, viability, motility, and normal morphology) in HFD rats (P < 0.05).
Conclusions
HFD-induced obesity in rats results in detrimental effects on spermatogenesis, semen quality, endogenous hormones, and testicular cell apoptosis. Metformin intervention improved the semen parameters, possibly due to its effects on weight loss, increased testicular weight, reduced testicular cell apoptosis, and resulted in restoration of hormonal homeostasis and correction of metabolic disorder.
Keywords: Male infertility, Metformin, Obesity, Spermatogenesis, Semen, Testis
Introduction
The prevalence of infertility is approximately 15 %, with male factors accounting for 30 to 50 % of this rate [1]. Although controversial, numerous studies show that the quality and quantity of male spermatozoa decline from 1 year to the next [2], with many factors including environmental effects, metabolic dysfunction, and genetic polymorphisms apparently associated with a decline in male reproductive ability [3]; however, only obesity has been shown conclusively to be involved in this phenomenon [4–6].
Due to improved living standards and dietary variation, the prevalence of obesity continues to increase. Previous studies suggested a close correlation between body mass index (BMI) and semen quality [6, 7]. Obesity has been reported to reduce semen quality and impact fertility by affecting spermatogenesis [5]. A high incidence of infertility in association with metabolic disturbances and hormonal dysregulation was confirmed in obese men [7].
Accumulating evidence demonstrates that obesity leads to insulin resistance (IR), resulting in a series of obesity-related diseases [8]. Moreover, low serum testosterone was demonstrated to be predictive of IR, type 2 diabetes, and metabolic syndrome in men [9]. These results imply that IR plays a vital role in the reduced semen quality and spermatogenic dysfunction induced by obesity.
Metformin, an oral insulin sensitizer, which improves insulin sensitivity effectively, reduces the incidence of the metabolic syndrome in overweight and obese patients as well helps in losing weight [10]. Long-term follow-up from the Diabetes Prevention Program demonstrated that metformin produced durable weight loss in several ways, with decreased food intake shown to be the primary mechanism [11]. Clinical trials showed that the mean total and free testosterone levels increased significantly after metformin treatment in men with metabolic syndrome. Similarly, there was a significant decrease in fasting insulin levels, which was more pronounced in male hypogonadism associated with metabolic syndrome [12]. Prompted by these observations, the present study was designed to reveal the changes in testicular spermatogenic function in high-fat diet (HFD)-induced obese rats and to evaluate the protective effects of metformin intervention. The purpose was to assess the variation in semen quality (including motility, vitality, and normal morphology), histological configuration of the seminiferous tubules, sex hormone levels, and testicular cell apoptosis with or without metformin treatment in a rat model of obesity induced by an HFD.
Materials and methods
Animal experiments
Eighteen male Sprague–Dawley (SD) rats (aged 3 months and weighing 200 ± 30 g, Permit number: 42000500002649) were included in the study. Rats were fed under specified-pathogen free (SPF) conditions, with a 12/12-h light/dark cycle and free access to food and water for 8 weeks. The rats were randomly divided into three groups (n = 6 per group). Rats in the control group received a standard diet, while the HFD group received an HFD, and the metformin group received an HFD plus metformin (Sigma-Aldrich) treatment (100 mg/kg, once daily by oral gavage) [13]; the control and HFD groups received saline simultaneously. The full compositions of the standard and HFDs have been reported previously [14]. The use of experimental animals in this study was approved by the Institutional Animal Care and Use Committee of Renmin Hospital of Wuhan University (China).
Morphology of testes and semen analysis
At the end of the experiment, following 12 h of starvation, the rats were anesthetized by intraperitoneal injection of sodium pentobarbital (45 mg/kg), then weighed using an electronic balance, and sacrificed by cervical dislocation. After incision of the abdominal wall along the midline, the testes were quickly removed, washed in cold saline, and blotted dry with filter paper before being observed and photographed. The weight of the testes was measured accurately using an electronic balance, and the testicle coefficient (g/kg) was defined as the weight ratio of the testes (the mean of the weight of the two testes) and the whole body. After being weighed, the testes were immediately fixed in Bouin’s solution (Wuhan Boster Biological Technology, Wuhan, China). Semen was obtained from the tail of the epididymis and transferred to Ham’s F10 medium (Wuhan Boster Biological Technology, Wuhan, China) for analysis of sperm count, viability, motility, and morphology according to routine protocols.
HE staining
The testicle samples were paraffin-embedded, sectioned (thickness, 5 μm), and stained with hematoxylin-eosin (HE) for evaluation by light microscopy. Photomicrographs were obtained using the Photo Imaging System (Canon 600D). The diameter of the seminiferous tubules was determined using Image-Pro Plus 6.0. In each group, 30 fields (five fields per rat, ×400 magnification) in six rats were randomly selected to count spermatogenetic cells, Sertoli cells, and Leydig cells, and the data mean values for each parameter were calculated.
Sex hormones and metabolic features
Blood samples were obtained from the abdominal aorta. After standing at room temperature for 1 h, the serum was collected after centrifugation at 300g for 15 min and stored at −70 °C until analyzed.
Serum levels of testosterone (T, Elabscience, E-EL-0072c), follicular stimulating hormone (FSH, Elabscience, E-EL-R0391c), luteinizing hormone (LH, Elabscience, E-EL-R0026c), and estradiol (E2, Elabscience, E-EL-0065c) were measured by enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instructions. Intra- and inter-assay coefficients of variation (CV) for measurements of both FSH and LH were 3.9 and 7.2 %, respectively. The intra- and inter-assay CV for T and E2 were 4.3 and 7.1 % and 6.9 and 9.3 %, respectively.
Blood glucose was estimated by using a glucometer (Accu-chek, Roche). Serum insulin and leptin were estimated using ELISA kits for rats (Mercodia, 10112401, Sweden; Elabscience, E-EL-R0582c, respectively). IR was measured through the homeostasis model assessment of IR (HOMA-IR) using the following formula: HOMA-IR = fasting insulin (μIU/mL) × fasting glucose (mg/dL)/405 [14].
TUNEL assay
Testicular cell apoptosis in tissue sections was measured using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay kit (Roche Applied Science, 11684817910) according to the instructions provided by the manufacturer. Positively labeled nuclei (apoptotic cells) were stained brown, while negatively labeled nuclei were stained blue. One hundred seminiferous tubule sections (×400 magnification, Nikon E100) were randomly selected in each group, and the total number of apoptotic cells was counted. Finally, the apoptosis index (AI) in each group was calculated according the following formula: AI = total number of apoptotic cells/100.
Statistical analysis
Statistical tests were performed using the Statistical Package for Social Sciences (SPSS), version 13.0 (SPSS, Chicago, IL, USA) for Windows XP. All data are expressed as the means ± standard error of the mean (S.E.M). Multiple group comparisons were carried using one-way ANOVA, followed by Tukey’s post hoc test. All statistical analyses were two-sided, and P < 0.05 was considered to indicate statistical significance.
Results
Induction of obesity and gross anatomy of the testes
The average final body weight and weight gain were significantly higher in the HFD group than in the control group. A tendency for decreased weight gain and body weight was observed in rats fed an HFD supplemented with metformin (Table 1); however, the opposite phenomenon was observed for testicle weight. The testes of the control group were oval and larger, with a smooth surface and plump appearance. They were opaque white and elastic with clear vascular texture. In contrast, the testes of the HFD group were atrophied, with visible differences observed between the HFD and metformin groups. Compared with the control group, the testicular coefficient of the HFD group was lower (8.01 ± 1.01 vs 4.72 ± 0.80, P < 0.05), and metformin treatment provided significant protection against the testicular atrophy observed in the HFD group (4.72 ± 0.80 vs 7.09 ± 0.65, P < 0.05, Table 1).
Table 1.
Effects of metformin on the body weight, weight gain, testicular weight, testicular coefficient, testicular cell, and sperm parameters after 8 weeks
| Control group (n = 6) | HFD group (n = 6) | Metformin group (n = 6) | |
|---|---|---|---|
| Final body weight (g) | 329.50 ± 29.99 | 401.50 ± 27.06a | 350.33 ± 41.36b |
| Weight gain (g) | 130.81 ± 15.30 | 196.26 ± 23.15a | 146.33 ± 28.17b |
| Testicular weight (g) | 2.64 ± 0.36 | 1.90 ± 0.32a | 2.44 ± 0.28b |
| Testicular coefficient (g/kg) | 8.01 ± 1.01 | 4.72 ± 0.80a | 7.09 ± 0.65b |
| Spermatogenesis | |||
| Spermatogonia | 25.23 ± 5.96 | 14.25 ± 3.58a | 23.35 ± 5.29b |
| Leydig cells | 8.18 ± 0.45 | 4.16 ± 0.18a | 5.57 ± 0.73b |
| Sertoli cells | 9.33 ± 0.85 | 5.18 ± 0.65a | 8.02 ± 1.01b |
| Sperm parameters | |||
| Concentration (×106/ml) | 56.62 ± 5.22 | 48.35 ± 4.36a | 52.81 ± 8.02b,c |
| Viability (%) | 97.33 ± 1.01 | 95.02 ± 0.81a | 98.26 ± 1.23b |
| Motility (%) | 68.52 ± 6.31 | 55.81 ± 5.19a | 66.72 ± 5.03b |
| Normal morphology (%) | 85.28 ± 2.11 | 80.46 ± 1.81a | 86.31 ± 1.82b |
All data are expressed as mean ± S.E.M
aIndicates a statistical difference when compared with the control group (P < 0.05)
bIndicates a statistical difference when compared with the HFD group (P < 0.05)
cIndicates a statistical difference when compared with the control group (P < 0.05)
Semen analysis
Compared to the control and metformin groups, the concentration, viability, and motility of sperm were significantly reduced in the HFD group with abnormal morphology (P < 0.05). Apart from sperm concentration, there were no significant differences in the other indexes between the metformin and control groups (Table 1).
Histological study
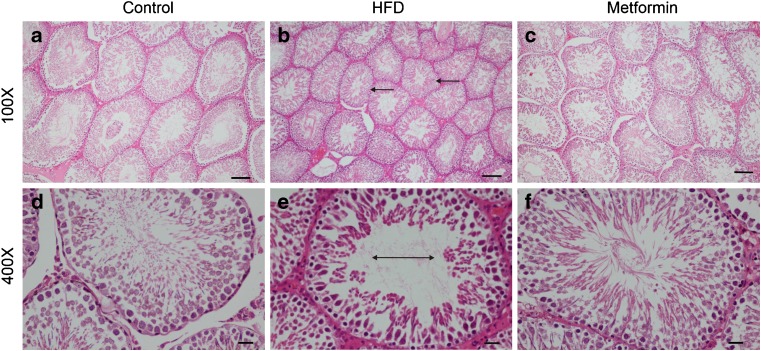
Under light microscopy, abundant seminiferous tubules with large diameters and intact basement membranes were observed in the testicular structure of the control group. Five to eight layers of aligned spermatogenetic cells were observed in the seminiferous tubules and the lumen of which was filled with numerous spermatozoa. The mesenchyme was composed of loose connective tissue with clustered Leydig cells, which were large, round, or polygonal in shape and rich in cytoplasm, with irregular nuclei and lightly stained chromatin. Compared with the control group, obvious pathological changes were observed in the testicular tissue of the HFD group, characterized by small, atrophic, and distorted seminiferous tubules and destroyed basement membrane. In each field of measurement, the average number of spermatogonia, Sertoli cells, and Leydig cells was significantly reduced in the HFD group (P < 0.05), while metformin treatment protected testicular tissue from the damage caused by the HFD (Fig. 1, Table 1).
Fig. 1.
Morphological changes in the testes in male rats. Hematoxylin-eosin staining of the testes in the three groups. Magnification ×100, scale bar 100 μm; magnification ×400, scale bar 20 μm. a, d Testicular section of a control rat showing abundant seminiferous tubules with large diameters, intact basement membranes, and normal spermatogenic cells. b, e Testicular section of an HFD rat showing atrophic seminiferous tubules with smaller diameters (arrows) and fewer spermatogenic cells (double-headed arrow). c, f Testicular section of a metformin group rat showing seminiferous tubular structures with normal diameters and spermatogenic cells in almost all the seminiferous tubules. HFD high-fat diet
Sex hormones and metabolic features
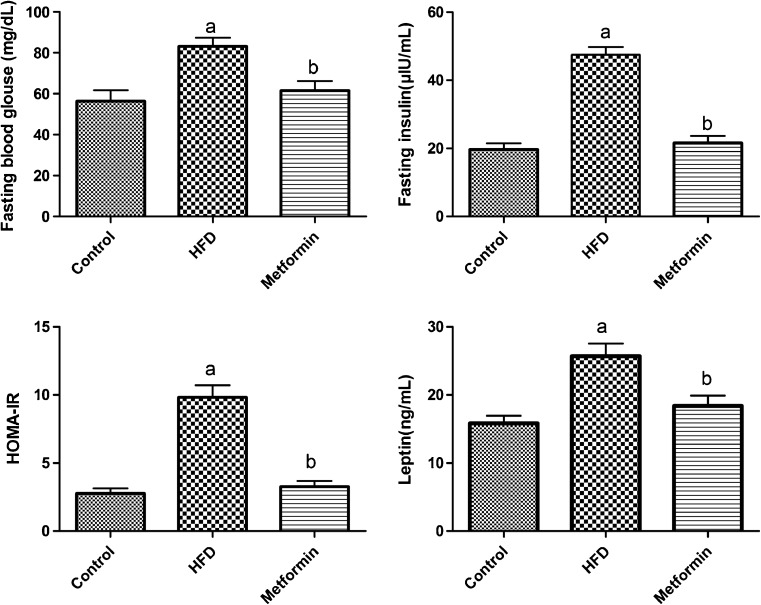
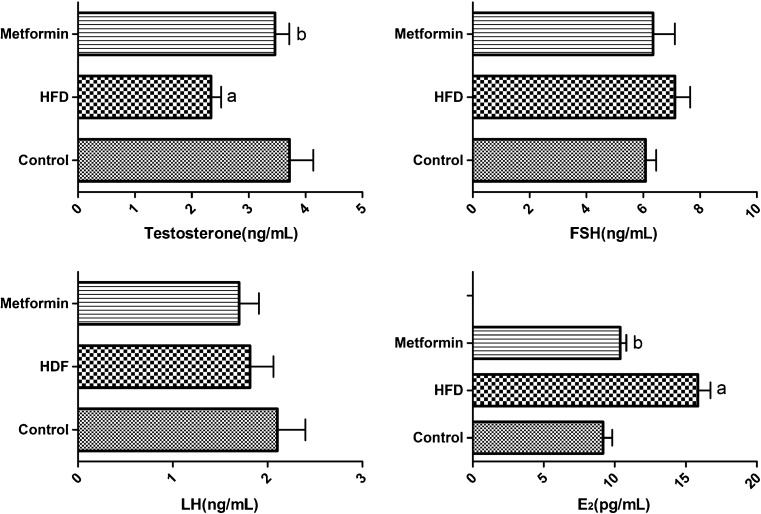
SD rats fed an HFD presented significantly increased serum levels of glucose, insulin, and leptin accompanied by body weight gain. The HOMA-IR results, which are a sensitive reflection of the degree of IR, were also significantly elevated in HFD rats (P < 0.05). After metformin intervention, all these indexes of metabolic abnormalities decreased, and no significant differences were observed between the metformin and control groups (Fig. 2, Table 2). Furthermore, abnormalities in serum sex hormone levels were observed, with obviously decreased T and increased E2 levels in the HFD group (P < 0.05). However, there were no significant differences in the levels of FSH and LH among the three groups (Fig. 3, Table 2).
Fig. 2.
Levels of fasting blood glucose, fasting insulin, HOMA-IR, and leptin. Data are expressed as mean ± S.E.M. a indicates a statistical difference when compared with the control group (P < 0.05); b indicates a statistical difference when compared with the HFD group (P < 0.05). HOMA-IR homeostasis model assessment of insulin resistance, HFD high-fat diet
Table 2.
Sex hormones and metabolic features in the three groups
| Control group (n = 6) | HFD group (n = 6) | Metformin group (n = 6) | |
|---|---|---|---|
| Metabolic features | |||
| Fasting glucose (mg/dL) | 56.35 ± 13.17 | 83.19 ± 10.55a | 61.48 ± 11.66b |
| Fasting insulin (μIU/mL) | 19.69 ± 4.50 | 47.53 ± 5.62a | 21.58 ± 5.15b |
| HOMA-IR | 2.77 ± 0.94 | 9.83 ± 2.15a | 3.27 ± 1.01b |
| Leptin (ng/mL) | 15.83 ± 2.73 | 25.68 ± 4.53a | 18.39 ± 3.71b |
| Sex hormones | |||
| FSH (ng/mL) | 6.08 ± 0.92 | 7.12 ± 1.30 | 6.35 ± 1.89 |
| LH (ng/mL) | 2.10 ± 0.73 | 1.78 ± 0.58 | 1.70 ± 0.51 |
| T (ng/mL) | 3.72 ± 1.02 | 2.34 ± 0.45a | 3.46 ± 0.61b |
| E2 (pg/mL) | 9.18 ± 1.60 | 15.85 ± 2.16a | 10.39 ± 1.04b |
All data are expressed as mean ± S.E.M
aIndicates a statistical difference when compared with the control group (P < 0.05)
bIndicates a statistical difference when compared with the HFD group (P < 0.05)
Fig. 3.
Serum levels of T, FSH, LH, and E2. All data are expressed as mean ± S.E.M. a indicates a statistical difference when compared with the control group (P < 0.05); b indicates a statistical difference when compared with the HFD group (P < 0.05). FSH follicle-stimulating hormone, LH luteinizing hormone, E 2 estradiol, HFD high-fat diet
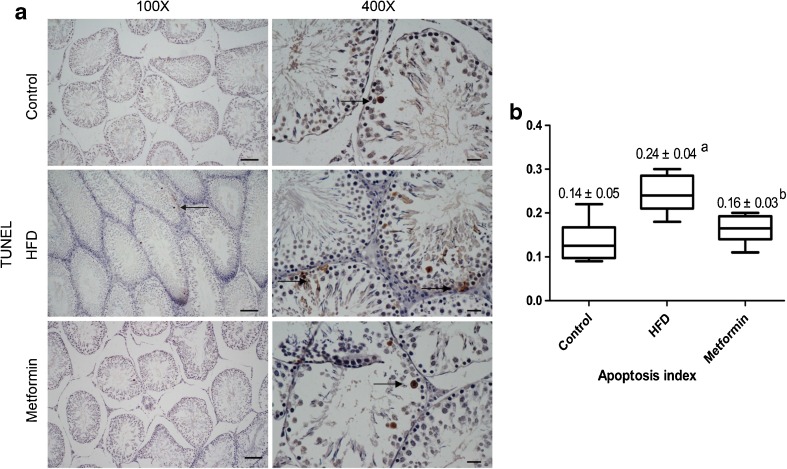
Testicular cell apoptosis
The rate of testicular cell apoptosis was measured by TUNEL staining. The percentage of apoptotic cells was calculated, and apoptosis index was used to represent the average of them, which could show the apoptosis status of each group. The AI was found to be higher in the HFD group compared with the control group; after metformin treatment, AI went down markedly, and no significant difference was seen between the metformin group and control group (Fig. 4).
Fig. 4.
TUNEL and quantitative analysis of the apoptosis index (AI). a TUNEL staining of the apoptotic cells in testes. Magnification ×100, scale bar 100 μm; magnification ×400, scale bar 20 μm. Arrows indicate apoptotic cells. b AI of the three groups. Data are expressed as mean ± S.E.M. a indicates a statistical difference when compared with the control group (P < 0.05); b indicates a statistical difference when compared with the HFD group (P < 0.05). HFD high-fat diet
Discussion
Obesity is a major health problem which has proved to be a high risk factor for IR, type 2 diabetes, cardiovascular diseases, endocrine disorders, and decreased fertility [8, 15]. Previous evidence demonstrated that low serum T concentrations and poor semen quality were associated with visceral obesity [7, 16], IR, and type 2 diabetes [17, 18]. Additionally, obesity can cause and aggravate IR. All of this evidence indicated that high insulin levels or IR may play an important role in infertility in obese males.
Metformin, which is the most common drug used to treat type 2 diabetes and IR, improves peripheral insulin sensitivity through transporter-stimulated tissue uptake of glucose. Despite the known glucose-lowering effects of metformin, more recent clinical interest lies in its potential as a weight loss drug based primarily on its ability to stimulate a reduction in food intake. In addition to appetite suppression, metformin improves leptin sensitivity, changes gastrointestinal physiology, and regulates fat oxidation and storage [11]. Clinical studies revealed that metformin treatment of oligo-terato-asthenozoospermic men with metabolic syndrome obtained satisfactory effects, including significant reductions in insulin and sex-hormone-binding globulin levels, increased serum androgen levels, and a consequent improvement in semen characteristics [19]. However, the mechanisms by which obesity impairs spermatogenic function and the protective effects of metformin on obesity-induced damage in the testes, sperm parameters, sex hormones, and metabolism remain to be elucidated. To address these issues, we established an HFD-induced obese male rat model to investigate the impact of metformin on spermatogenic and testicular function. Normal testicular weight and functional spermatogenic-related cells, such as spermatogonia, Leydig cells, and Sertoli cells, are essential for sperm production. In the present study, the gross morphology and HE staining of testicular tissues demonstrated that HFD not only results in rat obesity but also leads to atrophy of the testes. This was supported by the reduction in the testicular weight and coefficient in the HFD group compared with the control group. Furthermore, the decreased concentration, viability, motility, and morphology of sperm in HFD rats indicated poor sperm quality. Moreover, the number of spermatogonia, Leydig cells, and Sertoli cells in the metformin group was significantly higher than that in the HFD group.
Serum analysis reflected the metabolic and sex hormone changes in each group. FSH, LH, and T are known to be regulatory factors of spermatogenesis. FSH elevates the number and function of Sertoli cells and directly activates the intracellular signaling pathway leading to the secretion of paracrine factors that indirectly promote spermatogenesis. LH acts on Leydig cells and promotes the secretion of T, which regulates the critical steps of spermatogenesis and participates in the intracellular signaling pathways. Our study revealed that, along with the increased body mass, the serum levels of hormones such as insulin, E2, and leptin increased in the HFD group, while T decreased, indicating that obesity impairs male reproductive function by disrupting the homeostasis of these hormones. The lower T levels observed in HFD rats may result from the reduced number of Leydig and Sertoli cells and the enhanced negative feedback on gonadotropins mediated by increased E2.
Metformin treatment of HFD rats had beneficial effects on the serum indexes. Furthermore, the levels of blood glucose, insulin, and HOMA-IR were significantly higher in the HFD group than those in the control and metformin groups, demonstrating dysregulated glycometabolism and IR in HFD rats. The relationship between obesity and IR is multifactorial. Visceral obesity is associated with decreased basal cortisol secretion and increased cortisol response to exogenous adrenocorticotropin stimulation, which may lead to higher insulin levels just as our results suggest [20]. Studies have shown that bioavailable, free, and total levels of T are all inversely correlated with IR and that this effect is mediated through body fat [21]. More interestingly, recent reports have shown that T treatment induces dramatic changes in weight, waist circumference, insulin sensitivity, and hemoglobin A1c levels and improvements in each of the components of metabolic syndrome [22]. In this study, after metformin treatment, the hormone and glucose concentrations were restored to approximately normal levels. This is consistent with the report of Kapoor, in which the effects of IR on serum androgen levels appeared to be restored when hypogonadal men accompanied with type 2 diabetes mellitus were treated with an insulin sensitizer [17]. Taken together, these results provide further evidence of a close interactional relationship between IR and T.
Leptin, which is the product of obese (ob) gene, is synthesized by adipocytes. Several animal models had been used to demonstrate the importance of leptin in the regulation of the hypothalamic–pituitary–gonadal (HPG) axis [23]. An adequate concentration of leptin is necessary for normal reproductive function, while overproduction of leptin, resulting in hormonal resistance, may be an important mechanism of androgen deficiency in obese men [24]. Isidori et al. found that circulating leptin correlated with total T and identified leptin as the best hormonal predictor of lower androgen levels in obese men [24]. An endocrine and/or direct paracrine effect of leptin on the gonads inhibits T production in Leydig cells [25]. Moreover, high leptin levels are associated with IR and metabolic syndrome, and these associations are significantly mediated through the effects of central obesity [26], which can further affect the production of T. Thus, multiple endocrine variations in HFD rats, such as low T levels, high E2, insulin, and leptin levels, contribute to adverse effects on spermatogenesis and semen quality. The results of the present study indicate that metformin intervention could restore hormonal homeostasis and dramatically improve metabolic disorder.
Spermatogenesis is a continuous and productive process supported by the self-renewal and differentiation of spermatogonial stem cells. Moderate apoptosis of testicular cells is identified as a physiological phenomenon during spermatogenesis, which may lead to dislodgment of deformed sperms in meiosis. However, excessive apoptosis is harmful to sperm production and semen quality, which can result in oligozoospermia and asthenozoospermia. In this study, AI was found to be higher in the HFD group compared with that in the control group. Metformin intervention reduced the apoptotic rate remarkably.
Based on the above-mentioned results of this animal study, a novel therapeutic method for obese patients with male infertility may be put forward. We hypothesize that metformin therapy at an optimal dose represents an effective treatment for male infertility and hypogonadism accompanied with obesity and/or IR by improving the semen quality and correcting endocrine disorder. However, larger, prospective, case-controlled studies are required to elucidate the effects of metformin on male reproductive function in obese patients. This information will help fertility specialists in counseling their patients and in tailoring the appropriate infertility treatment.
The limitations of the present study should be noted. Although we demonstrated that HFD induced detrimental effects on spermatogenesis, semen quality, endogenous hormone levels, and apoptosis of testicular cells in rats, we did not investigate the effects of obesity on fertilization ability by mating the male rats in the three groups with normal female rats and comparing the pregnancy and abortion rates. Additionally, the influence of other mechanisms of weight loss, such as caloric restriction and physical activity, on spermatogenesis should also be investigated and compared with the effects of metformin of male reproductive function. Furthermore, more cellular, biochemical, and molecular studies are required to clarify the effects of HFD and metformin on the reproductive system. These issues will be addressed systematically in subsequent studies.
Conclusion
The present study indicates that obesity induced by HFD results in detrimental effects on spermatogenesis, semen quality, endogenous hormone levels, and apoptosis of testicular cells in rats. Metformin intervention improves semen parameters in obese male rats, possibly due to its effects on weight loss, increased testicular weight, reduced testicular cell apoptosis, and restoration of hormonal homeostasis and correction of metabolic disorder.
Acknowledgments
This study was partially supported by the key research project of the Ministry of Public Security (2010 ZDYJHBST007).
Author contributions
WJY and JY conceived and designed the study. YZ collected the data. YM, XLP, and DC performed the animal experiments and statistical analyses. WJY, NY, and JY drafted and revised the manuscript. All authors read and approved the final manuscript.
Conflict of interest
All authors declare no competing interest.
Footnotes
Capsule
Metformin intervention improved the semen parameters, possibly due to its effects on weight loss, increased testicular weight, reduced testicular cell apoptosis, and resulted in restoration of hormonal homeostasis and correction of metabolic disorder.
References
- 1.Anawalt BD. Approach to male infertility and induction of spermatogenesis. J Clin Endocrinol Metab. 2013;98:3532–42. doi: 10.1210/jc.2012-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.John AR. Falling sperm counts twenty years on: where are we now? Asian J Androl. 2013;15:204–7. doi: 10.1038/aja.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper TG, Handelsman DJ. Falling sperm counts and global oestrogenic pollution: postscript. Asian J Androl. 2013;15:208–11. doi: 10.1038/aja.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupont C, Faure C, Sermondade N, et al. Obesity leads to higher risk of sperm DNA damage in infertile patients. Asian J Androl. 2013;15:622–5. doi: 10.1038/aja.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen TK, Heitmann BL, Blomberg Jensen M, et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am J Clin Nutr. 2013;97:411–8. doi: 10.3945/ajcn.112.042432. [DOI] [PubMed] [Google Scholar]
- 6.Ramlau-Hansen CH, Hansen M, Jensen CR, et al. Semen quality and reproductive hormones according to birthweight and body mass index in childhood and adult life: two decades of follow-up. Fertil Steril. 2010;94:610–8. doi: 10.1016/j.fertnstert.2009.01.142. [DOI] [PubMed] [Google Scholar]
- 7.Hammoud AO, Gibson M, Peterson CM, et al. Impact of male obesity on infertility: a critical review of the current literature. Fertil Steril. 2008;90:897–904. doi: 10.1016/j.fertnstert.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Barth RJ. Insulin resistance, obesity and the metabolic syndrome. SD Med. 2011; Spec No: 22–7. [PubMed]
- 9.Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036–41. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 10.Desilets AR, Dhakal-Karki S, Dunican KC. Role of metformin for weight management in patients without type 2 diabetes. Ann Pharmacother. 2008;42:817–26. doi: 10.1345/aph.1K656. [DOI] [PubMed] [Google Scholar]
- 11.Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21:323–9. doi: 10.1097/MED.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 12.Casulari LA, Caldas AD, Domingues Casulari Motta L, et al. Effects of metformin and short-term lifestyle modification on the improvement of male hypogonadism associated with metabolic syndrome. Minerva Endocrinol. 2010;35:145–51. [PubMed] [Google Scholar]
- 13.Ayuob NN, Murad HA, Ali SS. Impaired expression of sex hormone receptors in male reproductive organs of diabetic rat in response to oral antidiabetic drugs. Folia Histochem Cytobiol. 2015 doi: 10.5603/FHC.a2015.0005. [DOI] [PubMed] [Google Scholar]
- 14.Szulinska M, Musialik K, Suliburska J, et al. The effect of L-arginine supplementation on serum resistin concentration in insulin resistance in animal models. Eur Rev Med Pharmacol Sci. 2014;18:575–80. [PubMed] [Google Scholar]
- 15.Pi-Sunyer FX. The obesity epidemic: pathophysiology and consequences of obesity. Obes Res. 2002;10:97S–104. doi: 10.1038/oby.2002.202. [DOI] [PubMed] [Google Scholar]
- 16.Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diab Obes. 2010;17:224–32. doi: 10.1097/MED.0b013e3283398ee2. [DOI] [PubMed] [Google Scholar]
- 17.Kapoor D, Channer KS, Jones TH. Rosiglitazone increases bioactive testosterone and reduces waist circumference in hypogonadal men with type 2 diabetes. Diab Vasc Dis Res. 2008;5:135–7. doi: 10.3132/dvdr.2008.022. [DOI] [PubMed] [Google Scholar]
- 18.Kelsey MM, Bjornstad P, McFann K, et al. Testosterone concentration and insulin sensitivity in young men with type 1 and type 2 diabetes. Pediatr Diabetes. 2015 doi: 10.1111/pedi.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgante G, Tosti C, Orvieto R, et al. Metformin improves semen characteristics of oligo-terato-asthenozoospermic men with metabolic syndrome. Fertil Steril. 2011;95:2150–2. doi: 10.1016/j.fertnstert.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Hautanen A. Synthesis and regulation of sex hormone-binding globulin in obesity. Int J Obes Relat Metab Disord. 2000;24:S64–70. doi: 10.1038/sj.ijo.0801281. [DOI] [PubMed] [Google Scholar]
- 21.Tsai EC, Matsumoto AM, Fujimoto WY, et al. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. 2004;27:861–8. doi: 10.2337/diacare.27.4.861. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham GR. Testosterone and metabolic syndrome. Asian J Androl. 2015;17:192–6. doi: 10.4103/1008-682X.148068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teerds KJ, de Rooij DG, Keijer J. Functional relationship between obesity and male reproduction: from humans to animal models. Hum Reprod Update. 2011;17:667–83. doi: 10.1093/humupd/dmr017. [DOI] [PubMed] [Google Scholar]
- 24.Isidori AM, Caprio M, Strollo F, et al. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab. 1999;84:3673–80. doi: 10.1210/jcem.84.10.6082. [DOI] [PubMed] [Google Scholar]
- 25.Ramos CF, Zamoner A. Thyroid hormone and leptin in the testis. Front Endocrinol (Lausanne) 2014;5:198. doi: 10.3389/fendo.2014.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esteghamati A, Khalilzadeh O, Anvari M, et al. Association of serum leptin levels with homeostasis model assessment-estimated insulin resistance and metabolic syndrome: the key role of central obesity. Metab Syndr Relat Disord. 2009;7:447–52. doi: 10.1089/met.2008.0100. [DOI] [PubMed] [Google Scholar]