Abstract
We have previously demonstrated that B lymphocyte specific somatic mutations are introduced into the variable regions of immunoglobulin kappa transgenes in two independent transgenic mouse lines. The frequency, distribution and nature of these mutations strongly suggest that they arose as a result of the process of somatic hypermutation, which is responsible, in part, for affinity maturation during an immune response. Unexpectedly, in these multiple copy transgenic lines, many of the transgene copies showed no evidence of somatic mutation. This paradox was addressed by determining the sequence of each transgene copy in several B cell hybridomas derived from a mouse line carrying three copies of the kappa transgene. It was found that the somatic hypermutation process in different B cells from the same mouse preferentially targets one, but not the same, transgene copy. We present a model, based on the pattern of this targeting, which links somatic hypermutation to the orientation of the Ig gene relative to the direction of DNA replication.
Full text
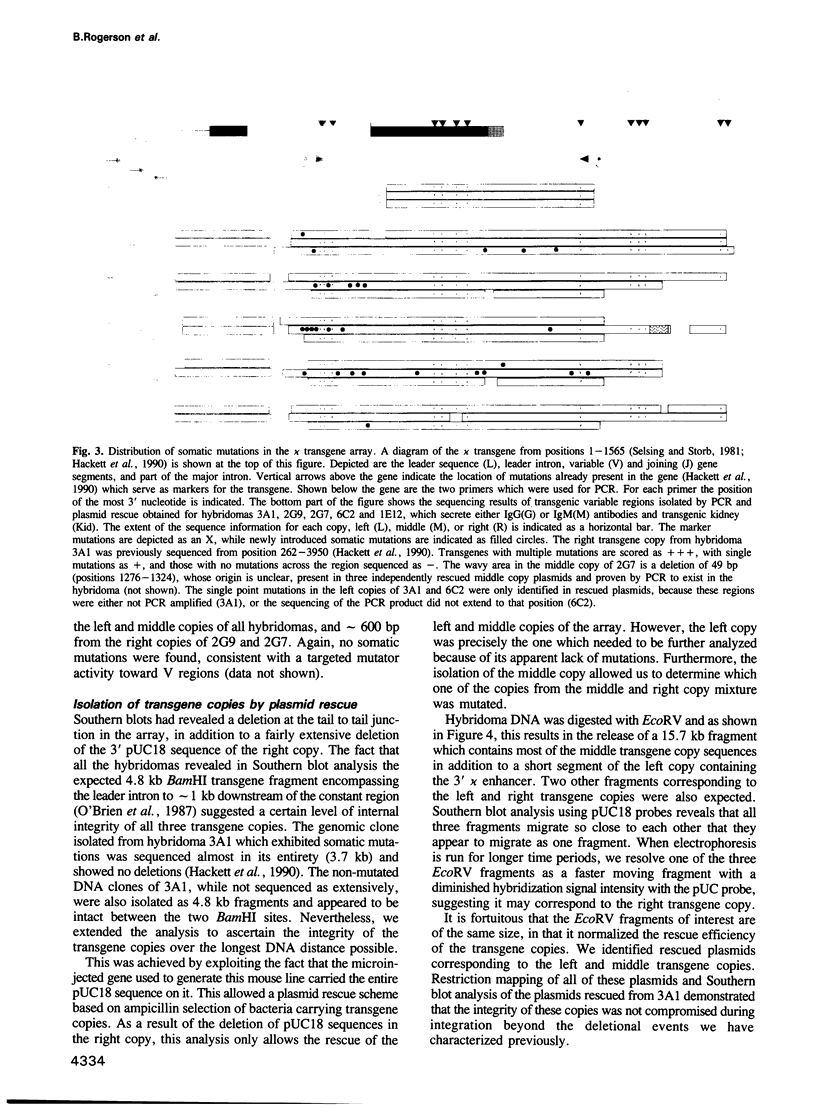
PDF

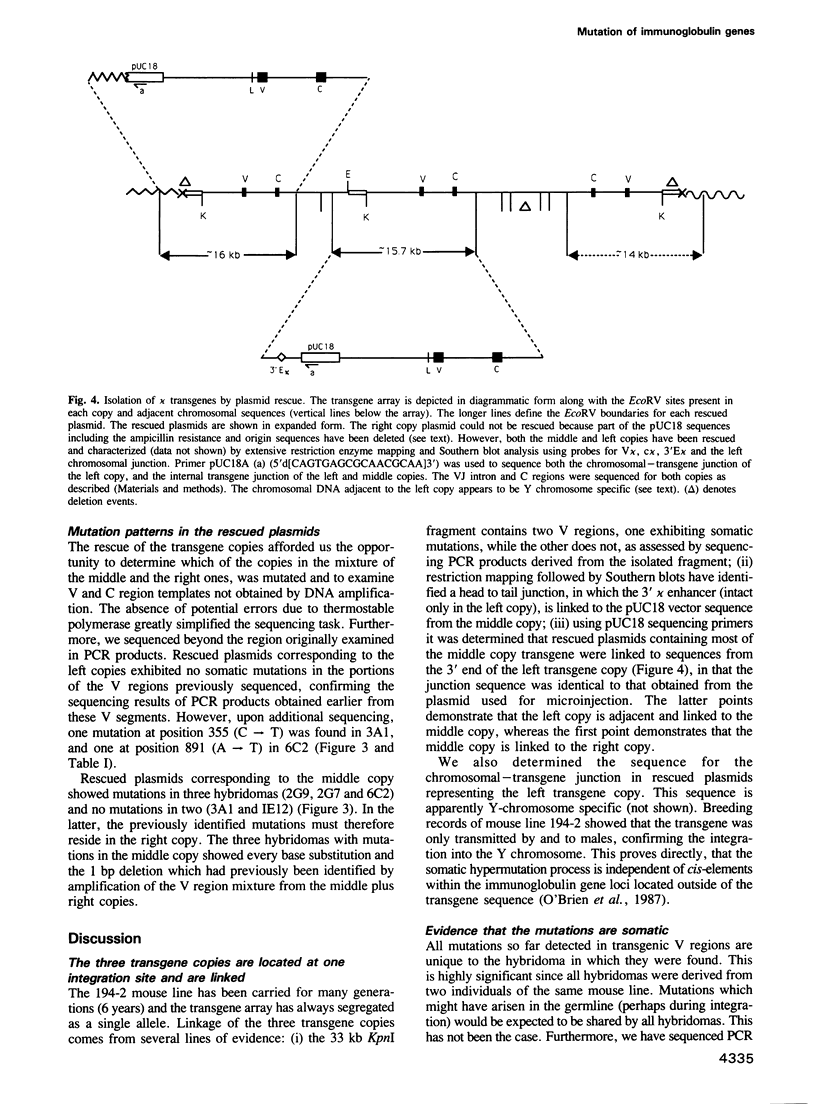




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Gene conversion: some implications for immunoglobulin genes. Cell. 1981 Jun;24(3):592–594. doi: 10.1016/0092-8674(81)90082-9. [DOI] [PubMed] [Google Scholar]
- Becker R. S., Knight K. L. Somatic diversification of immunoglobulin heavy chain VDJ genes: evidence for somatic gene conversion in rabbits. Cell. 1990 Nov 30;63(5):987–997. doi: 10.1016/0092-8674(90)90502-6. [DOI] [PubMed] [Google Scholar]
- Both G. W., Taylor L., Pollard J. W., Steele E. J. Distribution of mutations around rearranged heavy-chain antibody variable-region genes. Mol Cell Biol. 1990 Oct;10(10):5187–5196. doi: 10.1128/mcb.10.10.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., Milstein C. Origin of antibody variation. Nature. 1966 Jul 16;211(5046):242–243. doi: 10.1038/211242a0. [DOI] [PubMed] [Google Scholar]
- Brown E. H., Iqbal M. A., Stuart S., Hatton K. S., Valinsky J., Schildkraut C. L. Rate of replication of the murine immunoglobulin heavy-chain locus: evidence that the region is part of a single replicon. Mol Cell Biol. 1987 Jan;7(1):450–457. doi: 10.1128/mcb.7.1.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock P. A., Seo Y. S., Hurwitz J. Initiation of simian virus 40 DNA synthesis in vitro. Mol Cell Biol. 1991 May;11(5):2350–2361. doi: 10.1128/mcb.11.5.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza R. E., Eckhardt L. A., DelGiudice T., Schildkraut C. L. Changes in gene position are accompanied by a change in time of replication. Cell. 1984 Mar;36(3):689–696. doi: 10.1016/0092-8674(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Carlson L. M., McCormack W. T., Postema C. E., Humphries E. H., Thompson C. B. Templated insertions in the rearranged chicken IgL V gene segment arise by intrachromosomal gene conversion. Genes Dev. 1990 Apr;4(4):536–547. doi: 10.1101/gad.4.4.536. [DOI] [PubMed] [Google Scholar]
- Carson S., Wu G. E. A linkage map of the mouse immunoglobulin lambda light chain locus. Immunogenetics. 1989;29(3):173–179. doi: 10.1007/BF00373642. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Chien N. C., Pollock R. R., Desaymard C., Scharff M. D. Point mutations cause the somatic diversification of IgM and IgG2a antiphosphorylcholine antibodies. J Exp Med. 1988 Mar 1;167(3):954–973. doi: 10.1084/jem.167.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. H., Huppi K., Ruezinsky D., Staudt L., Gerhard W., Weigert M. Inter- and intraclonal diversity in the antibody response to influenza hemagglutinin. J Exp Med. 1985 Apr 1;161(4):687–704. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durdik J., Gerstein R. M., Rath S., Robbins P. F., Nisonoff A., Selsing E. Isotype switching by a microinjected mu immunoglobulin heavy chain gene in transgenic mice. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2346–2350. doi: 10.1073/pnas.86.7.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Gahn T. A., Schildkraut C. L. The Epstein-Barr virus origin of plasmid replication, oriP, contains both the initiation and termination sites of DNA replication. Cell. 1989 Aug 11;58(3):527–535. doi: 10.1016/0092-8674(89)90433-9. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Bogenhagen D. F. Clusters of point mutations are found exclusively around rearranged antibody variable genes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3439–3443. doi: 10.1073/pnas.80.11.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Gerstein R. M., Frankel W. N., Hsieh C. L., Durdik J. M., Rath S., Coffin J. M., Nisonoff A., Selsing E. Isotype switching of an immunoglobulin heavy chain transgene occurs by DNA recombination between different chromosomes. Cell. 1990 Nov 2;63(3):537–548. doi: 10.1016/0092-8674(90)90450-s. [DOI] [PubMed] [Google Scholar]
- Golding G. B., Gearhart P. J., Glickman B. W. Patterns of somatic mutations in immunoglobulin variable genes. Genetics. 1987 Jan;115(1):169–176. doi: 10.1093/genetics/115.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Rollini P., Mach B. Somatic mutations of immunoglobulin variable genes are restricted to the rearranged V gene. Science. 1983 Jun 10;220(4602):1179–1181. doi: 10.1126/science.6857243. [DOI] [PubMed] [Google Scholar]
- Grant S. G., Jessee J., Bloom F. R., Hanahan D. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4645–4649. doi: 10.1073/pnas.87.12.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. M., Berek C., Kaartinen M., Milstein C. Somatic mutation and the maturation of immune response to 2-phenyl oxazolone. Nature. 1984 Nov 15;312(5991):271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- Hackett J., Jr, Rogerson B. J., O'Brien R. L., Storb U. Analysis of somatic mutations in kappa transgenes. J Exp Med. 1990 Jul 1;172(1):131–137. doi: 10.1084/jem.172.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman J., Rudin C. M., Haasch D., Chaplin D., Storb U. A novel enhancer in the immunoglobulin lambda locus is duplicated and functionally independent of NF kappa B. Genes Dev. 1990 Jun;4(6):978–992. doi: 10.1101/gad.4.6.978. [DOI] [PubMed] [Google Scholar]
- Hamlin J. L., Vaughn J. P., Dijkwel P. A., Leu T. H., Ma C. Origins of replication: timing and chromosomal position. Curr Opin Cell Biol. 1991 Jun;3(3):414–421. doi: 10.1016/0955-0674(91)90068-a. [DOI] [PubMed] [Google Scholar]
- Hatton K. S., Schildkraut C. L. The mouse immunoglobulin kappa light-chain genes are located in early- and late-replicating regions of chromosome 6. Mol Cell Biol. 1990 Aug;10(8):4314–4323. doi: 10.1128/mcb.10.8.4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Davis M., Sinn E., Patten P., Hood L. Antibody diversity: somatic hypermutation of rearranged VH genes. Cell. 1981 Dec;27(3 Pt 2):573–581. doi: 10.1016/0092-8674(81)90399-8. [DOI] [PubMed] [Google Scholar]
- Kocks C., Rajewsky K. Stable expression and somatic hypermutation of antibody V regions in B-cell developmental pathways. Annu Rev Immunol. 1989;7:537–559. doi: 10.1146/annurev.iy.07.040189.002541. [DOI] [PubMed] [Google Scholar]
- Lebecque S. G., Gearhart P. J. Boundaries of somatic mutation in rearranged immunoglobulin genes: 5' boundary is near the promoter, and 3' boundary is approximately 1 kb from V(D)J gene. J Exp Med. 1990 Dec 1;172(6):1717–1727. doi: 10.1084/jem.172.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N. S., Malipiero U. V., Lebecque S. G., Gearhart P. J. Early onset of somatic mutation in immunoglobulin VH genes during the primary immune response. J Exp Med. 1989 Jun 1;169(6):2007–2019. doi: 10.1084/jem.169.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton PJ L., Decker D. J., Klinman N. R. Primary antibody-forming cells and secondary B cells are generated from separate precursor cell subpopulations. Cell. 1989 Dec 22;59(6):1049–1059. doi: 10.1016/0092-8674(89)90761-7. [DOI] [PubMed] [Google Scholar]
- Maizels N. Might gene conversion be the mechanism of somatic hypermutation of mammalian immunoglobulin genes? Trends Genet. 1989 Jan;5(1):4–8. doi: 10.1016/0168-9525(89)90004-8. [DOI] [PubMed] [Google Scholar]
- Manser T. Evolution of antibody structure during the immune response. The differentiative potential of a single B lymphocyte. J Exp Med. 1989 Oct 1;170(4):1211–1230. doi: 10.1084/jem.170.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser T. The efficiency of antibody affinity maturation: can the rate of B-cell division be limiting? Immunol Today. 1990 Sep;11(9):305–308. doi: 10.1016/0167-5699(90)90124-r. [DOI] [PubMed] [Google Scholar]
- Manz J., Denis K., Witte O., Brinster R., Storb U. Feedback inhibition of immunoglobulin gene rearrangement by membrane mu, but not by secreted mu heavy chains. J Exp Med. 1988 Oct 1;168(4):1363–1381. doi: 10.1084/jem.168.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHeyzer-Williams M. G., Nossal G. J., Lalor P. A. Molecular characterization of single memory B cells. Nature. 1991 Apr 11;350(6318):502–505. doi: 10.1038/350502a0. [DOI] [PubMed] [Google Scholar]
- Meyer K. B., Neuberger M. S. The immunoglobulin kappa locus contains a second, stronger B-cell-specific enhancer which is located downstream of the constant region. EMBO J. 1989 Jul;8(7):1959–1964. doi: 10.1002/j.1460-2075.1989.tb03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama N., Okada H., Azuma T. Somatic mutation in constant regions of mouse lambda 1 light chains. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7933–7937. doi: 10.1073/pnas.88.18.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. L., Brinster R. L., Storb U. Somatic hypermutation of an immunoglobulin transgene in kappa transgenic mice. 1987 Mar 26-Apr 1Nature. 326(6111):405–409. doi: 10.1038/326405a0. [DOI] [PubMed] [Google Scholar]
- Pech M., Höchtl J., Schnell H., Zachau H. G. Differences between germ-line and rearranged immunoglobulin V kappa coding sequences suggest a localized mutation mechanism. Nature. 1981 Jun 25;291(5817):668–670. doi: 10.1038/291668a0. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Anquez V., Dahan A., Weill J. C. A single rearrangement event generates most of the chicken immunoglobulin light chain diversity. Cell. 1985 Feb;40(2):283–291. doi: 10.1016/0092-8674(85)90142-4. [DOI] [PubMed] [Google Scholar]
- Roberts J. D., Thomas D. C., Kunkel T. A. Exonucleolytic proofreading of leading and lagging strand DNA replication errors. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3465–3469. doi: 10.1073/pnas.88.8.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roes J., Hüppi K., Rajewsky K., Sablitzky F. V gene rearrangement is required to fully activate the hypermutation mechanism in B cells. J Immunol. 1989 Feb 1;142(3):1022–1026. [PubMed] [Google Scholar]
- Rudikoff S., Pawlita M., Pumphrey J., Heller M. Somatic diversification of immunoglobulins. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2162–2166. doi: 10.1073/pnas.81.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schvartzman J. B., Adolph S., Martín-Parras L., Schildkraut C. L. Evidence that replication initiates at only some of the potential origins in each oligomeric form of bovine papillomavirus type 1 DNA. Mol Cell Biol. 1990 Jun;10(6):3078–3086. doi: 10.1128/mcb.10.6.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsing E., Storb U. Somatic mutation of immunoglobulin light-chain variable-region genes. Cell. 1981 Jul;25(1):47–58. doi: 10.1016/0092-8674(81)90230-0. [DOI] [PubMed] [Google Scholar]
- Shan H., Shlomchik M., Weigert M. Heavy-chain class switch does not terminate somatic mutation. J Exp Med. 1990 Aug 1;172(2):531–536. doi: 10.1084/jem.172.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe M. J., Milstein C., Jarvis J. M., Neuberger M. S. Somatic hypermutation of immunoglobulin kappa may depend on sequences 3' of C kappa and occurs on passenger transgenes. EMBO J. 1991 Aug;10(8):2139–2145. doi: 10.1002/j.1460-2075.1991.tb07748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele E. J., Pollard J. W. Hypothesis: somatic hypermutation by gene conversion via the error prone DNA----RNA----DNA information loop. Mol Immunol. 1987 Jun;24(6):667–673. doi: 10.1016/0161-5890(87)90049-6. [DOI] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Storb U., Pinkert C., Arp B., Engler P., Gollahon K., Manz J., Brady W., Brinster R. L. Transgenic mice with mu and kappa genes encoding antiphosphorylcholine antibodies. J Exp Med. 1986 Aug 1;164(2):627–641. doi: 10.1084/jem.164.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak H. F., Flavell R. A. A method for the recovery of DNA from agarose gels. Nucleic Acids Res. 1978 Jul;5(7):2321–2332. doi: 10.1093/nar/5.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Neiman P. E. Somatic diversification of the chicken immunoglobulin light chain gene is limited to the rearranged variable gene segment. Cell. 1987 Feb 13;48(3):369–378. doi: 10.1016/0092-8674(87)90188-7. [DOI] [PubMed] [Google Scholar]
- Weber J. S., Berry J., Litwin S., Claflin J. L. Somatic hypermutation of the JC intron is markedly reduced in unrearranged kappa and H alleles and is unevenly distributed in rearranged alleles. J Immunol. 1991 May 1;146(9):3218–3226. [PubMed] [Google Scholar]
- Weber J. S., Berry J., Manser T., Claflin J. L. Position of the rearranged V kappa and its 5' flanking sequences determines the location of somatic mutations in the J kappa locus. J Immunol. 1991 May 15;146(10):3652–3655. [PubMed] [Google Scholar]
- Weiss S., Wu G. E. Somatic point mutations in unrearranged immunoglobulin gene segments encoding the variable region of lambda light chains. EMBO J. 1987 Apr;6(4):927–932. doi: 10.1002/j.1460-2075.1987.tb04840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Gefter M. L. Gene conversion and the generation of antibody diversity. Annu Rev Biochem. 1989;58:509–531. doi: 10.1146/annurev.bi.58.070189.002453. [DOI] [PubMed] [Google Scholar]




