Abstract
Quality of air in the clinical embryology laboratory is considered critical for high in vitro fertilization (IVF) success rates, yet evidence for best practices is lacking. Predominantly anecdotal reports on relationships between air quality and IVF success rates have resulted in minimal authentic clinical laboratory guidelines or in recommendations that are based on industrial cleanroom particulate standards with little attention to chemical air filtration. As a result, a nascent industry of costly, specialized air handling equipment for IVF laboratories has emerged to provide air quality solutions that have not been clearly assessed or verified. Clinics are embracing such technology because their embryology laboratories have become epicenters of assisted reproductive technology as the practice of IVF has moved to blastocyst transfers and utilization of trophectoderm biopsy for preimplantation genetic testing (PGT). Thus, a laboratory’s ability to culture, biopsy, and freeze blastocysts is a rate-limiting step that depends on technical proficiency and a supportive and stable culture environment based on a foundation of high-quality ambient air. This review aims to describe how evidence for the importance of air quality, in particular the role of volatile organic compounds (VOC), has resulted in an evolution of clinical practice that has arguably contributed to improved outcomes.
Keywords: IVF, Air quality, Assisted reproduction, Embryo culture, Volatile organic compounds
Air quality and IVF: historical perspective
During the 1990s, two approaches to indoor air quality emerged in the IVF laboratory: the clean room philosophy that focused on filtration of particles using high efficiency particulate air filters (HEPA) and the chemical air filtration philosophy that focused on removal of volatile organic compounds (VOCs) with solid-phase filtration (e.g., activated carbon and potassium permanganate (KMnO4)). Though the Boone laboratory initially reported an adverse effect of VOCs on mouse embryo development [1], their laboratory responded to perceived adverse air quality caused by neighboring construction by converting their IVF laboratory and its heating, ventilation, and air conditioning (HVAC) unit into a Class 100 clean room [2]. A Class 100 clean room comprises numerous design features that includes a maximum of 100 particles ≥0.5 μm per cubic foot of air (ISO Class 5). While the system described by Boone [2] included carbon pre-filters, the extent of chemical air filtration appears minimal and no VOC testing was reported. In contrast, Cohen and colleagues [3] first described a detailed assessment of chemical air contamination and linked pregnancy rates to VOCs generated from neighboring space, construction, and cleaning activity. While it is likely that HEPA filters were used in the Cohen study, details of particle counts and the room classification were not provided.
The impact of these two approaches on laboratory design over the next two decades is illustrated in Fig. 1 and can be grouped into three eras: awareness, application, and clinical evidence. While there are more than 30 reports, few are well-controlled, peer-reviewed papers. During the awareness era, the first published reports of adverse effects of poor indoor air quality (IAQ) on IVF outcomes appeared. In response to these mostly anecdotal observations, a boutique/cottage industry of both HVAC and portable air purification systems emerged, with the first report of a portable air filtration product for the IVF laboratory in 1999.
Fig. 1.
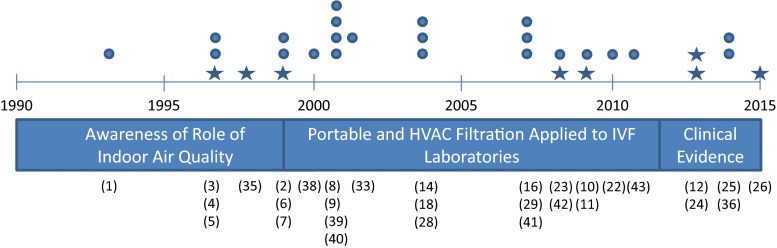
Historical representation of three periods of study of the relationship between air quality and assisted reproduction. Peer-reviewed papers are represented by stars, abstracts and other reports are listed as circles
Research on factors that impact air quality identified medical gasses (CO2), incubators [4], and plastic ware [5] as contributors to VOCs in the lab, which led to the development of the Coda® incubator chamber air filter. Preliminary results of the Coda® were promising, with reports of increased clinical pregnancy rates [6] and decreased miscarriage rates [7]. Other groups did not observe a benefit of incubator filtration units [8, 9], and the popularity of the incubator chamber unit at present is unknown. More recently, introduction of HEPA [10] or HEPA + VOC [11] filtration to incubators has not shown an improvement in IVF outcomes, results that likely contribute to their limited popularity. In contrast, another product developed in the late 1990s—the inline carbon filter for medical gasses—is ubiquitous in IVF laboratories, despite the fact that its effectiveness is largely unknown.
The second era of air quality in IVF laboratories began at the start of the twenty-first century, as awareness of a relationship between indoor air quality and IVF outcomes resulted in new air quality products and additional studies. During this phase, new standards for embryology laboratories in Europe and Brazil were introduced (reviewed by Esteves and Bento [12]). It should be noted, however, that the EU tissue directive does not include solid-phase chemical filtration for VOCs and though the Brazilian regulatory directive includes the use of filters embedded with activated carbon, specifications are lacking. The standards for laboratories in the USA as published by the American Society for Reproductive Medicine (ASRM) are notably lacking in specifics regarding air quality [13]. Prior to the EU guidelines, a survey in Europe found that only 1 of 10 clinics had a system with VOC filtration [14]. In contrast, Van Voorhis et al. [15] reported that at least 7 of 10 consistently high-performing IVF centers in the USA used filtered laboratory air with HEPA and chemical, solid-phase filtration for VOCs. The difference between the two regions could be due to the fact that the US report was several years after the EU survey, or it may represent different practice patterns.
Air quality and IVF outcomes
The most recent era as presented in Fig. 1 represents a period where the impact of improving air quality on clinical IVF outcomes is gaining traction in the form of peer-reviewed studies. The studies in Table 1 compared clinical outcomes before and after a change in air quality, with most of the peer-reviewed papers appearing in the past 5 years. This section will discuss the evidence for the different approaches to air filtration.
Table 1.
Impact of air filtration on clinical outcomes
| Study | Patients (n) | Era | Particulates | Chemical air filtration | Variables | Outcome |
|---|---|---|---|---|---|---|
| Boone et al., 1999 [2] | 275 | 1993–1997 | Class 100 | CIF | FR, CPR | Increased over time |
| Knaggs et al., 2007 [16] | NA | 2006 | Grade B | NA | CPR | 42.6 vs 30.6 |
| Jindal et al., 2008 [23] | 380 | 2006–2007 | NA | Carbon + KMnO4 | CPR | 46.8 vs 32.9 |
| Dickey et al., 2010 [22] | 324 | 2005–2009 | NA | Carbon + KMnO4 | CPR, IR | 63.4 vs 46.4 |
| Esteves and Bento, 2013 [12] | 2315 | 1999–2010 | ISO 5 | CIF, Carbon | FR, BR, CPR | 47.0 vs 40.0 |
| Khoudja et al., 2013 [24] | 1403 | 2011 | HEPA | Carbon | FR, CR, BR, CPR, IR | All improved |
| Forman et al., 2014 [25] | 1245 | 2012–2013 | NA | Carbon, UVPCO | BR, IR, CPR, OPR | All Improved |
| Munch et al., 2015 [26] | 524 | 2010–2012 | HEPA | Lack of carbon | FR, CR, BR, CPR, IR, LBR | FR, CR, BR decreased |
FR fertilization rate, CR cleavage rate, BR blastocyst rate, CPR clinical pregnancy rate, IR implantation rate, LBR live birth rate, OP ongoing pregnancy rate, CIF carbon-impregnated filter, KMnO 4 potassium permanganate, UVPCO ultraviolet photocatalytic oxidation
Particulate filtration and cleanroom methods
Particulate filtration is often associated with cleanrooms because specifications are well established in sectors such as the semiconductor industry. Cleanroom classification is based on several factors, including the class of HEPA filter, room air exchanges per hour, and air pressure relative to neighboring rooms. International standards exist for clean rooms (ISO; reviewed by [12]), and in general, these rooms are validated with particulate counts and microbiological monitoring. The specific requirements for IVF laboratories vary by country/region [12]. Only 3 of the 8 studies listed in Table 1 indicate clean room specifications with appropriate particulate count monitoring: two as Class 100/ISO Class 5 [2, 12] and one Class B [16]. The other five studies indicate use of HEPA filtration without providing particle counts, suggesting that particle counts and cleanroom designation were not a primary consideration for the design of the systems. The lack of a strong association between particulates and IVF outcomes may be due in part to the nature of IVF: relatively short culture duration, culture under mineral oil, and the presence of antibiotics in culture media.
Chemical air filtration and the role of VOCs
Chemical air filtration, unlike filtration for particulates, is far from standardized. The amount and form of activated carbon and oxidizing media (KMnO4) varies considerably among different types of filters [17]. Carbon-impregnated filters, used by Boone and Esteves [2, 18], were at one time considered sufficient but likely do not contain enough carbon to provide the required surface area and air residence time for effective VOC removal [17]. A more common approach is the use of activated carbon filter beds with KMnO4 as a separate filter or impregnated on the carbon itself. The challenge of chemical air filtration is effective scrubbing—handling a large and variable VOC load in the presence of varying relative humidity. A relatively new method for removing VOCs is ultraviolet photocatalytic oxidation (UVPCO; [19]), often combined with carbon filters. UVPCO uses the energy of UV lights absorbed by a semiconductor metal oxide (e.g., titanium oxide) to produce reactive species on the surface of the photocatalyst that then react with adsorbed VOCs. The photo-oxidation of VOCs leads to partial mineralization (i.e., conversion to CO2, water and other inorganic species) and also produces partially oxidized byproducts. Volatile byproducts can be released as secondary pollutants [20] and non-volatile byproducts remain attached to the surface of the catalyst, leading to partial deactivation [21]. The relative effectiveness of carbon media vs UVPCO is debatable, though both approaches provide considerably more air scrubbing capacity compared to standard carbon-impregnated filters.
While each study listed in Table 1 noted an improvement in either laboratory or clinical outcomes or both, nearly every study performed an unmatched, retrospective analysis that did not account for possible differences in patient populations and practice changes that could be responsible for the observed differences in outcomes [2, 12, 22]. The report by Esteves and Bento [12] highlights the challenge of analyzing outcomes over a long period of time (>10 years). From 2000 to 2003, the authors found an improvement in cleavage rate, embryo quality, and clinical pregnancy rate after they changed from a class 1000 (ISO 6) to a class 100 (ISO 5) cleanroom using carbon-impregnated filters in the HVAC [18]. During the subsequent years up to 2011, VOC filtration was changed to KMnO4-impregnated carbon-activated filter beds and the number of embryos transferred per patient decreased, leading to no change in clinical pregnancy rates although there was an increase in high-quality embryos, particularly during the final 3 years. This study along with the other long-term cleanroom study (Boone) provides little evidence for benefit of the particulate filtration approach.
Evidence for benefits of VOC filtration is derived from mostly circumstantial, retrospective reports that lack statistical adjustment for patient population differences and practice changes [22–25]. While these examples add to the limited evidence that air quality is positively associated with IVF outcomes, the study designs limit the strength of the conclusions. A notable exception is the recent report by Munch et al. where inadvertent removal of carbon filters resulted in poor embryo development compared to periods before or after the affected period [26]. Though key performance indicators (KPI) for laboratory variables such as fertilization rate and embryo utilization rate have not been developed industry-wide, the rate that fertilized oocytes cleaved to 2 cell embryos is sub-optimal at <90 % during the period without carbon filtration. Routine KPIs for cleavage rate are >90 % and typically >95 %. Two studies listed in Table 1 that reported this variable had similarly poor cleavage rates that improved after a change in air filtration (84.6 vs 94.7 %, [18]; 90.8 vs 97.4 %, [24]). Unlike the mostly retrospective studies in Table 1, Munch et al. did an excellent job of matching sample sizes, study duration, and season. Furthermore, the clinic has a strong history of stable pregnancy rates and practice patterns [15, 27]. They applied statistical measures to account for multiple embryos per patient and performed subgroup analyses, all of which indicated that embryo development was adversely affected during the period lacking carbon filtration. This provides the best evidence to date for the importance of chemical air filtration.
Portable filtration applications
Another development from the air quality studies of the late 1990s was the introduction of the stand-alone VOC filtration unit (first proposed by Cohen et al. [3]), raising the question of whether it is possible to overcome poor air quality with a less expensive in-room, portable solution. Similar to HVAC approaches, these portable air filtration units are available with carbon filtration or with UVPCO. Data on effectiveness of these portable units is minimal, though portable carbon filtration [28] and UVPCO units [29] in an IVF laboratory reduced concentrations of VOCs. There is even less evidence of benefit for clinical outcomes. Two of the studies in Table 1 included stand-alone systems in use at the start of the study and thus provide an opportunity to compare stand-alone units with a new system. In both studies, three periods were compared: pre-filtration, in-room portable filters, and in-room filters with a new air handler system [22] or specialty in-room purifier [24]. Dickey et al. [22] saw limited benefit after addition of in-room UVPCO units in an environment that did not contain VOC filtration. In contrast, Khoudja et al. [24] observed a decrease in some VOCs such as benzene but no change in formaldehyde after replacing carbon filters in several portable units. In spite of minimal changes in measured air quality in this latter study, fertilization and cleavage rate increased while implantation rate was not different. In both studies, addition of a final layer of VOC filtration resulted in improved embryo and pregnancy outcomes. Since portable air filtration units have significantly less capacity than HVAC systems, both in terms of carbon surface area and air flowrate, it is unlikely that they can provide the same degree of air quality in all conditions, though this requires further research.
Future directions
The recent multicenter, preliminary report on purpose-built HVAC systems for IVF laboratories [25] coupled with the activity of a well-financed HVAC specialty company will likely lead to more evidence that supports the importance of air quality in the IVF laboratory. These new systems appear robust and should offer world-class air quality, though at a significant cost upfront, operationally, and with ongoing maintenance. The question that many clinics are asking is whether such an extensive and expensive system is necessary. Implantation rates of >50 % in young patients are not uncommon in clinics without a specialized system. For high-performing centers, this new approach to air quality is akin to insurance, since a program never knows when it will need the filtering/cleansing power to combat a sudden drop in air quality. Like all areas of medicine, a cost-benefit analysis is needed.
In order to determine the extent that a laboratory should filter its air, we need to know what the species and the concentration of VOCs that are harmful for gametes and embryos. This is a challenge because of the variable nature of different VOCs in room air. Since indoor air quality is impacted by outdoor air, which varies regionally, the observation that air pollution affects fertility [30–32] provides further evidence for the importance of air filtration.
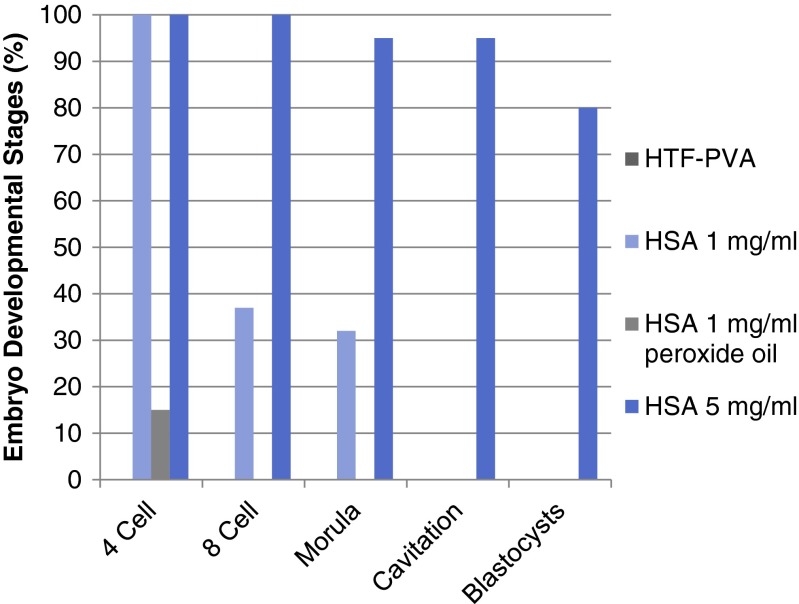
Beyond room air, the quality of medical-grade gas is critical since in many incubators, particularly the benchtop styles, 100 % of the air is from premixed tanks. While the United States Pharmacopeia (USP) has limits for some chemical air contaminants in medical-grade gas, these limits are likely higher than what is safe for an embryo, an organism that lacks the defenses and organ systems of an adult human. In fact, one report suggested that toluene at 2.2–2.7 parts per billion (ppb) in room air affected implantation rates [33]. When acrolein, a reactive aldehyde that is a common air pollutant [34], was added to embryo culture media, mouse embryos arrested at the 8-cell stage at 2.1 parts per million (ppm; 0.0375 mM) and live birth rate was reduced in as little as 0.58 ppm [35]. In addition, Karagouga et al. [36] found that 0.5 ppm acrolein in air for just 24 h during the zygote stage inhibited mouse embryo development. This effect was dependent on the concentration of albumin present in the culture media (Fig. 2). Zygotes degenerated in protein-free medium, developed to the morula stage in medium containing 1 mg/mL albumin, and developed to blastocysts at a high rate in 5 mg/mL albumin. Since laboratory conditions are variable among clinics, a fourth group was included to test if the effects of VOCs are exacerbated by sub-optimal conditions. Zygotes were cultured under mineral oil with known sublethal toxicity [37]. Zygotes lysed after reaching the 4-cell stage in medium containing 1 mg/mL albumin but again, development was protected when 5 mg/mL of albumin was included. Developmental competence was not tested, so it is possible that cell numbers or implantation rate was decreased in the latter group. These results illustrate the multifactorial nature of air quality and its relationship with culture conditions.
Fig. 2.
Effect of acrolein in air (0.5 ppm for 24 h at the zygote stage) on mouse embryo development. HTF-PVA control is protein-free medium. HSA human serum albumin. Peroxide oil passed a standard one-cell mouse embryo assay [36]
Conclusion
The evidence is clear that air quality impacts IVF outcomes, yet we have neglected to perform controlled studies on air quality. As a consequence, the field is left with minimal industry standards and costly solutions that are not evidence based. Assisted reproduction has experienced remarkable improvement over the past 20 years, progress that is the result of continuous research and development in many areas of fertility care. As reviewed by Van Voorhis et al., improvements in stimulation medications and protocols, fertilization rates with sperm injection, embryo culture media, embryo selection, incubator quality, and embryo transfer technique all contribute to the current state of clinical assisted reproduction [15]. Air quality, first addressed extensively in the late 1990s, has undoubtedly had a role in these improved outcomes. While there is little evidence that particulate filtration alone improves IVF outcomes, available evidence suggests that filtration systems should focus on robust VOC filtration. Further research is needed to effectively balance the benefits of air filtration systems with the costs.
Acknowledgments
The author would like to thank Hugo Destaillats, Ph.D. of the Lawrence Berkeley National Laboratory and Jack H. Britt, Ph.D. for their helpful suggestions.
Footnotes
Capsule Quality of air in the clinical embryology laboratory is considered critical for high in vitro fertilization (IVF) success rates, yet evidence for best practices is lacking. This review aims to describe how evidence for the importance of air quality, in particular the role of volatile organic compounds (VOC), has resulted in an evolution of clinical practice that has arguably contributed to improved outcomes.
References
- 1.Johnson JE, Boone WR, Bernard RS. The effects of volatile compounds (VC) on the outcome of in vitro mouse embryo culture. Fertil Steril. 1993;60:S98–9. [Google Scholar]
- 2.Boone WR, Johnson JE, Locke AJ, Crane MM, Price TM. Control of air quality in an assisted reproductive technology laboratory. Fertil Steril. 1999;71:150–4. doi: 10.1016/S0015-0282(98)00395-1. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J, Gilligan A, Esposito W, Schimmel T, Dale B. Ambient air and its potential effects on conception in vitro. Hum Reprod. 1997;12:1742–9. doi: 10.1093/humrep/12.8.1742. [DOI] [PubMed] [Google Scholar]
- 4.Schimmel T, Gilligan A, Garrisi GJ, Esposito B, Jr, Cecchi M, Dale B. Removal of volatile organic compounds from incubators used for gamete and embryo culture. Fertil Steril. 1997;68(Supplement 1):S165. doi: 10.1016/S0015-0282(97)90966-3. [DOI] [Google Scholar]
- 5.Gilligan A, Schimmel T, Esposito B, Jr, Cohen J. Release of volatile organic compounds such as styrene by sterile petri dishes and flasks used for in-vitro fertilization. Fertil Steril. 1997;68(Supplement 1):S52–S3. doi: 10.1016/S0015-0282(97)90737-8. [DOI] [Google Scholar]
- 6.Mayer JF, Nehchiri F, Weedon VM, Jones EL, Kalin HL, Oehninger SC, et al. Prospective randomized crossover analysis of the impact of an IVF incubator filtration system (Coda, GenX) on clinical pregnancy rates. Fertil Steril. 1999;72:S42–3. [Google Scholar]
- 7.Racowsky C, Jackson K, Nurredin A, Balint C, de los Santos M, Kelley J et al. Carbon-activation air filtration results in reduced in reduced spontaneous abortion rates following IVF. 11th World Congress on In Vitro Fertilization and Human Reproductive Genetics 1999;May 9–14 1999, Sydney, Australia O-59.
- 8.Battaglia DE, Khabani A, Rainer C, Moore DE. Prospective randomized trial of incubator CODA filtration units revealed no effect on outcome parameters for IVF. Fertil Steril. 2001;75:S6. doi: 10.1016/S0015-0282(01)01710-1. [DOI] [Google Scholar]
- 9.McLellan ST, Panagoulopoulos C, Dickinson KA, Wright DL, Toth TL, Lanzendorf SE. Effect of incubator air filtration system on IVF outcomes. Fertil Steril. 2001;76:S103. doi: 10.1016/S0015-0282(01)02303-2. [DOI] [Google Scholar]
- 10.Souza MCB, Mancebo ACA, da Rocha CA, Henriques CA, Souza MM, Cardoso FFO. Evaluation of two incubation environments—ISO class 8 versus ISO class 5—on intracytoplasmic sperm injection cycle outcome. Fertil Steril. 2009;91:1780–4. doi: 10.1016/j.fertnstert.2008.02.130. [DOI] [PubMed] [Google Scholar]
- 11.Sene IS, Carvalho BF, Freitas TAF, Pádua LEM, Sousa GNS, Bona LN. Comparing HEPA versus HEPA-VOC filtration system: influence on embryo quality and clinical outcomes of in vitro fertilization. Fertil Steril. 2009;92:S234. doi: 10.1016/j.fertnstert.2009.07.1573. [DOI] [Google Scholar]
- 12.Esteves SC, Bento FC. Implementation of air quality control in reproductive laboratories in full compliance with the Brazilian cells and germinative tissue directive. Reprod Biomed Online. 2013;26:9–21. doi: 10.1016/j.rbmo.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 13.ASRM Revised minimum standards for practices offering assisted reproductive technologies. Fertil Steril. 2008;90:S165–S8. doi: 10.1016/j.fertnstert.2008.08.098. [DOI] [PubMed] [Google Scholar]
- 14.von Wyl S, Bersinger NA. Air quality in the IVF laboratory: results and survey. J Assist Reprod Genet. 2004;21:283–4. doi: 10.1023/B:JARG.0000043700.70912.0b. [DOI] [PubMed] [Google Scholar]
- 15.Van Voorhis BJ, Thomas M, Surrey ES, Sparks A. What do consistently high-performing in vitro fertilization programs in the U.S. do? Fertil Steril. 2010;94:1346–9. doi: 10.1016/j.fertnstert.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 16.Knaggs P, Birch D, Drury S, Morgan M, Kumari S, Sriskandakumar R, et al. Full compliance with the EU directive air quality standards does not compromise IVF outcome. Hum Reprod. 2007;22:i164–5. [Google Scholar]
- 17.Fisk WJ. Can sorbent-based gas phase air cleaning for VOCs substitute for ventilation in commercial buildings? IAQ 2007 Healthy and Sustainable Buildlings 2007;American Society of Heating, Refrigerating and Air Conditioning Engineers, Inc.
- 18.Esteves SC, Gomes AP, Verza S., Jr Control of air pollution in assisted reproductive technology laboratory and adjacent areas improves embryo formation, cleavage and pregnancy rates and decreases abortion rate: comparison between a class 100 (ISO 5) and a class 1.000 (ISO 6) cleanroom for micromanipulation and embryo culture. Fertil Steril. 2004;82(Supplement 2):S259–S60. doi: 10.1016/j.fertnstert.2004.07.691. [DOI] [Google Scholar]
- 19.Hodgson AI, Destaillats H, Sullivan DP, Fisk WJ. Performance of ultraviolet photocatalytic oxidation for indoor air cleaning applications. Indoor Air. 2007;17:305–16. doi: 10.1111/j.1600-0668.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- 20.Destaillats H, Sleiman M, Sullivan DP, Jacquiod C, Sablayrolles J, Molins L. Key parameters influencing the performance of photocatalytic oxidation (PCO) air purification under realistic indoor conditions. Appl Catal B-Environ. 2012;128:159–70. doi: 10.1016/j.apcatb.2012.03.014. [DOI] [Google Scholar]
- 21.Hay SO, Obee TN, Thibaud-Erkey C. The deactivation of photocatalytic based air purifiers by ambient siloxanes. Appl Catal B-Environ. 2010;99:435–41. doi: 10.1016/j.apcatb.2010.06.018. [DOI] [Google Scholar]
- 22.Dickey RP, Wortham JWE, Jr, Potts A, Welch A. Effect of IVF laboratory air quality on pregnancy success. Fertil Steril. 2010;94:S151. doi: 10.1016/j.fertnstert.2010.07.605. [DOI] [Google Scholar]
- 23.Jindal SK, Polotsky AJ, Buyuk ER, Lieman HJ, Gilligan A. Improved pregnancy rates following introduction of engineering controls of lab air quality. Fertil Steril. 2008;90(Supplement):S403. doi: 10.1016/j.fertnstert.2008.07.1412. [DOI] [Google Scholar]
- 24.Khoudja RY, Xu Y, Li T, Zhou C. Better IVF outcomes following improvements in laboratory air quality. J Assist Reprod Genet. 2013;30:69–76. doi: 10.1007/s10815-012-9900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forman M, Sparks AET, Degelos S, Koulianos G, Worrilow KC. Statistically significant improvements in clinical outcomes using engineered molecular media and genomically modeled ultraviolet light for comprehensive control of ambient air (AA) quality. Fertil Steril. 2014;102:e91. doi: 10.1016/j.fertnstert.2014.07.307. [DOI] [Google Scholar]
- 26.Munch EM, Sparks AE, Van Voorhis BJ, Duran EH. Lack of carbon filtration impacts early embryo development. J Assist Reprod Genet. 2015:1–9. [DOI] [PMC free article] [PubMed]
- 27.Kresowik JD, Stegmann BJ, Sparks AE, Ryan GL, van Voorhis BJ. Five-years of a mandatory single-embryo transfer (mSET) policy dramatically reduces twinning rate without lowering pregnancy rates. Fertil Steril. 2011;96:1367–9. doi: 10.1016/j.fertnstert.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Forman M, Polanski V, Horvath P, Gilligan A, Rieger D. Reductions in volatile organic compounds, aldehydes, and particulate air contaminants in an IVF laboratory by centralized and stand-alone air filtration systems. Fertil Steril. 2004;82(Supplement 2):S324. doi: 10.1016/j.fertnstert.2004.07.877. [DOI] [Google Scholar]
- 29.Lawrence C. VOC levels in a new IVF laboratory with both central and in-laboratory photocatalytic air purification units. Alpha Scientists Reproduct Med. 2007;36:1–5. [Google Scholar]
- 30.Legro RS, Sauer MV, Mottla GL, Richter KS, Li X, Dodson WC, et al. Effect of air quality on assisted human reproduction. Hum Reprod. 2010;25:1317–24. doi: 10.1093/humrep/deq021. [DOI] [PubMed] [Google Scholar]
- 31.Perin PM, Maluf M, Czeresnia CE, Januario DA, Saldiva PH. Impact of short-term preconceptional exposure to particulate air pollution on treatment outcome in couples undergoing in vitro fertilization and embryo transfer (IVF/ET) J Assist Reprod Genet. 2010;27:371–82. doi: 10.1007/s10815-010-9419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perin PM, Maluf M, Czeresnia CE, Nicolosi Foltran Januario DA, Nascimento Saldiva PH. Effects of exposure to high levels of particulate air pollution during the follicular phase of the conception cycle on pregnancy outcome in couples undergoing in vitro fertilization and embryo transfer. Fertil Steril. 2010;93:301–3. doi: 10.1016/j.fertnstert.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 33.Worrilow KC, Huynh HT, Bower JB, Schillings W, Peters AJ. A retrospective analysis: seasonal decline in implantation rates (IR) and its correlation with increased levels of volatile organic compounds (VOC) Fertil Steril. 2002;78(Supplement 1):S39. doi: 10.1016/S0015-0282(02)03482-9. [DOI] [Google Scholar]
- 34.Destaillats H, Spaulding RS, Charles MJ. Ambient air measurement of acrolein and other carbonyls at the Oakland-San Francisco Bay Bridge toll plaza. Environ Sci Technol. 2002;36:2227–35. doi: 10.1021/es011394c. [DOI] [PubMed] [Google Scholar]
- 35.Hall J, Gilligan A, Schimmel T, Cecchi M, Cohen J. The origin, effects and control of air pollution in laboratories used for human embryo culture. Hum Reprod. 1998;13:146–55. doi: 10.1093/humrep/13.suppl_4.146. [DOI] [PubMed] [Google Scholar]
- 36.Karagouga G, Fredrickson JR, Morbeck DE. Interaction of air quality and culture environment: role of protein concentration and oil quality on effects of volatile organic compounds (VOCs) on embryo development. Fertil Steril. 2014;102:e212.
- 37.Wolff HS, Fredrickson JR, Walker DL, Morbeck DE. Advances in quality control: mouse embryo morphokinetics are sensitive markers of in vitro stress. Hum Reprod. 2013;28:1776–82. doi: 10.1093/humrep/det102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Worrilow KC, Huynh HT, Peters AJ. The innovative marriage between cleanroom and assisted reproductive technologies (ART)—the Design, Construction and National Environmental Balancing Bureau (NEBB) certification of a prototype class 100/Class 10 IVF laboratory cleanroom. Fertil Steril. 2000;74:S103. doi: 10.1016/S0015-0282(00)01004-9. [DOI] [Google Scholar]
- 39.Hill DL. Role of the in vitro fertilization laboratory in a negative pregnancy outcome. Fertil Steril. 2001;75:249–51. doi: 10.1016/S0015-0282(00)01713-1. [DOI] [PubMed] [Google Scholar]
- 40.Worrilow KC, Huynh HT, Gwozdziewicz JB, Schillings WA, Peters AJ. A retrospective analysis: the examination of a potential relationship between particulate (P) and volatile organic compound (VOC) levels in a class 100 IVF laboratory cleanroom (CR) and specific parameters of embryogenesis and rates of implantation (IR) Fertil Steril. 2001;76:S15–S6. doi: 10.1016/S0015-0282(01)02057-X. [DOI] [Google Scholar]
- 41.Worrilow KC, Huynh HT, Bower JB, Schillings WJ. Dissection of the high velocity air control (HVAC) system serving the in vitro fertilization (IVF) laboratory: the impact of ultraviolet-c band (UVC) irradiation on clinical pregnancy rates (CPR) Fertil Steril. 2007;88(Supplement 1):S89. doi: 10.1016/j.fertnstert.2007.07.294. [DOI] [Google Scholar]
- 42.Higdon HL, 3rd, Blackhurst DW, Boone WR. Incubator management in an assisted reproductive technology laboratory. Fertil Steril. 2008;89:703–10. doi: 10.1016/j.fertnstert.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 43.Morbeck DE, Fredrickson JR, Walker DL, Daftary GS. Culture in a benchtop incubator reduces in vitro stress in a sensitive mouse embryo QC assay: potential role of air quality. Fertil Steril. 2011;96:S249. doi: 10.1016/j.fertnstert.2011.07.957. [DOI] [Google Scholar]