Abstract
Purpose
We report on the results of the whole-genome analysis performed in a patient who developed severe ovarian hyperstimulation syndrome (OHSS) following gonadotropin-releasing hormone (GnRH) agonist triggering in a “freeze-all” protocol.
Methods
A 30-year-old patient with polycystic ovary syndrome who developed severe early-onset OHSS with clinical ascites, and slight renal and hepatic dysfunction was admitted for monitoring and treatment with cabergoline and intravenous albumin. Exome sequencing to assess for any known genetic predisposition for OHSS was performed.
Results
No known genetic variants associated with OHSS predisposition were found.
Conclusions
Case reports of severe OHSS following a “freeze-all” strategy are starting to arise, showing that OHSS has not been completely eliminated with this approach. Further studies should be conducted to confirm if such cases may be due to genetic predisposition or not.
Keywords: GnRH antagonist, Ovarian hyperstimulation syndrome, GnRH agonist triggering, Freeze-all strategy, OHSS-free clinic
Introduction
Ovarian hyperstimulation syndrome (OHSS) is an almost exclusive iatrogenic complication related to ovarian stimulation with gonadotropins in otherwise healthy women [1]. While its general incidence is approximately 2 to 3 % per cycle [2, 1], OHSS can occur in up to a third of all cases of high-risk patients [3, 4], notably those with a previous history of OHSS or polycystic ovary morphology (PCOM)/polycystic ovary syndrome (PCOS). In its severe form, this syndrome has the potential to cause serious morbidity or mortality, mainly due to the increased occurrence of ovarian torsion and thromboembolism [5].
OHSS seems to result from an excessive secretion of vasoactive substances during ovarian stimulation (OS), namely vascular endothelial growth factor (VEFG) and factors that derive from the renin–angiotensin system [6]. This over-secretion of vasoactive substances is almost entirely dependent on the activity of luteinizing-hormone (LH). In in vitro fertilization (IVF), final oocyte maturation and ovulation are typically triggered with exogenous human chorionic gonadotropin (hCG), which is structurally and functionally similar to LH. However, hCG has a substantially longer half-life (over 24 h versus approximately 60 min for LH) and its administration seems to play a key-role in the development of OHSS [7]. For this reason, many authors have proposed to replace hCG with a gonadotropin-releasing hormone (GnRH) agonist for triggering [4, 8–13]. In a GnRH antagonist co-treated cycle, the GnRH agonist causes the displacement of the GnRH antagonist from the pituitary receptors, resulting in a LH flare-up/“surge” that lasts for approximately 24–36 h [7]. This approach, combined with the elective cryopreservation of all oocytes/embryos, referred to by some as the “OHSS-free clinic” [14], had effectively abolished the incidence of severe early OHSS [14, 15, 10] until recently, when the first cases of OHSS requiring hospitalization were reported [16–19]. These cases include both patients with either grade 4 (3 cases) and grade 5 (4 patients) severe early-onset OHSS according to the OHSS classification proposed by Golan et al. [adopted by the European Society of Human Reproduction and Embryology (ESHRE)] [20]. However, it has been questioned if the first case report did indeed represent a bona fide case of severe OHSS [21] and if OHSS after a “freeze-all” protocol may not be limited to extremely rare genetically-prone women [19]. In fact, evidence of genetic predisposition for OHSS has been previously described, namely through reports of familial and isolated cases of severe OHSS in women with natural conceptions [22–24].
In this article we report on a case and the results of the whole-exome sequencing (WES) analysis performed in a patient who developed severe early-onset OHSS following GnRH agonist triggering with elective cryopreservation of all available viable embryos.
Materials and methods
Case report
A 30-year-old otherwise healthy patient with PCOS according to the revised [25] Rotterdam criteria (PCOM and oligomenorrhoea) visited our outpatient clinic due to primary infertility during the last 12 months. We performed preliminary diagnostic testing which revealed a high ovarian reserve (anti-Müllerian hormone serum level of 18.28 ng/mL, Immunotech kit), hyperandrogenemia (free testosterone 14.8 ng/L, testosterone 0.73 μg/L and androstenedione 4750 ng/L) and a total antral follicle count of 40. We also performed a semen analysis to her partner, which revealed the presence of severe oligoasthenoteratozoospermia (sperm cell concentration 2.15 × 106/mL, 21 % total progressive sperm of which 0 % had a normal morphology).
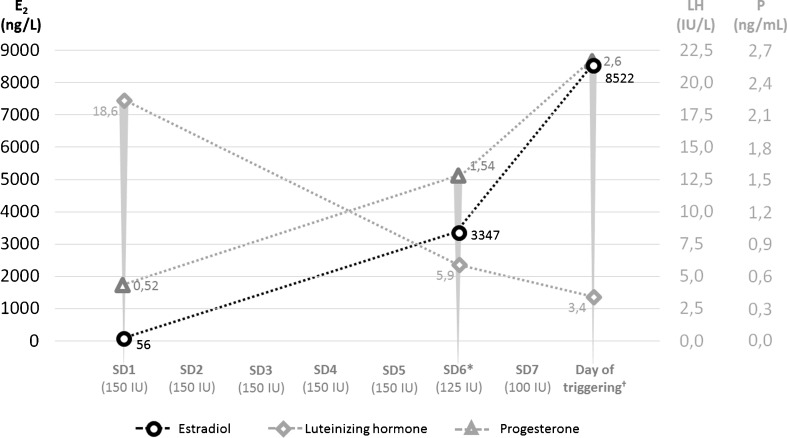
She consented for her first attempt of OS under GnRH antagonist co-treatment for IVF. The patient started OS with 150 IU of recombinant follicle-stimulating hormone (Puregon®, Merck Sharp & Dohme, New Jersey, USA) and daily ganirelix (Orgalutran®, Merck Sharp & Dohme, New Jersey, USA) was administered from day 6 of the stimulation onwards, as described in detail in Fig. 1. Due to the excessive ovarian response noted on stimulation day 6 (SD6), a “step-down” in the protocol was performed with the administration of 125 and 100 IU on SD7 and SD8, respectively. A single dose of 0.2 mg of triptorelin (Gonapeptyl®, Ferring Pharmaceuticals, Geneva, Switzerland) was administered the following day for final oocyte maturation and ovulation triggering. At that time, the patient had over 70 follicles during pelvic ultrasound examination, 3 of which were ≥ 17 mm and 25 between 11 and 16 mm.
Fig. 1.
Endocrine profile of the patient during stimulation. The x axis represents the daily dose of recombinant FSH. *First day of daily GnRH antagonist co-treatment with ganirelix; †with 0.2 mg of Triptorelin; E2, estradiol; LH, luteinizing hormone; P, progesterone; SD, stimulation day; FSH, folliclestimulating hormone; GnRH, Gonadotropin-releasing hormone; IU, international units
Thirty-six hours after triggering, a transvaginal ultrasound-guided oocyte retrieval of 59 cumulus-oophorus-complexes was performed, 29 of which were metaphase II. At that time, clinical ascites was already evident by a positive “fluid wave” test on abdominal examination and the patient complained of nausea with abdominal pain/distention and had a diminished urinary output (0.48 mL/Kg/h). The serum assessment revealed a normal hematocrit (36.9 %), normal prothrombin time (98 %) and a slight decrease in creatinine clearance (34 mL/min). According to the OHSS classification proposed by Golan et al., this patient developed early-onset severe grade 4 OHSS [20].
Intracytoplasmic sperm injection (ICSI) was performed on all mature oocytes and 13 day-5 blastocysts of good morphological quality were eventually cryopreserved. No fresh embryo transfer or exogenous luteal support was ever performed and the patient was hospitalized for observation after being prescribed a daily dose of 0.5 mg cabergoline (Dostinex®, Pfizer Inc, New York, USA) for 8 consecutive days. On the second day of hospitalization, although her urinary output improved (0.64 mL/Kg/h), her clinical ascites worsened (objectively, an abdominal circumference increase of 5 cm and weight gain of 1 Kg) and hypoalbuminemia (2.8 mg/L) was also now evident. We administered intravenous albumin on the third day of hospitalization and the patient was discharged on the following day since she was substantially less symptomatic and her condition had clinically and analytically improved. Progression to grade 5 severe OHSS fortunately did not occur as her hematocrit remained stable and her urinary output progressively improved with time (on the 4th day of hospitalization, she had hematocrit and urinary output of 35.6 % and 1.02 mL/Kg/h, respectively).
In a subsequent cycle one embryo was thawed and transferred. The patient became pregnant and a healthy baby was delivered vaginally at term.
In order to gain insight into a potential genetic background of the patient for OHSS, whole exome sequencing was performed on a HiSeq 1500 (Illumina, San Diego, California, USA), using the NEBNext Ultra DNA Library Prep Kit (New England Biolabs, Ipswich, Massachusetts, USA) and SeqCap EZ Human Exome v3.0 (Roche Applied Science, Penzberg, Germany) target enrichment according to the manufacturer’s instructions. Variants were assessed through Alamut HT® (Interactive Biosoftware). This evaluation was performed upon signed informed consent of the patient.
In a first step, we focused on genes known to potentially influence ovarian stimulation or to be involved in OHSS: FSHR, LHR/LHCGR, LHB, CYP11A1, CYP19A1, ESR1, ESR2, PGR, VEGFR1/VEGFR2, VEGF, AMH, AMHR, GDF9, BMP15, SOD2, SHBG, MTHFR, FOLR1, P53, PAI and TNF [26–29]. The patient was a (heterozygous) carrier of the c.2039G > A, p.Ser680Asn and c.919G > A, p.Ala307Thr polymorphisms in the FSHR gene [30, 31] and of the c.665C > T, p.Ala222Val mutation in the MTHFR gene [32]. These and the other detected alterations, however, were present in a high frequency in the general population and are unlikely to be causal for OHSS (Table 1). Consequently, the data of the exome analysis of the patient were re-assessed for the presence of relevant mutations in the complete exome sequence. Rare variants [with a less than 1 % average heterozygosity in the population, according to the Average Heterozygosity Database of Single Nucleotide Polymorphisms – dbSNP [33]] that are presumably pathogenic in silico were selected and a recessive inheritance pattern was assumed. To this extent, the prediction software SIFT® [34] and PolyPhen2® [35] were used, which use both validated in silico algorithms to predict the possible impact of an amino acid change on a protein’s function. The SIFT® algorithm is based on the degree of conservation of an amino acid residue in sequence alignments derived from closely related sequences, while PolyPhen2® compares amino acid sequences, phylogenetic and structural information to predict the impact of a substitution on the structure and function of the protein. It has to be emphasized that although the data from these in silico models are a computational simulation for the best, they might represent an important overestimation in their conclusions in one direction or the other. No relevant candidate gene was identified as having any potential role in the development of OHSS in our patient.
Table 1.
Exonic variants found in the patient which could potentially influence ovarian stimulation
| Gene | RefSeq | Variant found* | Nt change | AA change | Patient status | MAF† (%) | SIFT prediction | PolyPhen prediction |
|---|---|---|---|---|---|---|---|---|
| LHCGR | NM_00023.3 | rs2293275 | c.935A > G | p.Asn312Ser | Homozygous | 36,5 | Tolerated | Benign |
| FSHR | NM_000145.3 | rs6166 | c.2039G > A | p.Ser680Asn | Carrier | 40,2 | Tolerated | Benign |
| rs6165 | c.919G > A | p.Ala307Thr | Carrier | 48,6 | Tolerated | Benign | ||
| CYP19A1 | NM_031226.2 | rs700518 | c.240A > G | p.= | Homozygous | 36,8 | NR | NR |
| ESR1 | NM_000125.3 | rs2077647 | c.30 T > C | p.= | Carrier | 43,2 | NR | NR |
| PGR | NM_000926.4 | rs500760 | c.2658A > G | p.= | Carrier | 29,1 | NR | NR |
| AMH | NM_000479.3 | rs10407022 | c.146G > T | p.Ser49Ile | Homozygous | 32,7‡ | Deleterious | Possibly damaging |
| MTHFR | NM_005957.4 | rs4846051 | c.1305C > T | p.= | Homozygous | 7,4 | NR | NR |
| rs1801131 | c.1286A > C | p.Glu429Ala | Carrier | 22,8 | Tolerated | Benign | ||
| rs1801133 | c.665C > T | p.Ala222Val | Carrier | 32,5 | Deleterious | Probably damaging |
Although variants rs10407022 and rs1801133 were predicted as potentially deleterious/damaging, these variants are present in at least one third of the general population, making them unlikely candidate variations to justify the occurrence of severe OHSS
*no variants were found for the following genes: AMHR2, LHB, CYP11A1, ESR2, VEGFR1/VEGFR2/VEGF, SOD2, SHBG, GDF9, BMP15, FOLR1, P53, PAI and TNF
†Minor Allele Frequency, assessed on September 26th, 2014
‡In Europe, 75 % of the population is homozygous TT (30), making
Discussion
The widespread use of GnRH antagonist co-treatment for primary prevention of OHSS has been, until now, the single most effective method in preventing OHSS, halving the risk of severe OHSS without hindering clinical outcomes [36]. However, OHSS still occurs in current clinical practice [14] and its overall importance increases with the steady worldwide proliferation of IVF. GnRH agonist ovulation triggering has contributed to the further reduction of severe OHSS [37, 10, 15, 14] and, in conjunction with elective cryopreservation of all viable embryos, is considered to be the safest way to avoid OHSS after OS for IVF [38, 12, 14]. In view of this, elective cryopreservation of embryos has been proposed for women undergoing IVF/ICSI who develop ≥18 follicles with a diameter of at least 10–14 mm [39] to minimize the risk of the development of severe OHSS. OHSS in a GnRH agonist triggered “freeze-all” has been, until now, such a unique event that some have considered it to be likely limited to women genetically prone to develop this complication [19].
Since 2014, an increasing amount of clinicians have reported on cases of severe (grade 4 or 5) early-onset OHSS following a “freeze-all” protocol. These cases have come from all over the globe, including India, the United Arab Emirates [19], Singapore [17], Turkey [18], and now Belgium. In all instances, the patients were administered total doses of exogenous FSH within the normal ranges that one could expect for ovarian stimulation during a conventional IVF treatment (between 775 and 2025 IU). Hence, the occurrence of a uniquely extreme ovarian response did not seem to be related with any unwarranted excessive stimulation and was, for this reason, otherwise unexpected. To this extent, Ling et al. [17] postulated if extreme AMH levels could serve as a warning sign for this sort of complication, since they were the first to report that, in their case, the patient had an exceptionally high AMH level of 64.5 ng/mL (Beckman DSL, AMH Gen II assay kit). Our patient also had an abnormally high AMH of 18.28 ng/mL, once more raising one to question if AMH might serve as a means to flag women who are at high risk of developing severe OHSS even after a freeze-all protocol.
The strength of our case report is that it was the first to perform genome-wide analysis to test this hypothesis. Whole exome sequencing technologies allow us to investigate all previously reported variants involved in OHSS in a single approach. Moreover, the analysis is not restricted to these specific sequences/genes as the complete human exome was interrogated. However, this genetic analysis through whole exome sequencing did not allow us to identify any definitive genetic predisposition for OHSS in our patient. A rare mutation in a gene with an unassigned function can, however, not completely be ruled out. Performing more genetic analyses in “extreme” severe OHSS cases similar to these could allow us to further understand the pathologic pathway of OHSS and if specific women may have a genetic predisposition to develop it.
In conclusion, IVF/ICSI using GnRH antagonist treatment followed by GnRH agonist triggering and oocyte/embryo cryopreservation is efficacious and safe. Nevertheless, severe early OHSS cannot be eliminated completely in current clinical practice by this strategy alone and a genetic predisposition is yet to be confirmed.
Acknowledgments
Conflict of interest
The authors have nothing to disclose
Funding
This case report was financially supported by the Willy Gepts Fund of the Universitair Ziekenhuis Brussel
Footnotes
Capsule Whole-genome sequencing performed in a patient who developed early-onset severe OHSS following a freeze-all strategy with GnRH agonist triggering did not reveal any known genetic predisposition for this ART-related complication.
References
- 1.Papanikolaou EG, Pozzobon C, Kolibianakis EM, Camus M, Tournaye H, Fatemi HM, et al. Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril. 2006;85(1):112–20. doi: 10.1016/j.fertnstert.2005.07.1292. [DOI] [PubMed] [Google Scholar]
- 2.Mathur RS, Akande AV, Keay SD, Hunt LP, Jenkins JM. Distinction between early and late ovarian hyperstimulation syndrome. Fertil Steril. 2000;73(5):901–7. doi: 10.1016/S0015-0282(00)00492-1. [DOI] [PubMed] [Google Scholar]
- 3.Engmann L, DiLuigi A, Schmidt D, Nulsen J, Maier D, Benadiva C. The use of gonadotropin-releasing hormone (GnRH) agonist to induce oocyte maturation after cotreatment with GnRH antagonist in high-risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomized controlled study. Fertil Steril. 2008;89(1):84–91. doi: 10.1016/j.fertnstert.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Acevedo B, Gomez-Palomares JL, Ricciarelli E, Hernandez ER. Triggering ovulation with gonadotropin-releasing hormone agonists does not compromise embryo implantation rates. Fertil Steril. 2006;86(6):1682–7. doi: 10.1016/j.fertnstert.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 5.Delvigne A, Rozenberg S. Review of clinical course and treatment of ovarian hyperstimulation syndrome (OHSS) Hum Reprod Update. 2003;9(1):77–96. doi: 10.1093/humupd/dmg005. [DOI] [PubMed] [Google Scholar]
- 6.Vloeberghs V, Peeraer K, Pexsters A, D’Hooghe T. Ovarian hyperstimulation syndrome and complications of ART. Best Pract Res Clin Obstet Gynaecol. 2009;23(5):691–709. doi: 10.1016/j.bpobgyn.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Humaidan P, Kol S, Papanikolaou EG. GnRH agonist for triggering of final oocyte maturation: time for a change of practice? Hum Reprod Update. 2011;17(4):510–24. doi: 10.1093/humupd/dmr008. [DOI] [PubMed] [Google Scholar]
- 8.Engmann L, Benadiva C. Agonist trigger: what is the best approach? Agonist trigger with aggressive luteal support. Fertil Steril. 2012;97(3):531–3. doi: 10.1016/j.fertnstert.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Velasco JA. Agonist trigger: what is the best approach? Agonist trigger with vitrification of oocytes or embryos. Fertil Steril. 2012;97(3):527–8. doi: 10.1016/j.fertnstert.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Griesinger G, Diedrich K, Devroey P, Kolibianakis EM. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: a systematic review and meta-analysis. Hum Reprod Update. 2006;12(2):159–68. doi: 10.1093/humupd/dmi045. [DOI] [PubMed] [Google Scholar]
- 11.Herrero L, Pareja S, Losada C, Cobo AC, Pellicer A, Garcia-Velasco JA. Avoiding the use of human chorionic gonadotropin combined with oocyte vitrification and GnRH agonist triggering versus coasting: a new strategy to avoid ovarian hyperstimulation syndrome. Fertil Steril. 2011;95(3):1137–40. doi: 10.1016/j.fertnstert.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 12.Iliodromiti S, Blockeel C, Tremellen KP, Fleming R, Tournaye H, Humaidan P, et al. Consistent high clinical pregnancy rates and low ovarian hyperstimulation syndrome rates in high-risk patients after GnRH agonist triggering and modified luteal support: a retrospective multicentre study. Hum Reprod. 2013;28(9):2529–36. doi: 10.1093/humrep/det304. [DOI] [PubMed] [Google Scholar]
- 13.Melo M, Busso CE, Bellver J, Alama P, Garrido N, Meseguer M, et al. GnRH agonist versus recombinant HCG in an oocyte donation programme: a randomized, prospective, controlled, assessor-blind study. Reprod Biomed Online. 2009;19(4):486–92. doi: 10.1016/j.rbmo.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Devroey P, Polyzos NP, Blockeel C. An OHSS-free clinic by segmentation of IVF treatment. Hum Reprod. 2011;26(10):2593–7. doi: 10.1093/humrep/der251. [DOI] [PubMed] [Google Scholar]
- 15.Bodri D, Guillén JJ, Trullenque M, Schwenn K, Esteve C, Coll O. Early ovarian hyperstimulation syndrome is completely prevented by gonadotropin releasing-hormone agonist triggering in high-risk oocyte donor cycles: a prospective, luteal-phase follow-up study. Fertil Steril. 2010;93(7):2418–20. doi: 10.1016/j.fertnstert.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 16.Griesinger G, Schultz L, Bauer T, Broessner A, Frambach T, Kissler S. Ovarian hyperstimulation syndrome prevention by gonadotropin-releasing hormone agonist triggering of final oocyte maturation in a gonadotropin-releasing hormone antagonist protocol in combination with a “freeze-all” strategy: a prospective multicentric study. Fertil Steril. 2011;95(6):2029–33. doi:10.1016/j.fertnstert.2011.01.163. [DOI] [PubMed]
- 17.Ling LP, Phoon JW, Lau MS, Chan JK, Viardot-Foucault V, Tan TY, et al. GnRH agonist trigger and ovarian hyperstimulation syndrome: relook at ‘freeze-all strategy’. Reprod Biomed Online. 2014;29(3):392–4. doi: 10.1016/j.rbmo.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Gurbuz AS, Gode F, Ozcimen N, Isik AZ. Gonadotrophin-releasing hormone agonist trigger and freeze-all strategy does not prevent severe ovarian hyperstimulation syndrome: a report of three cases. Reprod Biomed Online. 2014 doi: 10.1016/j.rbmo.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Fatemi HM, Popovic-Todorovic B, Humaidan P, Kol S, Banker M, Devroey P, et al. Severe ovarian hyperstimulation syndrome after gonadotropin-releasing hormone (GnRH) agonist trigger and “freeze-all” approach in GnRH antagonist protocol. Fertil Steril. 2014;101(4):1008–11. doi: 10.1016/j.fertnstert.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv. 1989;44(6):430–40. doi: 10.1097/00006254-198906000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Kol S. A case of severe early-onset OHSS after GnRH-agonist triggering. Fertil Steril. 2011;96(3):e151. doi: 10.1016/j.fertnstert.2011.06.057. [DOI] [PubMed] [Google Scholar]
- 22.Abu-Louz SK, Ahmed AA, Swan RW. Spontaneous ovarian hyperstimulation syndrome with pregnancy. Am J Obstet Gynecol. 1997;177(2):476–7. doi: 10.1016/S0002-9378(97)70225-4. [DOI] [PubMed] [Google Scholar]
- 23.Ayhan A, Tuncer ZS, Aksu AT. Ovarian hyperstimulation syndrome associated with spontaneous pregnancy. Hum Reprod. 1996;11(8):1600–1. doi: 10.1093/oxfordjournals.humrep.a019452. [DOI] [PubMed] [Google Scholar]
- 24.Di Carlo C, Bruno P, Cirillo D, Morgera R, Pellicano M, Nappi C. Increased concentrations of renin, aldosterone and Ca125 in a case of spontaneous, recurrent, familial, severe ovarian hyperstimulation syndrome. Hum Reprod. 1997;12(10):2115–7. doi: 10.1093/humrep/12.10.2115. [DOI] [PubMed] [Google Scholar]
- 25.group E-A-sPcw Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19(1):41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 26.Altmae S, Hovatta O, Stavreus-Evers A, Salumets A. Genetic predictors of controlled ovarian hyperstimulation: where do we stand today? Hum Reprod Update. 2011;17(6):813–28. doi: 10.1093/humupd/dmr034. [DOI] [PubMed] [Google Scholar]
- 27.Moron FJ, de Castro F, Royo JL, Montoro L, Mira E, Saez ME, et al. Bone morphogenetic protein 15 (BMP15) alleles predict over-response to recombinant follicle stimulation hormone and iatrogenic ovarian hyperstimulation syndrome (OHSS) Pharmacogenet Genomics. 2006;16(7):485–95. doi: 10.1097/01.fpc.0000215073.44589.96. [DOI] [PubMed] [Google Scholar]
- 28.Rizk B. Symposium: Update on prediction and management of OHSS. Genetics of ovarian hyperstimulation syndrome. Reprod Biomed Online. 2009;19(1):14–27. doi: 10.1016/S1472-6483(10)60041-7. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien TJ, Kalmin MM, Harralson AF, Clark AM, Gindoff I, Simmens SJ, et al. Association between the luteinizing hormone/chorionic gonadotropin receptor (LHCGR) rs4073366 polymorphism and ovarian hyperstimulation syndrome during controlled ovarian hyperstimulation. Reprod Biol Endocrinol RB&E. 2013;11(1):71. doi: 10.1186/1477-7827-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Follicle-stimulating hormone receptor polymorphism (Thr307Ala) is associated with variable ovarian response and ovarian hyperstimulation syndrome in Indian women. Fertil Steril. 2009;91(2):432–9. doi: 10.1016/j.fertnstert.2007.11.093. [DOI] [PubMed] [Google Scholar]
- 31.Daelemans C, Smits G, de Maertelaer V, Costagliola S, Englert Y, Vassart G, et al. Prediction of severity of symptoms in iatrogenic ovarian hyperstimulation syndrome by follicle-stimulating hormone receptor Ser680Asn polymorphism. J Clin Endocrinol Metab. 2004;89(12):6310–5. doi: 10.1210/jc.2004-1044. [DOI] [PubMed] [Google Scholar]
- 32.Dulitzky M, Cohen SB, Inbal A, Seidman DS, Soriano D, Lidor A, et al. Increased prevalence of thrombophilia among women with severe ovarian hyperstimulation syndrome. Fertil Steril. 2002;77(3):463–7. doi: 10.1016/S0015-0282(01)03218-6. [DOI] [PubMed] [Google Scholar]
- 33.Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29(1):308–11. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31(13):3812–4. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Inany HG, Youssef MA, Aboulghar M, Broekmans F, Sterrenburg M, Smit J, et al. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2011;5:CD001750. doi: 10.1002/14651858.CD001750.pub3. [DOI] [PubMed] [Google Scholar]
- 37.Humaidan P. Agonist trigger: what is the best approach? Agonist trigger and low dose hCG. Fertil Steril. 2012;97(3):529–30. doi: 10.1016/j.fertnstert.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Griesinger G, von Otte S, Schroer A, Ludwig AK, Diedrich K, Al-Hasani S, et al. Elective cryopreservation of all pronuclear oocytes after GnRH agonist triggering of final oocyte maturation in patients at risk of developing OHSS: a prospective, observational proof-of-concept study. Hum Reprod. 2007;22(5):1348–52. doi: 10.1093/humrep/dem006. [DOI] [PubMed] [Google Scholar]
- 39.Seyhan A, Ata B, Polat M, Son WY, Yarali H, Dahan MH. Severe early ovarian hyperstimulation syndrome following GnRH agonist trigger with the addition of 1500 IU hCG. Hum Reprod. 2013;28(9):2522–8. doi: 10.1093/humrep/det124. [DOI] [PubMed] [Google Scholar]