Abstract
Purpose
The ability to accurately estimate a woman’s ovarian reserve by non-invasive means is the goal of ovarian reserve prediction. It is not known whether a correlation exists between model-predicted estimates of ovarian reserve and data generated by direct histological analysis of ovarian tissue. The aim of this study was to compare mean non-growing follicle density values obtained from analysis of ovarian cortical tissue samples against ovarian volume models.
Methods
Non-growing follicle density values were obtained from 13 ovarian cortical biopsies (16-37 years). A mean non-growing follicle density was calculated for each patient by counting all follicles in a given volume of biopsied ovarian cortex. These values were compared to age-matched model generated densities (adjusted to take into consideration the proportion of ovary that is cortex) and the correlation between data sets tested.
Results
Non-growing density values obtained from fresh biopsied ovarian cortical samples closely matched model generated data with low mean difference, tight agreement limits and no proportional error between the observed and predicted results.
Conclusion
These findings validate the use of the adjusted population and ovarian volume models, to accurately predict mean follicle density in the ovarian cortex of healthy adult women.
Keywords: Ovary, Modelling, Ageing, Follicles
Introduction
The human ovary contains a population of non-growing follicles (NGFs). This population declines after an average peak of 300,000 NGFs at 18–22 weeks gestation to fewer than 1000 NGFs at menopause [1, 2], with the decrease largely due to atresia following follicular recruitment towards maturation. Follicle density is an important parameter in studies that compare normal and polycystic ovaries [3, 4], and in studies involving fertility preservation for women at risk of ovarian insufficiency due to surgery and/or aggressive radio- and chemotherapies [5–12], where subsequent low follicle density limits the success of in vivo and in vitro approaches to fertility restoration. In this study, mean follicle density (MFD) is taken to be the average number of NGFs in each cubic millimetre of the cortex of the ovary. This is the standard definition of MFD [4], although there are studies that define MFD as NGFs per quarter cubic millimetre [13], NGFs per gram [14], and primary or preantral follicles per cubic millimetre [6]. Cryopreservation techniques have been compared by calculating MFD before and after different freezing methods [6]; the cytotoxicity of drugs has been assessed by comparing MFD before and after in vitro exposure [15].
Deriving accurate predictions of follicle numbers from indirect methods is complicated by the uneven distribution of follicles within the ovary. Although indirect biochemical and ultrasound tests correlate with individual MFD [16], the gold standard method of determination is to directly examine the ovarian tissue sample [17]. Histological examination of ovarian cortical samples allows accurate calculation of individual MFDs and therefore follicle reserve at any age as >90 % of the NGF population is located in this region [14]. Direct examination however is an expensive and inherently destructive technique, requiring surgical procedures, tissue storage and the preparation and examination of histological samples. Indirect ex vivo methods have not been shown to be accurate to more than qualitative levels (low, medium or high density), and can also be destructive, with exposure to shortwave light from the use of fluorescent probes possibly damaging ovarian cells [18], and the use of compounds such as dimethylsulphoxide, ethanol and methanol to help the uptake of biochemical markers can also decrease NGF numbers during in situ assessment [19].
Currently there are no models for MFD calculation available: previous analyses have however separately derived the total NGF population of the ovary and ovarian volume. We have therefore combined and modified these to present a simple age-related model for MFD of the normal human ovarian cortex, needing only age as an input. We show that the predictive model provides a good approximation to MFDs calculated by direct histological examination of ovarian tissue from healthy women free from ovarian pathology and not using hormonal contraception, and hence that the model can be used as safe and inexpensive proxy for MFDs for ages from mid-teens to late thirties. The model will facilitate analysis of MFD in states of disease and following interventions, e.g., in relation to cytotoxic and other chemical exposures, as the pre-exposure MFD can be reliably estimated using the model with no tissue or examination needed until the post-exposure stage.
Materials and methods
The predictive model
The predictive model was derived by combining the predictions of two models in the published literature (i) an age-related normative model for the NGF population in the human ovary [1], and (ii) an age-related normative model for the volume of the human ovary [20]. For each age we set P (Age) and V (Age) as the predicted NGF population and the predicted volume respectively. The predicted mean follicle density (MFD) for an entire ovary at that age is then P (Age) divided by V (Age).
To estimate the proportion of the volume of a typical healthy ovary that consists of cortical tissue, we use the standard formula for the volume of a prolate ellipsoid: V0 = 4/3π × a × b × c where a, b and c are the length, breadth and depth values, with the estimate that b = c = 3/5 × a approximates the usual relationship between the three values for the human ovary.
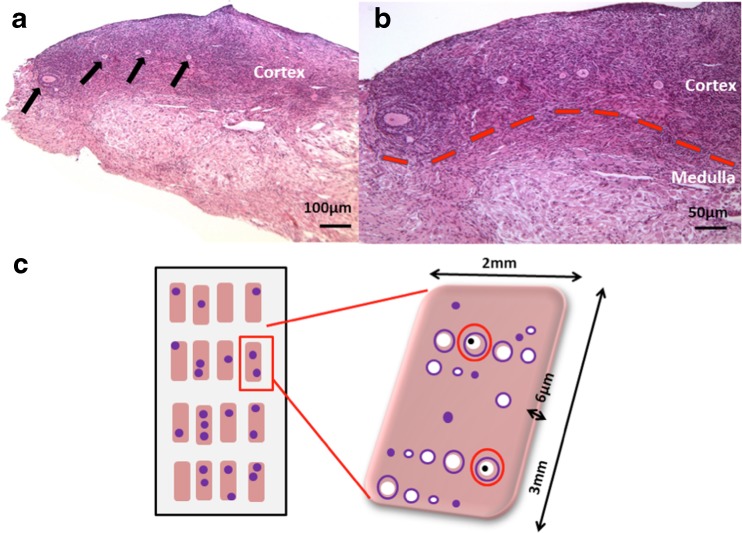
The interfaces between the epithelial, cortical and medullary regions of the ovary can be indistinct (Fig. 1a and b) however by analysis of the pixels in two-dimensional histological images of human ovarian tissue, we estimate that 3 % of the tissue area consists of the epithelial and connective layers, 10 % consists of cortex, and the remaining 87 % is the medulla. To convert these values into three dimensions, we set V1 = 4/3π × 0.97 × a × 3/5a × 3/5a and V2 = 4/3π × 0.87 × a × 3/5a × 3/5a. The volume of the cortex is then V1 – V2. We relate these values to chronological age by taking V0 to be Vol(age) – i.e., the predicted age-related volume – and re-arranging the first formula to give a as the cube root of 75 × Vol(age) divided by 36π. Substituting this value into V1 and V2 gives 23.8 % as the three-dimensional proportion of the ovarian volume that consists of cortical tissue, and hence the predicted MFD for a given chronological age is P(age) divided by 23.8 % of V(Age).
Fig. 1.
a. Photomicrograph of ovarian tissue from a woman aged 30 years. Growing follicles are visible in the upper cortical region (indicated by arrows). b. Follicles are present in the cortex but not in the medulla; cortical medullary interface indicated by dotted line. c. Diagrammatic representation of tissue analysis methodology. All tissue sections were examined for the presence of follicles; only sections of follicles containing the oocyte nucleolus (black dot) were analysed (red circles)
Ovarian cortical biopsies
Ovarian cortical biopsies were obtained from 13 patients with informed consent and Ethical Committee approval (Table 1). All adult biopsies (n = 12, mean age 28.7 ± 1.4 SEM, range 22–37 years) were taken from healthy women at the time of caesarean section. The 16 year-old patient had a biopsy collected during laparoscopic ovarian cortical harvesting for fertility preservation purposes. All patients were healthy with no ovarian pathology and none were using hormonal contraception. Tissue was transferred to the laboratory in holding medium (Leibovitz medium, Life Technologies, Paisley, UK supplemented with 2 mM sodium pyruvate, 2 mM glutamine 3 mg/ml human serum albumin, 75 μg/ml penicillin G and 50 μg/ml streptomycin; all chemicals from Sigma Chemicals, Poole, UK), removed to fresh pre-warmed holding medium and examined under light microscopy. Any damaged or haemorrhagic areas were removed and the tissue was divided into fine fragments using a scalpel and fixed in neutral buffered saline for 48 h. Tissue was processed and prepared microscopic evaluation as previously described (McLaughlin et al., 2014).
Table 2.
Predicted MFD for the age range 16–37 years
| Age (years) | NGFs | Ovarian Vol. (mm3) | Cortical MFD |
|---|---|---|---|
| 16 | 147,912 | 6358 | 98 |
| 17 | 137,487 | 6896 | 84 |
| 18 | 127,238 | 7308 | 73 |
| 19 | 117,221 | 7576 | 65 |
| 20 | 107,493 | 7700 | 59 |
| 21 | 98,106 | 7695 | 54 |
| 22 | 89,105 | 7584 | 49 |
| 23 | 80,532 | 7399 | 46 |
| 24 | 72,419 | 7169 | 42 |
| 25 | 64,794 | 6924 | 39 |
| 26 | 57,676 | 6684 | 36 |
| 27 | 51,075 | 6467 | 33 |
| 28 | 44,997 | 6283 | 30 |
| 29 | 39,438 | 6137 | 27 |
| 30 | 34,387 | 6029 | 24 |
| 31 | 29,831 | 5956 | 21 |
| 32 | 25,747 | 5911 | 18 |
| 33 | 22,112 | 5887 | 16 |
| 34 | 18,897 | 5873 | 14 |
| 35 | 16,073 | 5856 | 12 |
| 36 | 13,607 | 5825 | 10 |
| 37 | 11,468 | 5770 | 8 |
Assessment of mean follicle density in ovarian biopsies
Each biopsy obtained measured between 2.5 and 8 mm3. Random samples measuring 1.2–2.2 mm in total were taken for histological analysis and each histological section of every tissue fragment was examined under light microscopy. Follicles were categorised according to their stage of development as previously described [21, 22]. To avoid over-counting, only follicle sections containing the nucleolus were assessed (Fig. 1c). The volume of tissue analysed per patient was calculated as described previously [23, 24]. Briefly, tissue volume was calculated as the sum of the area in mm2 of all tissue sections analyzed per patient, multiplied by 0.006 mm (the thickness of each section) to give a value in mm3. Mean follicle density was determined by dividing the total number of follicles per patient by the volume of tissue analyzed and expressing this value as follicles per cubic millimeter [23].
Comparative published data on mean follicle densities
Observed MFDs were taken from Table 2 of Schmidt et al., 2003 [25] (n = 15, age range 13–38 years), excluding subjects who had had previous chemotherapy. These MFD values were obtained by counting follicles in every second slide, with correction factors used to account for missing follicles and follicles counted more than once. The cortical volume was estimated from two-dimensional values taken from every fourth section.
Table 1.
Subject data for observed MFDs (n = 13)
| Age (years) | Procedure | Tissue analysed (mm3) | No. NGFs counted | Observed MFD NGF/mm3 | Predicted MFD NGF/mm3 |
|---|---|---|---|---|---|
| 16 | Laparoscopy for fertility preservation | 2.1 | 189 | 90.0 | 98 |
| 22 | ECS | 1.17 | 95 | 81.8 | 49 |
| 23 | ECS | 1.63 | 81 | 49.7 | 46 |
| 23 | ECS | 2.23 | 62 | 27.8 | 46 |
| 25 | ECS | 1.70 | 61 | 35.9 | 39 |
| 27 | ECS | 1.70 | 57 | 33.5 | 33 |
| 29 | ECS | 1.70 | 41 | 24.0 | 27 |
| 30 | ECS | 1.68 | 33 | 19.7 | 24 |
| 30 | ECS | 1.55 | 46 | 29.6 | 24 |
| 32 | ECS | 1.62 | 40 | 24.7 | 18 |
| 33 | ECS | 1.75 | 44 | 25.1 | 16 |
| 33 | ECS | 1.52 | 42 | 27.7 | 16 |
| 37 | ECS | 1.64 | 16 | 9.8 | 8 |
ECS denotes elective caesarean section
Statistical analysis
The a priori criteria for external validation of the model by the observed data, both derived for this study and previously published [25], were (i) at least 80 % positive correlation between observed and predicted values, (ii) low average difference (5 follicles or fewer) between observed and predicted values across the entire age range, (iii) tight limits of agreement (a standard deviation of 15 follicles or fewer), and (iv) low proportional error (i.e., means and differences of observed and predicted values do not have a coefficient of determination r2 greater than 10 %).
Correlation between observed MFDs and predicted MFDs was measured by calculating the Pearson product-moment correlation coefficient. The mean difference between observed and predicted values, and limits of agreement at one and two standard deviations from mean were derived from Bland-Altman analysis [26, 27]. Proportional error was calculated as the correlation between the means and differences derived from the Bland-Altman plot, with absence of correlation indicating low proportional error. All analyses were performed using R version 2.15.3.
Results
Predicted NGF populations, ovarian volumes, and cortical MFD for ages 16 through 37 are given in Table 2. Predicted cortical MFD is obtained by dividing predicted NGFs by 23.8 % of the predicted ovarian volume.
Own data
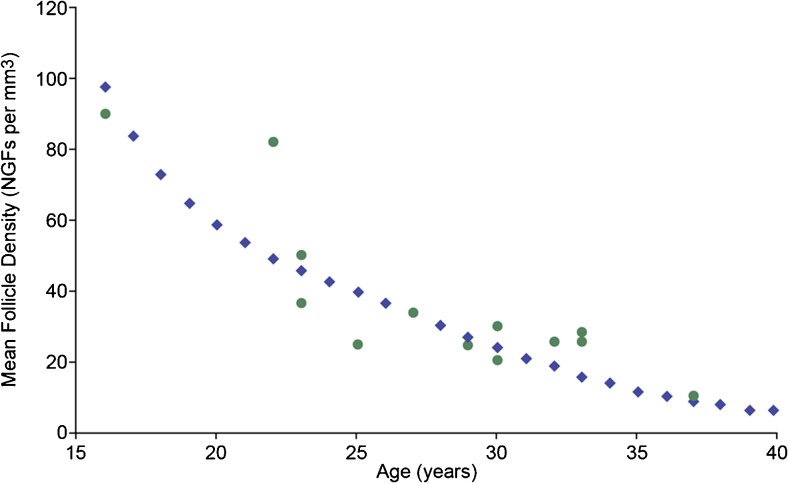
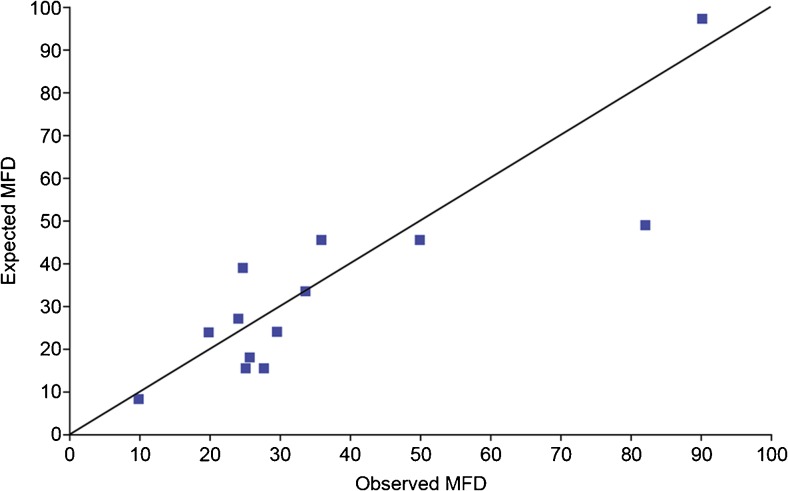
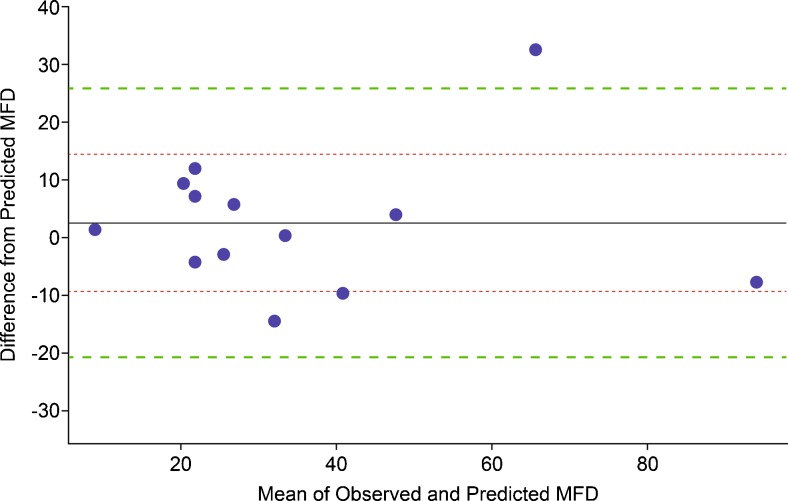
Visual comparison of the observed and expected MFDs for the age range 16–37 years shows good agreement (Fig. 2), as does the plot of the observed and predicted MFD values compared to the line denoting an exact match between observed and predicted MFD (Fig. 3). The Pearson product-moment correlation coefficient is 0.87 (95 % CI 0.62–0.96, p = 0.001). Bland-Altman analysis shows no proportional error when using predicted MFD values to estimate observed MFD values, since the coefficient of determination is close to zero, with r2 = 0.003 (95 % CI 0.000–0.038) for means of observed and predicted values against differences between observed and predicted values (Fig. 4). The mean difference between observed and predicted MFD values is 2.6 NGFs, with 68 % of new values expected to lie within one standard deviation (i.e., 11.9 NGFs) of this mean, and 95 % of new values expected to lie within 1.96 standard deviations (i.e., 23.3 NGFs) (Fig. 4, dashed lines).
Fig. 2.
Observed and predicted MFD – predicted values are shown for ages 15 through 40 years in blue. Observed values (n = 13) are shown as green dots
Fig. 3.
Observed vs predicted MFD - the line of identity represents the idealised confluence of observed and predicted values. Blue squares indicate observed against predicted MFD values (n = 13). The Pearson product-moment correlation coefficient for observed against predicted MFDs is 0.87 (95 % CI 0.62–0.96, p = 0.001)
Fig. 4.
Bland-Altman Plot – the x –axis represents means (i.e., half the sum of observed and predicted MFD values); the y – axis represents the differences (i.e., predicted MFD values subtracted form observed values). The solid horizontal line is the mean difference (2.6 NGFs) for our data (n = 13) which have coefficient of determination r2 = 0.003 (95 % CI 0.000–0.038), showing no trend between means and differences. The inner dotted horizontal lines are at one standard deviation (11.9 NGFs) from the mean difference; the outer dotted lines are at 1.96 standard deviations (23.3 NGFs). About 68 % of observations will have a difference in MFD between the inner limits of agreement; about 95 % of observations will have a difference in MFD between the outer limits of agreement
This combination of high correlation, low mean difference, tight limits of agreement and lack of proportional error satisfies the criteria for external validation of the predictive model by the observations.
Published data
Identical analyses were performed using 15 age-related MFD values [25]. Two of the four criteria for external validation were satisfied: the Pearson product-moment correlation coefficient for these data is 0.90 (95 % CI 0.73–0.97, p < 0.001), and the mean difference between observed and predicted MFD values is 3.3 NGFs.
Proportional error is small - r2 = 0.012 (95 % CI 0.0003–0.273) - but is slightly over the criterion value of 10 %. Limits of agreement at one standard deviation are 20.8 NGFs, which is outside the criterion cut-off of 15 NGFs.
We conclude that these data come close to externally validating the predictive model, failing only on marginally higher proportional error and Bland-Altman limits of agreement. The criteria and results for both sets of observed data are shown in Table 3.
Table 3.
Criteria for external validation of the predictive model by the observations, together with results obtained from correlation and Bland-Altman analysis
| Criterion | Cutoff value | Own data (n = 13) | Published data (n = 15) |
|---|---|---|---|
| Correlation | r > 0.80 | r = 0.87 | r = 0.90 |
| Mean difference | <5 NGFs | 2.6 NGFs | 3.3 NGFs |
| Limits of agreement | 1 SD < 15 NGFs | 1 SD = 11.9 NGFs | 1 SD = 20.8 NGFs |
| Proportional error | r2 < 0.10 | r 2 = 0.003 | r2 = 0.12 |
Discussion
We show a novel predictive model for MFD in the human ovary, and that this has high correlation with our own observed MFD calculations, low mean difference, tight limits of agreement and lack of proportional error. The model can therefore be used to accurately and reliably predict the actual MFD in the cortex of healthy women aged 16 through 37 years (Table 2). The obvious advantage of using the model is that it is non-destructive: no cortical tissue is needed to make the prediction.
Our model depends on one assumption and a calculated estimate of a key parameter. The assumption is that larger ovaries contain more NGFs than small ones, with the proportion roughly linear with ovarian volume. Indirect supporting evidence for this assumption is provided by studies that show strong correlation between ovarian volume and NGF populations [28, 29]. The key parameter is our estimate of the volume of the cortex as a proportion of the entire ovary, which we take to be close to the typical case; it should be noted that these data derive from normal healthy women, in the absence of factors known to affect the key variables such as the use of hormonal contraception which has been shown to significantly reduce ovarian volume [30]. Our estimate of roughly 24 % of ovarian volume can be compared to descriptions of average widths of the cortex seen in histological samples. Our calculations suggest that the width of the cortex is typically between 0.75 and 1.75 mm. There is a lack of homogeneity in the literature regarding these values. Our lower bound is in good agreement with the 0.75 mm quoted in a recent review by Silber [7], and broadly concordant with the 1 to 2 mm range described by von Woolf et el. [8], but our entire range is below the 2–3 mm described by Barrett and Woodruff [31]. We believe that it is reasonable to assume that the volume of the cortex relative to the whole ovary changes little during the age range for this study (i.e., mid teen to late thirties) as there are no published reports of cortical loss due to thinning before menopause [32] however this assumption cannot be extrapolated to earlier ages, since the cortex volume corresponds to about 90 % of the fetal and 85 % of the infant ovary [33]. Moreover cortical follicle density has been reported as up to eight-fold greater in infant and very young ovaries compared to mid teenage tissue [23] highlighting the difference in follicle population at pre-reproductive age.
We combine two predictive models to predict MFDs. The normative model for ovarian volume [20] was validated during development, but the NGF population model [1] has not yet been directly validated. Indirect validation for the model (and hence its suitability for use in this study) follows from its ability to predict average age (and ranges) at menopause, and its close agreement at fertile ages with indirect markers of ovarian reserve such as AMH [2] and ovarian volume [28].
Our own data validated the model, achieving or exceeding the four criteria set out in advance. However we found only partial external validation when data from a published study, employing less stringent counting methods, was used. Two of the four validation criteria were satisfied, with the remaining two being close to the a priori cut-offs (Table 3). A plausible explanation for results that are similar but not quite as close-fitting as for our own data, is that the published MFDs were obtained by examination of half the tissue with correction factors applied, rather than examination of all the tissue. In all studies using differential counts of follicles there is a compromise reached between data accuracy and effort required to generate it and so most studies have opted for a sampling frequency. In this study every section was counted and repeat counts show a high degree of reproducibility with a low coefficient of variation. Hence the MFD calculations for our data are precise (modulo observer error), whereas the published data are reasonable approximations to the actual MFDs. Combining the internal and external data gives 28 observations for the age range 16–37 years. This relatively small sample size for a 21-year age range is a limitation of our study, however, a strength of this study is that we have counted and classified follicles in every section of all tissue samples. There is variation in follicle density and if a sampling frequency is used (e.g. every 10th or 20th section) then results will be skewed. High-quality estimates of MFD depend on access to tissue and intensive laboratory examination, and are therefore hard to obtain. Despite this, it should be possible to revise and improve our predictive model if and when new data become available.
In conclusion, we report development of a model of non-growing follicle density in the human ovarian cortex, which shows excellent fit with newly-derived experimental data. This model will be valuable for assessment of MFD in ovarian biopsies in a range of pathological and experimental situations, including assessment of gonadotoxicity induced by chemotherapy, the development of approaches to mitigate such damage, and potential effects of environmental exposures where only post-treatment/exposure sampling is possible.
Acknowledgments
The authors thank Joan Creiger and Anne Saunderson for patient recruitment and John Binnie for excellent technical assistance and all the patients who donated tissue. This study was in part supported by MRC grants G1100357 and G0901839 and EPSRC grant EP/H004092/1
Compliance with Ethical Standards
ᅟ
Conflict of interest
None of the authors have any conflicts of interest to declare.
Informed consent
Human ovarian material used in this study was obtained with informed consent from all participants. All procedures performed involving human participants were in accordance with the ethical standards of the University of Edinburgh and approved by Lothian Health Board’s ethics committee. These are fully compliant with the 1964 Helsinki declaration and its later amendments.
Footnotes
Capsule A non-invasive model can be used to accurately predict ovarian reserve in healthy adult women.
References
- 1.Wallace WH, Kelsey TW. Human ovarian reserve from conception to the menopause. PLoS One. 2010;5 doi: 10.1371/journal.pone.0008772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelsey TW, Anderson RA, Wright P, Nelson SM, Wallace WH. Data-driven assessment of the human ovarian reserve. Mol Hum Reprod. 2012;18:79–87. doi: 10.1093/molehr/gar059. [DOI] [PubMed] [Google Scholar]
- 3.Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, et al. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–21. doi: 10.1016/S0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- 4.Forsdike RA, Hardy K, Bull L, Stark J, Webber LJ, et al. Disordered follicle development in ovaries of prenatally androgenized ewes. J Endocrinol. 2007;192:421–8. doi: 10.1677/joe.1.07097. [DOI] [PubMed] [Google Scholar]
- 5.Dittrich R, Lotz L, Mueller A, Hoffmann I, Wachter DL, et al. Oncofertility: combination of ovarian stimulation with subsequent ovarian tissue extraction on the day of oocyte retrieval. Reprod Biol Endocrinol. 2013;11:19. doi: 10.1186/1477-7827-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isachenko V, Isachenko E, Reinsberg J, Montag M, Braun F, et al. Cryopreservation of human ovarian tissue: effect of spontaneous and initiated ice formation. Reprod Biomed Online. 2008;16:336–45. doi: 10.1016/S1472-6483(10)60593-7. [DOI] [PubMed] [Google Scholar]
- 7.Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18:59–67. doi: 10.1093/molehr/gar082. [DOI] [PubMed] [Google Scholar]
- 8.von Wolff M, Donnez J, Hovatta O, Keros V, Maltaris T, et al. Cryopreservation and autotransplantation of human ovarian tissue prior to cytotoxic therapy—a technique in its infancy but already successful in fertility preservation. Eur J Cancer. 2009;45:1547–53. doi: 10.1016/j.ejca.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Wallace WH, Kelsey TW, Anderson RA. Ovarian cryopreservation: experimental or established and a cure for the menopause? Reprod Biomed Online. 2012;25:93–5. doi: 10.1016/j.rbmo.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Campos JR, Rosa-e-Silva JC, Carvalho BR, Vireque AA, Silva-de-Sa MF, et al. Cryopreservation time does not decrease follicular viability in ovarian tissue frozen for fertility preservation. Clinics (Sao Paulo) 2011;66:2093–7. doi: 10.1590/S1807-59322011001200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.David A, Dolmans MM, Van Langendonckt A, Donnez J, Amorim CA. Immunohistochemical localization of growth factors after cryopreservation and 3 weeks’ xenotransplantation of human ovarian tissue. Fertil Steril. 2011;95:1241–6. doi: 10.1016/j.fertnstert.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Qu J, Godin PA, Nisolle M, Donnez J. Distribution and epidermal growth factor receptor expression of primordial follicles in human ovarian tissue before and after cryopreservation. Hum Reprod. 2000;15:302–10. doi: 10.1093/humrep/15.2.302. [DOI] [PubMed] [Google Scholar]
- 13.Geber S, Megale R, Vale F, Lanna AM, Cabral AC. Variation in ovarian follicle density during human fetal development. J Assist Reprod Genet. 2012;29:969–72. doi: 10.1007/s10815-012-9810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristensen SG, Rasmussen A, Byskov AG, Andersen CY. Isolation of pre-antral follicles from human ovarian medulla tissue. Hum Reprod. 2011;26:157–66. doi: 10.1093/humrep/deq318. [DOI] [PubMed] [Google Scholar]
- 15.Petrillo SK, Desmeules P, Truong TQ, Devine PJ. Detection of DNA damage in oocytes of small ovarian follicles following phosphoramide mustard exposures of cultured rodent ovaries in vitro. Toxicol Appl Pharmacol. 2011;253:94–102. doi: 10.1016/j.taap.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95:170–5. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Sills ES, Alper MM, Walsh AP. Ovarian reserve screening in infertility: practical applications and theoretical directions for research. Eur J Obstet Gynecol Reprod Biol. 2009;146:30–6. doi: 10.1016/j.ejogrb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Hockberger PE, Skimina TA, Centonze VE, Lavin C, Chu S, et al. Activation of flavin-containing oxidases underlies light-induced production of H2O2 in mammalian cells. Proc Natl Acad Sci U S A. 1999;96:6255–60. doi: 10.1073/pnas.96.11.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avery B, Greve T. Effects of ethanol and dimethylsulphoxide on nuclear and cytoplasmic maturation of bovine cumulus-oocyte complexes. Mol Reprod Dev. 2000;55:438–45. doi: 10.1002/(SICI)1098-2795(200004)55:4<438::AID-MRD12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 20.Kelsey TW, Dodwell SK, Wilkinson AG, Greve T, Andersen CY, et al. Ovarian volume throughout life: a validated normative model. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLaughlin M, Kinnell HL, Anderson RA, Telfer EE. Inhibition of phosphatase and tensin homologue (PTEN) in human ovary in vitro results in increased activation of primordial follicles but compromises development of growing follicles. Mol Hum Reprod. 2014;20:736–44. doi: 10.1093/molehr/gau037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–8. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 23.Anderson RA, McLaughlin M, Wallace WH, Albertini DF, Telfer EE. The immature human ovary shows loss of abnormal follicles and increasing follicle developmental competence through childhood and adolescence. Hum Reprod. 2014;29:97–106. doi: 10.1093/humrep/det388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lass A, Skull J, McVeigh E, Margara R, Winston RM. Measurement of ovarian volume by transvaginal sonography before ovulation induction with human menopausal gonadotrophin for in-vitro fertilization can predict poor response. Hum Reprod. 1997;12:294–7. doi: 10.1093/humrep/12.2.294. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt KL, Byskov AG, Nyboe Andersen A, Muller J, Yding Andersen C. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and in individual pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18:1158–64. doi: 10.1093/humrep/deg246. [DOI] [PubMed] [Google Scholar]
- 26.Eken C. Bland-Altman analysis for determining agreement between two methods. J Emerg Med. 2009;36:307. doi: 10.1016/j.jemermed.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Sedgwick P. Limits of agreement (Bland-Altman method) BMJ. 2013;346:f1630. doi: 10.1136/bmj.f1630. [DOI] [PubMed] [Google Scholar]
- 28.Kelsey TW, Wallace WH. Ovarian volume correlates strongly with the number of nongrowing follicles in the human ovary. Obstet Gynecol Int. 2012;2012:305025. doi: 10.1155/2012/305025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace WH, Kelsey TW. Ovarian reserve and reproductive age may be determined from measurement of ovarian volume by transvaginal sonography. Hum Reprod. 2004;19:1612–7. doi: 10.1093/humrep/deh285. [DOI] [PubMed] [Google Scholar]
- 30.Bentzen JG, Forman JL, Pinborg A, Lidegaard Ø, Larsen EC, et al. Ovarian reserve parameters: a comparison between users and non-users of hormonal contraception. Reprod Biomed Online. 2012;25:612–9. doi: 10.1016/j.rbmo.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Barrett SL, Woodruff TK. Gamete preservation. Cancer Treat Res. 2010;156:25–39. doi: 10.1007/978-1-4419-6518-9_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laszczyńska M, Brodowska A, Starczewski A, Masiuka M, Brodowski J. Human postmenopausal ovary –hormonally inactive fibrous connective tissue or more. Histol Histopathol. 2008;23:219–226. [DOI] [PubMed]
- 33.Forabosco A, Sforza C, De Pol A, Vizzotto L, Marzona L, et al. Morphometric study of the human neonatal ovary. Anat Rec. 1991;231:201–8. doi: 10.1002/ar.1092310208. [DOI] [PubMed] [Google Scholar]