Abstract
Purpose
Inflammation in chorionic villi is involved in the development of recurrent pregnancy loss (RPL). High mobility group box 1 protein (HMGB1) plays critical roles in inflammation and expression of the protein can be found in chorionic villi. The purpose of the study was to investigate the association between HMGB1 genetic polymorphisms and susceptibility to RPL and to examine the mechanism underlying this correlation.
Methods
Two HMGB1 polymorphisms, rs2249825C/G and rs1412125T/C, were examined in 112 RPL patients and 118 healthy controls by the polymerase chain reaction–restriction fragment length polymorphism assay.
Results
Percentage of rs2249825GG was significantly increased in patients than in controls (Odd ratio [OR] =2.33, 95 % confidence interval [CI]: 1.18–4.58, P = 0.013). Also, prevalence of rs2249825G allele was significantly higher in RPL cases (OR = 1.77, 95 % CI: 1.20–2.62, P = 0.004). Function analysis of rs2249825C/G revealed that the polymorphism did not affect serum level of HMGB1. Interestingly, we found significantly increased level of HMGB1 in chorionic villi from RPL patients. Moreover, patients with rs2249825GG genotype presented significantly elevated level of HMGB1 in chorionic villi compared to those with CG or CC genotypes.
Conclusions
These results suggest that HMGB1 rs2249825C/G polymorphism is associated with increased risk of RPL and can elevate gene expression in chorionic villi.
Keywords: HMGB1, Polymorphism, Chorionic villi, RPL
Introduction
Recurrent pregnancy loss (RPL) has been a challenging problem in reproductive medicine [13]. Approximately 2 % of reproductive couples in the world are affected by the disease [14]. Mechanism of the disease remains elusive [14, 13]. Immunopathological examinations of the placental implantation site often show decidual inflammation and decidual fibrin deposition in RPL cases [15]. In addition, literature has reported that thrombotic and inflammatory processes play a major role in spontaneous abortion [15].
Human high mobility group box 1 protein (HMGB1) is a ubiquitous nuclear protein [21], and can be found in different kinds of cells in mammalian tissues [17]. HMGB1 acts as a critical extracellular mediator in inflammation processes [16]. It can be automatically released from necrotic cells by simple diffusion into the extracellular milieu [11]. In addition, HMGB1 can be also produced by various immune cells including dendritic cells (DC), monocytes, neutrophils, etc. in response to different stimuli [8]. HMGB1 outside of cells can bind to different receptors, nucleosomes, and DNAs, which causes immune responses in pathological conditions. It has been reported that HMGB1 could be involved in many inflammatory and autoimmune diseases such as sepsis, atherosclerosis, and rheumatoid arthritis, and increased serum levels of HMGB1 have been identified in systemic lupus erythematosus (SLE) and chronic kidney disease [1, 5]. HMGB1 may also participate in the pathogenesis of different tumors. Elevated level of the protein has been reported in lymphoma, pancreatic cancer, and breast cancer [11, 21]. Studies have shown that HMGB1 is generally correlated with tumor metastasis [8]. In addition, HMGB1 may affect other diseases such as diabetes by modulating cell death and damage [5].
Since HMGB1 plays a major role in inflammation, whereas inflammatory processes are crucial to spontaneous abortion, it is possible that HMGB1 may involve in the pathogenesis of RPL. Single-nucleotide polymorphisms (SNPs) may affect the development of human diseases by interfering with gene expression [12]. In the current study, we first investigated the association between HMGB1 genetic polymorphisms and susceptibility to RPL. As a part of the border between maternal and fetal blood during pregnancy, chorionic villi may greatly influence the pregnancy process [4, 6]. Aberrant chorionic villi have been identified in many RPL patients [9, 10]. Meanwhile, recent study has shown a strong nuclear HMGB1 expression in almost all cells in placentas including chorionic villi [7]. It is possible that the deregulation of HMGB1 in chorionic villi works as a key factor for the development of RPL. Therefore, we further investigated whether HMGB1 polymorphisms could affect the gene expression in chorionic villi.
Materials and methods
Ethical statement
All procedures performed in studies were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Review Board of the Maternal and Child Health Hospital of Jinan City. Informed consent was obtained from all individual participants included in the study.
Study subjects
The patient group consisted of 112 women who were diagnosed with idiopathic RPL. Patients who had at least two pregnancy losses before 20 weeks of gestation were invited to participate in the study. All the cases were reported no previous full-term pregnancies. To exclude genetic causes of RPL, only RPL patients and their partners with normal karyotype results were included in the study. Also, all potential participants received a preliminary standard diagnostic protocol which included laparoscopy, hysteroscopy, ultrasound, and comprehensive determination of hormonal status. In addition, acquired thrombophilia was examined by evaluating lupus anticoagulant, anticardiolipin, and antinuclear antibodies. Patients with any maternal clinical conditions that could prevent full-term pregnancy (e.g., uterine abnormality) were excluded. The control group consisted of 118 women and was recruited from the same hospital. All the healthy controls had at least one biological living child and had no history of pregnancy loss or infertility.
DNA extraction and genotyping
Genomic DNA was extracted from 5 ml whole blood using DNA Extraction Kit (Qiagen, Germany). The HMGB1 SNPs were detected using the polymerase chain reaction–restriction fragment length polymorphism method [3]. In brief, PCR was performed in a total volume of 20 μl, which contained 0.5 uM of each primer, 2 μl of PCR buffer (Qiagen, Germany), Taq polymerase (Qiagen, Germany), 0.2 mM of dNTP, and 200 ng of genomic DNA. The primers for rs2249825C/G polymorphism were 5′ – TGCAAAGCCACGAATTGGCA – 3′ and 5′ – GGGTGCTTCTTCTTATGCTC – 3′. The primers for rs1412125T/C polymorphism were 5′ – TGCCCAAATCCACAGGCTGT – 3′ and 5′ – GAGGGAAGCAGAGGATAGTA – 3′. PCR products carrying the polymorphic sites were then digested with the restriction enzymes Taq I and Pst I (NEB, MA, USA), and tested by electrophoresis.
Real-time quantitative reverse transcription PCR (qRT–PCR) and western blot
Real-time qRT–PCR was performed using previously published method and some conditions were modified in this study [2]. In brief, total RNA were extracted from the chorionic villi of RPL patients by TRIzol method. Real-time qRT–PCR was the performed using the primers 5′ - AGTTAGATACTCATTCAGAGCAG -3′ and 5′ - TCTTCTTATGCTCCTCCCGACAA -3′ for rs2249825C/G; 5′ - CCTGAGCAGACCACGCCCCT -3′ and 5′ - TAAACACTGCATGTGCAGTATACC -3′ for rs1412125T/C. Experiments were performed in triplicates. Data of the real-time qRT–PCR experiments were further analyzed by the 2(−Delta Delta C(T)) method. Protein levels of HMGB1 in chorionic villi were measured by western blot method. In brief, chorionic villi were lysated with lysis buffer, and about 10 mg proteins were separated by 4–12 % gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDSPAGE). The proteins were then transferred to nitrocellulose membranes, and blocked in tris buffered saline-Tween-20 containing 5 % skim milk. Rabbit polyclonal anti-HMGB1 (Abcam, Cambridge, UK) antibody was diluted at the concentration of 1:1000 and was applied to the membranes. The membranes were then washed, incubated for 1 h with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA), washed again, and developed with ECL-Plus (Amersham Pharmacia Biotech, Uppsala, Sweden).
ELISA
Serum concentration of HMGB1 was evaluated using the HMGB1 ELISA Kit II (Shino-test, Tokyo, Japan). ELISA was performed according to the manufacturer′s instruction.
Statistical analyses
The SPSS statistical software package ver.17.0 (SPSS Inc., Chicago, USA) were used for statistical analyses. Hardy-Weinberg equilibrium of the polymorphisms was analyzed using the chi-square test. Distribution of the genotypes and alleles were calculated by the chi-square test. Odds ratios (OR) and 95 % confidence intervals (CIs) were analyzed by unconditional logistic regression. P < 0.05 was considered as statistical significance.
Results
Characteristics of the study population
As shown in Table 1, mean age of the patients and controls was 26.8 and 27.5 years old (P > 0.05). Smoking status was not significantly different between the study groups (P > 0.05). Each patient in our study group had two to six times of pregnancy loss, and none of them had a biological child. None of the healthy controls had pregnancy loss, and each of them had 1 or 2 biological children.
Table 1.
Characteristics of the patients and healthy controls
| Characteristics | Patients (n = 112) | Controls (n = 118) | p value |
|---|---|---|---|
| Age (mean ± SD) | 26.8 ± 4.5 | 27.5 ± 5.3 | >0.05 |
| Smokers | 12 (10.7 %) | 11 (9.3%) | >0.05 |
| a Times of pregnancy loss | 2–6 | 0 | <0.05 |
| b No. of biological children | 0 | 1–2 | <0.05 |
aTimes of pregnancy loss of each subject
bNumber of biological children of each subject
HMGB1 polymorphisms and susceptibility to RPL
We investigated the distribution of HMGB1 rs2249825 C/G and rs1412125 T/C SNPs in RPL patients and healthy donors (Table 2). For the HMGB1 rs2249825 C/G polymorphism, percentage of the heterozygous CG genotype was 22.3 % in patients and 23.7 % in controls; percentage of the GG genotype was 28.6 % in RPL cases and 15.3 % in healthy subjects. Distribution of the HMGB1 rs2249825 genotypes was significantly different between the RPL patients and controls (P = 0.046, Table 2). Further analysis revealed that prevalence of the GG genotype was significantly higher in RPL cases than in controls (OR = 2.33, 95 % CI: 1.18–4.58, P = 0.013). Also, the allele frequency of rs2249825G was significantly increased in patients compared to the healthy subjects (39.7 % vs 27.1 %, OR = 1.77, 95 % CI: 1.20–2.62, P = 0.004, Table 2). For the HMGB1 rs1412125 T/C polymorphism, distribution of the three genotypes was very similar between cases and controls (P = 0.905, Table 2). Also, prevalence of the rs1412125 alleles was not significantly different between the RPL patients and controls (P > 0.05).
Table 2.
Distribution of HMGB1 SNPs in patients and controls
| Polymorphisms | Patients (N = 112) (%) | Controls (N = 118) (%) | OR (95% CI) | P value | Overall P value a |
|---|---|---|---|---|---|
| rs2249825 C/G | |||||
| Genotype | 0.046* | ||||
| CC | 55 (49.1) | 72 (61.0) | Ref. | ||
| CG | 25 (22.3) | 28 (23.7) | 1.17 (0.61–2.23) | 0.635 | |
| GG | 32 (28.6) | 18 (15.3) | 2.33 (1.18–4.58) | 0.013* | |
| Allele | |||||
| C | 135 (60.3) | 172 (72.9) | Ref. | ||
| G | 89 (39.7) | 64 (27.1) | 1.77 (1.20–2.62) | 0.004* | |
| rs1412125 T/C | |||||
| Genotype | 0.905 | ||||
| TT | 62 (55.4) | 66 (55.9) | Ref. | ||
| TC | 40 (35.7) | 41 (34.7) | 1.04 (0.60–1.81) | 0.894 | |
| CC | 10 (8.9) | 11 (9.4) | 0.97 (0.38–2.44) | 0.945 | |
| Allele | |||||
| T | 164 (73.2) | 173 (73.3) | Ref. | ||
| C | 60 (26.8) | 63 (26.7) | 1.01 (0.66–1.52) | 0.983 | |
*P value < 0.05. aOverall p-value across the three genotypes of a polymorphism
HMGB1 polymorphisms and gene expression
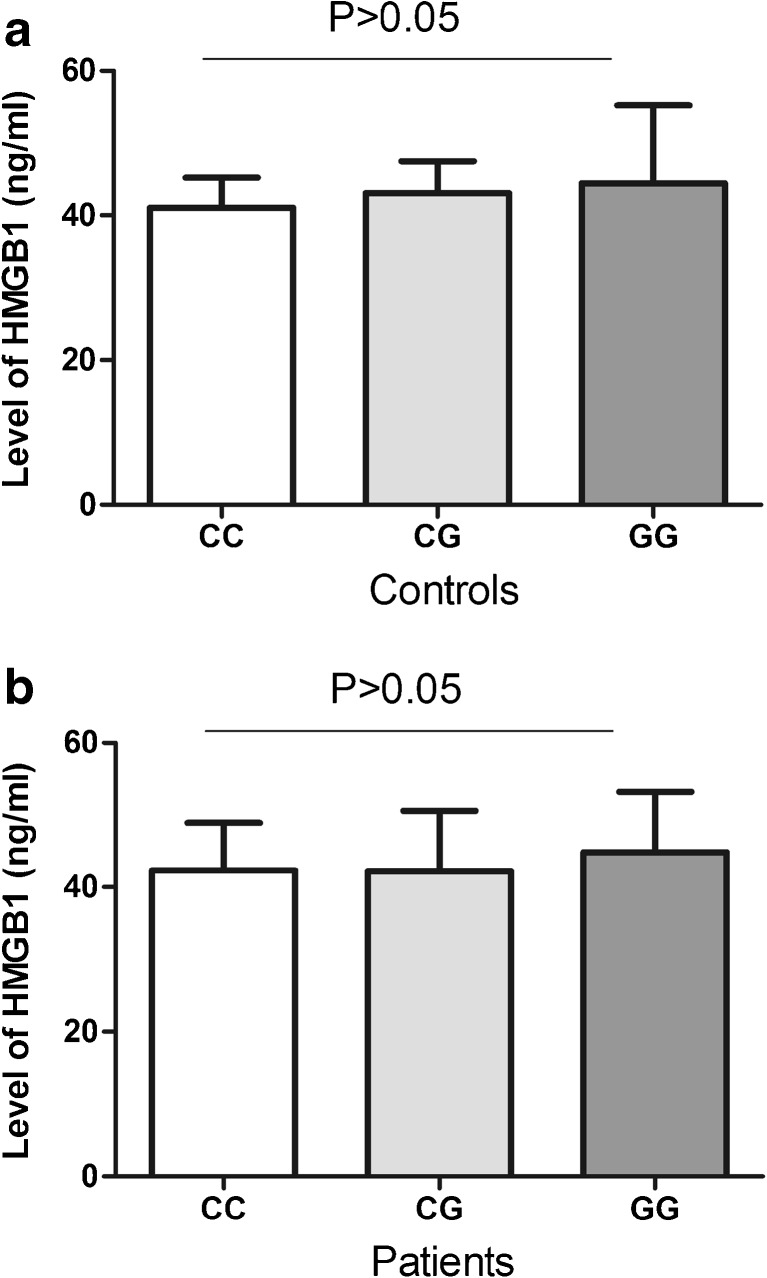
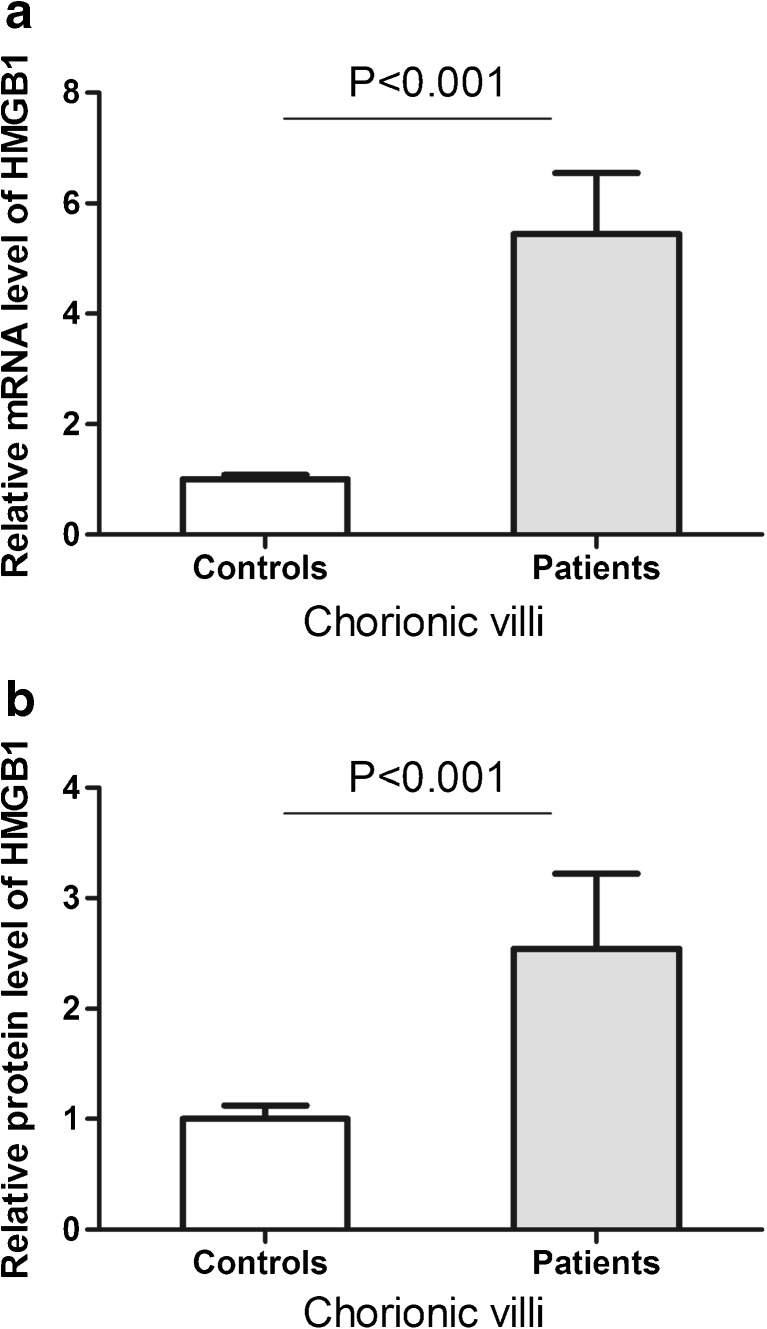
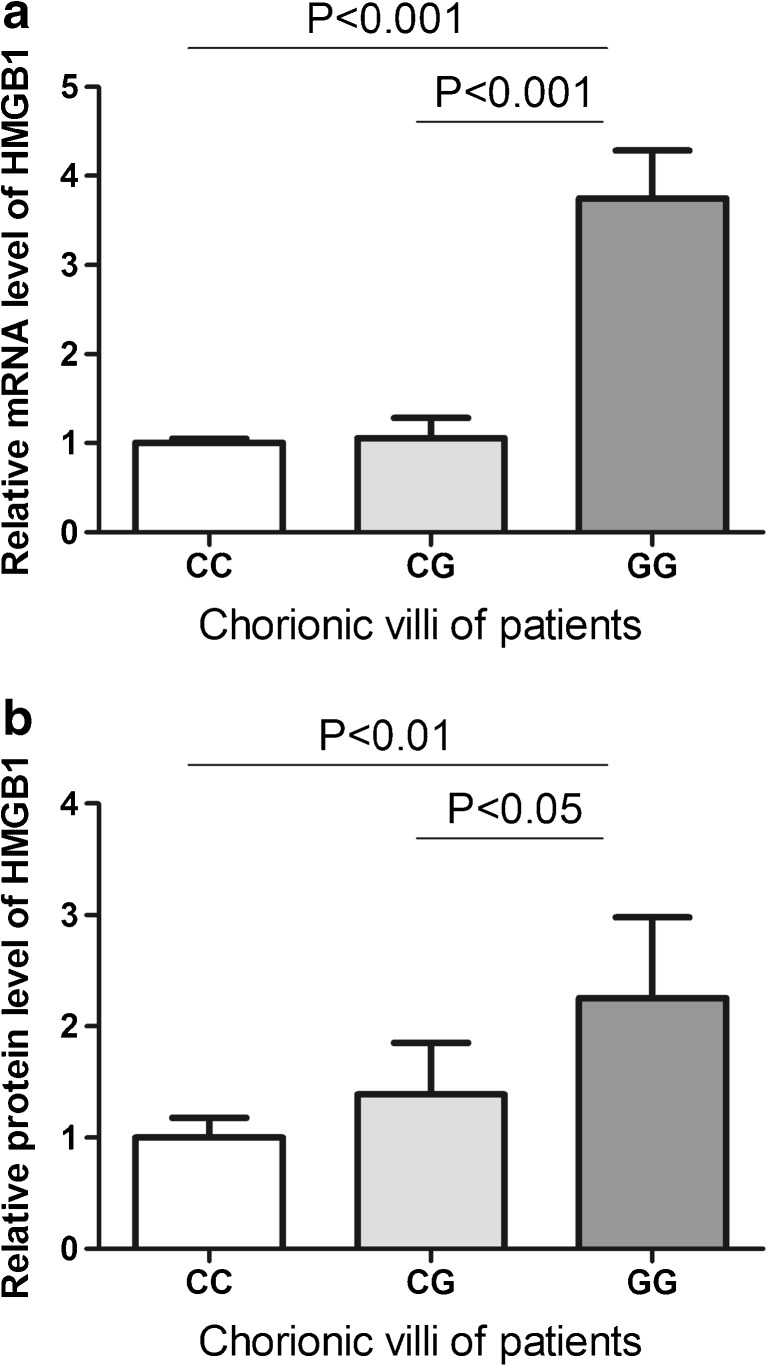
To understand the function of the rs2249825 C/G SNP, we first evaluated serum levels of HMGB1 in subjects with different genotypes. However, both patients (Fig. 1a) and controls (Fig. 1b) with different genotypes revealed similar level of HMGB1 (P > 0.05). Studies have shown that changes in chorionic villi may play critical roles in the development of RPL. We therefore examined expression of HMGB1 in the chorionic villi of RPL patients and controls. Data revealed that both mRNA and protein levels of HMGB1 were significantly increased in chorionic villi of RPL patients (Fig. 2). We further investigated whether the rs2249825 C/G SNP could affect the gene expression in chorionic villi. Results showed that patients with GG genotype had significantly higher level of HMGB1 mRNA than those with CC or CG genotypes (Fig. 3a). Protein level of HMGB1 was significantly elevated in patients with CG and GG genotypes compared to those with CC genotype (Fig. 3b).
Fig. 1.
Serum level of HMGB1 in subjects with different rs2249825 genotypes. a in controls. b in patients. Data are presented as mean ± standard deviation (SD)
Fig. 2.
Relative mRNA level (a) and protein level (b) of HMGB1 in chorionic villi from RPL patients and controls. Data are presented as mean ± SD
Fig. 3.
Relative mRNA level (a) and protein level (b) of HMGB1 in chorionic villi of patients with different rs2249825 genotypes. Data are presented as mean ± SD
Discussion
In the current study, we identified that the HMGB1 rs2249825 C/G SNP was associated with increased risk of RPL. Both mRNA level and protein level of HMGB1 were significantly elevated in the chorionic villi of RPL patients. Moreover, the HMGB1 rs2249825 C/G SNP could up-regulate gene expression in the chorionic villi of RPL patients. These data suggest that HMGB1 expression in chorionic villi may play a role in the pathogenesis of RPL, and HMGB1 SNP may affect the susceptibility to RPL through increasing gene expression in chorionic villi.
RPL is a condition when a woman has two or more clinical pregnancy losses (miscarriages) before the pregnancies reach 20 weeks. Many factors could contribute to the development of RPL such as anatomic problems, medical conditions, genetic abnormalities, etc. [13]. Recent evidences have shown that inflammatory processes play a major role in the pathogenesis of RPL. HMGB1 works as an important extracellular mediator in the progress of inflammation. We hypothesized that HMGB1 may be involved in the development of RPL. First of all, by investigating HMGB1 polymorphisms in RPL and healthy subjects, we identified that percentages of HMGB1 rs2249825GG and rs2249825G allele were significantly increased in patients than in controls, indicating an association between HMGB1 polymorphisms and the disease. The HMGB1 rs2249825 SNP has been reported in several literatures [20, 18, 19]. Data have suggested that the HMGB1 rs2249825 polymorphism is significantly associated with hypertension and diastolic blood pressure. Rs2249825 is associated with platinum-based chemotherapy responses in Chinese lung cancer patients. There are also significant differences in sepsis morbidity rate among patients with different genotypes of the rs2249825. The rs2249825 SNP is located at intron 1 of the HMGB1 gene. Study has reported that the SNP may be associated with HMGB1 production in peripheral blood leukocytes. In this study, we did not observe any changes of HMGB1 serum level among the subjects with different rs2249825 genotypes, probably due to that effect of the SNP is too subtle to make an obvious change in serum. Interestingly, we found elevated level of HMGB1 in the chorionic villi of patients with GG genotype (Fig. 3). It is possible that the effect of the SNP on gene expression is tissue specific.
Chorionic villi greatly influence the pregnancy process. Since HMGB1 expression can be detected in chorionic villi, it is possible that deregulation of HMGB1 in chorionic villi may result in abnormal pregnancy. Here, we identified significantly higher expression of HMGB1 in chorionic villi from RPL patients compared to controls (Fig. 2), suggesting that modulation of HMGB1 in chorionic villi may be important for the disease. Thus, the effect of HMGB1 rs2249825C/G polymorphism on the susceptibility to RPL could be through up-regulating gene expression in chorionic villi.
The rs1412125 is another HMGB1 polymorphism which we examined in the study. A literature has shown that the polymorphism is associated with the platinum-based chemotherapy response in both recessive and genotypic models [18]. Since the SNP is located in the 5′ upstream region of HMGB1 gene, it is possible that it can affect the promoter activity of HMGB1. However, our study did not identify any association between the polymorphism and the risk of RPL (Table 2).
In conclusion, we identified an association between HMGB1 rs2249825 C/G SNP and the increased risk of RPL. We also observed elevated HMGB1 expression in chorionic villi of RPL patients. In addition, we found that HMGB1 rs2249825 C/G SNP may up-regulate gene expression in the chorionic villi of RPL patients. These results provide important information on understanding HMGB1 in the pathogenesis of RPL. However, since the subject number of our study is relatively small, it would be necessary to conduct similar experiments in a larger study population.
Acknowledgments
This work was supported by Jinan Science and Technology Bureau Grant Award (201112003). Title: “The clinical application study for first-trimester villi prenatal diagnosis”.
Conflict of interest
None.
Footnotes
Capsule Inflammation in chorionic villi may play critical roles in the development of recurrent pregnancy loss (RPL). Here, we identified an association between HMGB1 rs2249825 C/G SNP and increased risk of RPL. Moreover, RPL patients carrying rs2249825 GG genotype revealed increased level of HMGB1 in the chorionic villi than those with CC and CG genotypes. These data suggest that HMGB1 rs2249825C/G polymorphism may affect susceptibility to RPL by up-regulating gene expression in chorionic villi.
Hua Jin and Jie Wu contributed equally to this work.
References
- 1.Branco-Madeira F, Lambrecht BN. High mobility group box-1 recognition: the beginning of a RAGEless era? EMBO Mol Med. 2010;2:193–5. doi: 10.1002/emmm.201000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui XS, Shen XH, Kim NH. High mobility group box 1 (HMGB1) is implicated in preimplantation embryo development in the mouse. Mol Reprod Dev. 2008;75:1290–9. doi: 10.1002/mrd.20694. [DOI] [PubMed] [Google Scholar]
- 3.Deng CQ, Deng GH, Wang YM. HMGB1 gene polymorphisms in patients with chronic hepatitis B virus infection. World J Gastroenterol. 2013;19:5144–9. doi: 10.3748/wjg.v19.i31.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dugoff L, Hobbins JC. Invasive procedures to evaluate the fetus. Clin Obstet Gynecol. 2002;45:1039–53. doi: 10.1097/00003081-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Gaggar A, Rowe SM, Matthew H, Blalock JE. Proline-Glycine-Proline (PGP) and High Mobility Group Box Protein-1 (HMGB1): potential mediators of cystic fibrosis airway inflammation. Open Respir Med J. 2010;4:32–8. doi: 10.2174/1874306401004010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill RM, Ni J, Hunt JS. Differential expression of LIGHT and its receptors in human placental villi and amniochorion membranes. Am J Pathol. 2002;161:2011–7. doi: 10.1016/S0002-9440(10)64479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmlund U, Wahamaa H, Bachmayer N, Bremme K, Sverremark-Ekstrom E, Palmblad K. The novel inflammatory cytokine high mobility group box protein 1 (HMGB1) is expressed by human term placenta. Immunology. 2007;122:430–7. doi: 10.1111/j.1365-2567.2007.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Deng J, Xu J, Wang H, Yuan M, Liu N, et al. High-mobility group box 1 (HMGB1) downregulates cardiac transient outward potassium current (Ito) through downregulation of Kv4.2 and Kv4.3 channel transcripts and proteins. J Mol Cell Cardiol. 2010;49:438–48. doi: 10.1016/j.yjmcc.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Reus AD, Stephenson MD, van Dunne FM, de Krijger RR, Joosten M, Steegers EA, et al. Chorionic villous vascularization related to phenotype and genotype in first trimester miscarriages in a recurrent pregnancy loss cohort. Hum Reprod. 2013;28:916–23. doi: 10.1093/humrep/det025. [DOI] [PubMed] [Google Scholar]
- 10.Reus AD, van Besouw NM, Molenaar NM, Steegers EA, Visser W, de Kuiper RP, et al. An immunological basis for chronic histiocytic intervillositis in recurrent fetal loss. Am J Reprod Immunol. 2013;70:230–7. doi: 10.1111/aji.12125. [DOI] [PubMed] [Google Scholar]
- 11.Schierbeck H, Wahamaa H, Andersson U, Harris HE. Immunomodulatory drugs regulate HMGB1 release from activated human monocytes. Mol Med. 2010;16:343–51. doi: 10.2119/molmed.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seal A, Gupta A, Mahalaxmi M, Aykkal R, Singh TR, Arunachalam V. Tools, resources and databases for SNPs and indels in sequences: a review. Int J Bioinform Res Appl. 2014;10:264–96. doi: 10.1504/IJBRA.2014.060762. [DOI] [PubMed] [Google Scholar]
- 13.Shahine L, Lathi R. Recurrent pregnancy loss: evaluation and treatment. Obstet Gynecol Clin North Am. 2015;42:117–134. doi: 10.1016/j.ogc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Shahine LK, Lathi RB. Embryo selection with preimplantation chromosomal screening in patients with recurrent pregnancy loss. Semin Reprod Med. 2014;32:93–9. doi: 10.1055/s-0033-1363550. [DOI] [PubMed] [Google Scholar]
- 15.Sharma S. Natural killer cells and regulatory T cells in early pregnancy loss. Int J Dev Biol. 2014;58:219–29. doi: 10.1387/ijdb.140109ss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsoyi K, Nizamutdinova IT, Jang HJ, Mun L, Kim HJ, Seo HG, et al. Carbon monoxide from CORM-2 reduces HMGB1 release through regulation of IFN-beta/JAK2/STAT-1/INOS/NO signaling but not COX-2 in TLR-activated macrophages. Shock. 2010;34:608–14. doi: 10.1097/SHK.0b013e3181e46f15. [DOI] [PubMed] [Google Scholar]
- 17.Volz HC, Kaya Z, Katus HA, Andrassy M. The role of HMGB1/RAGE in inflammatory cardiomyopathy. Semin Thromb Hemost. 2010;36:185–94. doi: 10.1055/s-0030-1251503. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Li XP, Yin JY, Zhang Y, He H, Qian CY, et al. Association of HMGB1 and HMGB2 genetic polymorphisms with lung cancer chemotherapy response. Clin Exp Pharmacol Physiol. 2014;41:408–15. doi: 10.1111/1440-1681.12232. [DOI] [PubMed] [Google Scholar]
- 19.Yao Y, Guo D, Yang S, Jin Y, He L, Chen J, Zhao X, Chen Y, Zhou, W, Shen C. HMGB1 gene polymorphism is associated with hypertension in Han Chinese population. Clin Exp Hypertens. 2014;1–6. [DOI] [PubMed]
- 20.Zeng L, Zhang AQ, Gu W, Chen KH, Jiang DP, Zhang LY, et al. Clinical relevance of single nucleotide polymorphisms of the high mobility group box 1 protein gene in patients with major trauma in southwest China. Surgery. 2012;151:427–36. doi: 10.1016/j.surg.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 21.Zhou M, Wu R, Dong W, Leong J, Wang P. Accelerated apoptosis contributes to aging-related hyperinflammation in endotoxemia. Int J Mol Med. 2010;25:929–35. doi: 10.3892/ijmm_00000424. [DOI] [PMC free article] [PubMed] [Google Scholar]