Abstract
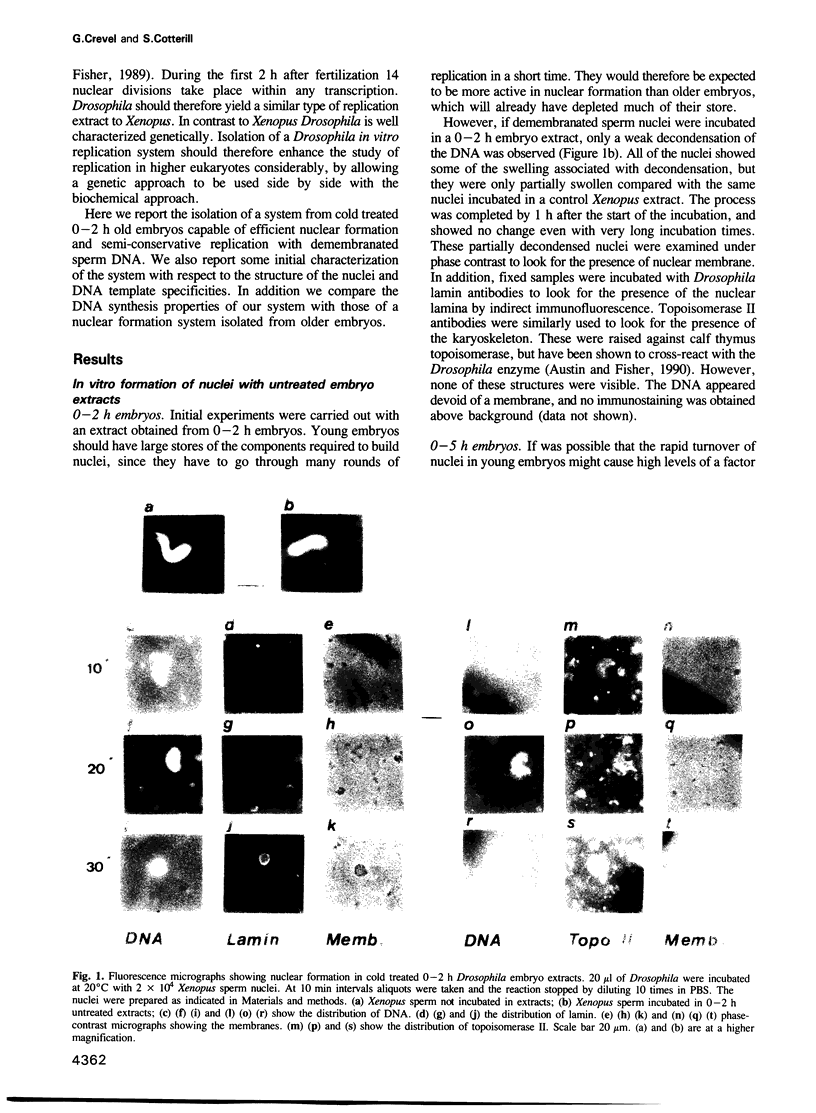
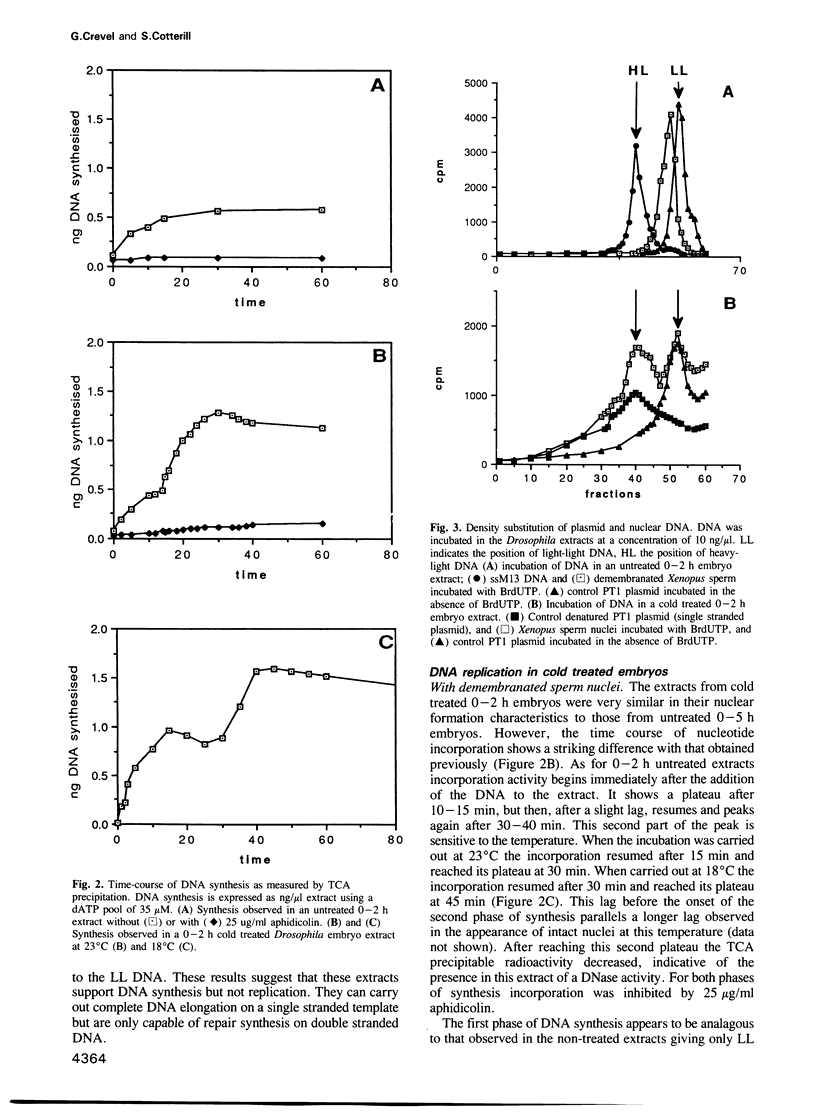
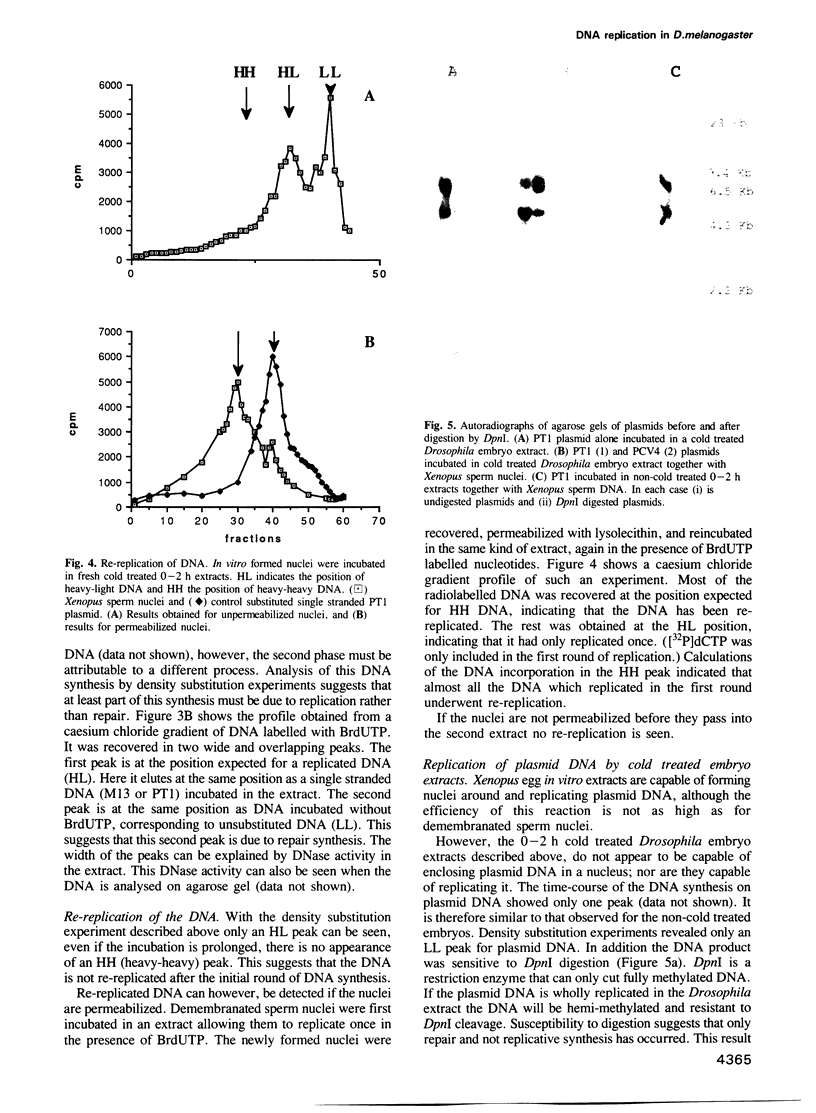
We have developed an efficient in vitro replication system from 0-2 h Drosophila melanogaster embryos. Demembranated Xenopus sperm DNA when incubated in such an extract first becomes enclosed in a nucleus-like structure with a nuclear envelope and a karyoskeleton. It then undergoes one round of semiconservative replication--this replication appears completely dependent on nuclear formation. Up to 30% of input DNA is nucleated in one reaction. Efficient nuclear formation and replication are dependent on a cold treatment step, prior to disruption of the embryos. They also depend on the age of the embryos used. Extracts from older embryos (0-5 h) are capable of nuclear formation, although at a much reduced efficiency, and repair synthesis, but seem to have lost the ability to initiate DNA replication. In addition to replicating sperm DNA this system appears capable of carrying out semi-conservative replication on some plasmids. However, it cannot use these to trigger nuclear formation; replication is only seen if the plasmids are coincubated with sperm DNA. The in vitro formed nuclei have not been observed to trigger nuclear envelope breakdown and entry into mitosis. However, they can re-replicate the DNA if the nuclei are permeabilized. This system should be a useful complement to the previously isolated Xenopus in vitro replication system. In addition the amenability of Drosophila to genetic study should open up new approaches not previously possible with Xenopus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allis C. D., Waring G. L., Mahowald A. P. Mass isolation of pole cells from Drosophila melanogaster. Dev Biol. 1977 Apr;56(2):372–381. doi: 10.1016/0012-1606(77)90277-9. [DOI] [PubMed] [Google Scholar]
- Austin C. A., Fisher L. M. Isolation and characterization of a human cDNA clone encoding a novel DNA topoisomerase II homologue from HeLa cells. FEBS Lett. 1990 Jun 18;266(1-2):115–117. doi: 10.1016/0014-5793(90)81520-x. [DOI] [PubMed] [Google Scholar]
- Berrios M., Avilion A. A. Nuclear formation in a Drosophila cell-free system. Exp Cell Res. 1990 Nov;191(1):64–70. doi: 10.1016/0014-4827(90)90036-a. [DOI] [PubMed] [Google Scholar]
- Blow J. J., Laskey R. A. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature. 1988 Apr 7;332(6164):546–548. doi: 10.1038/332546a0. [DOI] [PubMed] [Google Scholar]
- Blow J. J., Laskey R. A. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell. 1986 Nov 21;47(4):577–587. doi: 10.1016/0092-8674(86)90622-7. [DOI] [PubMed] [Google Scholar]
- Blow J. J., Nurse P. A cdc2-like protein is involved in the initiation of DNA replication in Xenopus egg extracts. Cell. 1990 Sep 7;62(5):855–862. doi: 10.1016/0092-8674(90)90261-c. [DOI] [PubMed] [Google Scholar]
- Blow J. J., Sleeman A. M. Replication of purified DNA in Xenopus egg extract is dependent on nuclear assembly. J Cell Sci. 1990 Mar;95(Pt 3):383–391. doi: 10.1242/jcs.95.3.383. [DOI] [PubMed] [Google Scholar]
- Blow J. J., Watson J. V. Nuclei act as independent and integrated units of replication in a Xenopus cell-free DNA replication system. EMBO J. 1987 Jul;6(7):1997–2002. doi: 10.1002/j.1460-2075.1987.tb02463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broek D., Bartlett R., Crawford K., Nurse P. Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature. 1991 Jan 31;349(6308):388–393. doi: 10.1038/349388a0. [DOI] [PubMed] [Google Scholar]
- Callaini G., Marchini D. Abnormal centrosomes in cold-treated Drosophila embryos. Exp Cell Res. 1989 Oct;184(2):367–374. doi: 10.1016/0014-4827(89)90336-4. [DOI] [PubMed] [Google Scholar]
- Cox L. S., Leno G. H. Extracts from eggs and oocytes of Xenopus laevis differ in their capacities for nuclear assembly and DNA replication. J Cell Sci. 1990 Sep;97(Pt 1):177–184. doi: 10.1242/jcs.97.1.177. [DOI] [PubMed] [Google Scholar]
- Dasso M., Newport J. W. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: studies in Xenopus. Cell. 1990 Jun 1;61(5):811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- Debec A., Courgeon A. M., Maingourd M., Maisonhaute C. The response of the centrosome to heat shock and related stresses in a Drosophila cell line. J Cell Sci. 1990 Jul;96(Pt 3):403–412. doi: 10.1242/jcs.96.3.403. [DOI] [PubMed] [Google Scholar]
- Harland R. M., Laskey R. A. Regulated replication of DNA microinjected into eggs of Xenopus laevis. Cell. 1980 Oct;21(3):761–771. doi: 10.1016/0092-8674(80)90439-0. [DOI] [PubMed] [Google Scholar]
- Hutchison C. J., Cox R., Drepaul R. S., Gomperts M., Ford C. C. Periodic DNA synthesis in cell-free extracts of Xenopus eggs. EMBO J. 1987 Jul;6(7):2003–2010. doi: 10.1002/j.1460-2075.1987.tb02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C., Kill I. Changes in the nuclear distribution of DNA polymerase alpha and PCNA/cyclin during the progress of the cell cycle, in a cell-free extract of Xenopus eggs. J Cell Sci. 1989 Aug;93(Pt 4):605–613. doi: 10.1242/jcs.93.4.605. [DOI] [PubMed] [Google Scholar]
- Kriegstein H. J., Hogness D. S. Mechanism of DNA replication in Drosophila chromosomes: structure of replication forks and evidence for bidirectionality. Proc Natl Acad Sci U S A. 1974 Jan;71(1):135–139. doi: 10.1073/pnas.71.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lohka M. J., Maller J. L. Induction of nuclear envelope breakdown, chromosome condensation, and spindle formation in cell-free extracts. J Cell Biol. 1985 Aug;101(2):518–523. doi: 10.1083/jcb.101.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. V., Lin T. T., Myers S. P., Leibo S. P., Macintyre R. J., Pitt R. E., Steponkus P. L. A two-step method for permeabilization of Drosophila eggs. Cryobiology. 1989 Oct;26(5):445–452. doi: 10.1016/0011-2240(89)90069-2. [DOI] [PubMed] [Google Scholar]
- Miake-Lye R., Newport J., Kirschner M. Maturation-promoting factor induces nuclear envelope breakdown in cycloheximide-arrested embryos of Xenopus laevis. J Cell Biol. 1983 Jul;97(1):81–91. doi: 10.1083/jcb.97.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills A. D., Blow J. J., White J. G., Amos W. B., Wilcock D., Laskey R. A. Replication occurs at discrete foci spaced throughout nuclei replicating in vitro. J Cell Sci. 1989 Nov;94(Pt 3):471–477. doi: 10.1242/jcs.94.3.471. [DOI] [PubMed] [Google Scholar]
- Minshull J., Blow J. J., Hunt T. Translation of cyclin mRNA is necessary for extracts of activated xenopus eggs to enter mitosis. Cell. 1989 Mar 24;56(6):947–956. doi: 10.1016/0092-8674(89)90628-4. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989 May 25;339(6222):275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Méchali M., Harland R. M. DNA synthesis in a cell-free system from Xenopus eggs: priming and elongation on single-stranded DNA in vitro. Cell. 1982 Aug;30(1):93–101. doi: 10.1016/0092-8674(82)90015-0. [DOI] [PubMed] [Google Scholar]
- Nakagawa J., Kitten G. T., Nigg E. A. A somatic cell-derived system for studying both early and late mitotic events in vitro. J Cell Sci. 1989 Nov;94(Pt 3):449–462. doi: 10.1242/jcs.94.3.449. [DOI] [PubMed] [Google Scholar]
- Sheehan M. A., Mills A. D., Sleeman A. M., Laskey R. A., Blow J. J. Steps in the assembly of replication-competent nuclei in a cell-free system from Xenopus eggs. J Cell Biol. 1988 Jan;106(1):1–12. doi: 10.1083/jcb.106.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Interconversion of Drosophila nuclear lamin isoforms during oogenesis, early embryogenesis, and upon entry of cultured cells into mitosis. J Cell Biol. 1989 Feb;108(2):255–265. doi: 10.1083/jcb.108.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. Fate of DNA injected into early Drosophila embryos. Dev Biol. 1985 May;109(1):54–62. doi: 10.1016/0012-1606(85)90345-8. [DOI] [PubMed] [Google Scholar]
- Stillman B. W. The replication of adenovirus DNA with purified proteins. Cell. 1983 Nov;35(1):7–9. doi: 10.1016/0092-8674(83)90201-5. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Melendy T., Stillman B. Sequential initiation of lagging and leading strand synthesis by two different polymerase complexes at the SV40 DNA replication origin. Nature. 1990 Aug 9;346(6284):534–539. doi: 10.1038/346534a0. [DOI] [PubMed] [Google Scholar]
- Ulitzur N., Gruenbaum Y. Nuclear envelope assembly around sperm chromatin in cell-free preparations from Drosophila embryos. FEBS Lett. 1989 Dec 18;259(1):113–116. doi: 10.1016/0014-5793(89)81507-8. [DOI] [PubMed] [Google Scholar]
- de Cicco D. V., Spradling A. C. Localization of a cis-acting element responsible for the developmentally regulated amplification of Drosophila chorion genes. Cell. 1984 Aug;38(1):45–54. doi: 10.1016/0092-8674(84)90525-7. [DOI] [PubMed] [Google Scholar]