Abstract
Objectives
The present study was aimed at exploring whether the pathogenesis of hypertension is related with an altered expression of nitric oxide synthase (NOS) isozymes, i.e., bNOS, iNOS and ecNOS.
Method
By Western blot analysis, the expression of NOS isozymes were determined in the kidney isolated from spontaneously hypertensive rats (SHR) and their normotensive control, Wistar-Kyoto rats (WKY). The NOx (nitrite/nitrate) contents were also determined in the kidney and plasma.
Results
The plasma NOx was significantly increased in SHR compared with that in WKY. The basal level of NOx was higher in the medulla and cortex of the kidney in SHR compared with that in WKY rat. bNOS proteins were expressed higher in the outer medulla and cortex, and iNOS proteins were higher in the inner medulla, outer medulla and cortex in SHR. ecNOS expression did not significantly differ between the SHR and WKY.
Conclusions
These results indicate that the NO generation may not be impaired, but rather increased. It is likely that the increased expression of NOS isozymes is a counter-reactive phenomenon secondary to the increased blood pressure in this model of hypertension.
Keywords: Pathogenesis of hypertension, Spontaneously hypertensive rats (SHR), Nitric oxide (NO)
INTRODUCTION
Nitric oxide (NO), produced by the conversion of the terminal guanidino nitrogen atoms from L-arginine, accounts for many of the biological properties of endothelium-derived relaxing factor (EDRF)1). NO synthesis is catalyzed by three distinct forms of nitric oxide synthases (NOS), i.e., brain (bNOS), inducible (iNOS) and endothelial constitutive (ecNOS) isozymes2). The importance of NO in the physiological control of blood pressure is now well established. It causes vascular relaxation by activation of soluble guanylate cyclase and, hence, increasing cyclic guanosine 3′, 5′-monophosphate (cGMP) levels in the smooth muscle3). Therefore, an alteration in NO synthesis may be a significant factor in the pathogenesis of hypertension. A decreased responsiveness to endothelium-dependent vasodilators is characteristically seen in isolated arteries from various models of experimental hypertension4–7).
A large number of studies evidence the capacity of the kidney to produce NO and its relevant role in renal function8–12). It has been demonstrated that the kidney is very sensitive to the reduction of NO, as low doses of NOS inhibitors reduce sodium and water excretion without affecting renal hemodynamics or systemic arterial pressure8). The kidney has an intrinsic mechanism, in which an increased renal perfusion pressure promotes sodium excretion, a phenomenon called pressure natriuresis13). It has been shown that chronic inhibition of NO synthesis impairs sodium excretion, resulting in right shift of the pressure-natriuresis curve and hypertension14). In spontaneously hypertensive rats (SHR), pressure-natriuresis is also blunted15) and papillary blood flow is reduced16). Recently, Larson et al.17) have shown that administration of L-arginine to SHR restores the pressure-dependent increase in renal medullary hemodynamics in association with restoration of pressure-natriuresis. These results suggest that a defective NO pathway may be involved in the blunted pressure-natriuresis. However, the precise status and role of the NO-cGMP pathway in the kidney in SHR is not clear.
The present study was aimed at investigating whether the hypertension is related with an altered activity of NO system in the kidney of SHR. The expression of NOS isozymes and tissue NO levels were determined in the kidney isolated from SHR and their normotensive control Wistar-Kyoto rats (WKY) by Western blot analysis.
MATERIALS AND METHODS
1. Animals
Experiments were carried out using adult (12-week old) male SHR and WKY. Systolic blood pressure was measured in a conscious state by the tail-cuff method. Rats were killed by decapitation. Blood was collected from the trunk, and centrifuged (3,000 rpm for 30 min) for the determination of plasma NO contents. Kidneys were rapidly removed, immediately frozen in liquid nitrogen and stored at −80°C until extraction.
2. Colorimetric Assay of Nitrite/Nitrate
NOx (nitrite/nitrate) contents in the kidney and plasma were measured with a colorimetric nitric oxide assay kit (Oxford). A microplate was used to perform enzyme reactions in vitro. For spectrophotometric assay of nitrite with Griess reagent, 80 ml MOPS (50 mmol/L)/EDTA (1 mmol/L) buffer and 5 μl tissue samples were added to wells. Nitrate reductase (0.01 U) and 10 μl NADH (2 mmol/L) were added to the reaction mixture, and the plate was shaken for 20 minutes at room temperature. Color reagents, sulfanilamide and N-(1-Naphthayl) ehylenediamine dihydrochloride were added, and absorbance values at 540 nm were read in a microtiter plate reader (Bio-Rad model 3550). The concentration of nitrite/nitrate was estimated from a standard curve, which was constructed with the use of standard reagents included in the assay kit.
3. Protein preparation
The cortex, outer medulla and inner medulla from frozen kidney tissues were dissected and were homogenized with Polytron homogenizer at 3,000 rpm in a solution containing 250 mmol/L sucrose, 1 mmol/L EDTA, 0.1 mmol/L phenylmethylsulfonyl fluoride and 50 mmol/L potassium phosphate buffer at pH 7.6. Large tissue debris and nuclear fragments were removed by two consecutive low speed centrifuge spins (3,000g, 5 min; 10,000g, 10 min). The protein concentration of the homogenate was determined by the method of Bradford18), with bovine serum albumin as a standard. In the case of cortex, the membrane-bound protein was further centrifuged at 100,000g for 60 min. The pellet was resuspended for protein blotting of ecNOS and the supernatant was used for blotting of bNOS and iNOS.
4. Western blot analysis
Protein samples were electrophoretically size-separated with a discontinuous system consisting of a 7.5% polyacrylamide resolving gel and 5% polyacrylamide stacking gel. High-range molecular weight markers (Biorad; Hercules, CA, USA) were loaded as size standard. An equivalent amount of total tissue protein (100 μg) was loaded on each lane. After separation, the proteins were electrophoretically transferred to a nitrocellulose membrane at 20 V overnight. The membranes were washed in Tris-based saline buffer (pH 7.4) containing 1% Tween-20 (TBST), blocked with 5% nonfat milk in TBST for one hour and incubated with a 1:2,000 dilution of monoclonal mouse anti-bNOS, anti-ecNOS and anti-iNOS antibodies (Transduction Laboratories; Lexington, KY, USA) in 2% nonfat milk/TBST for one hour at room temperature. The membranes were then incubated with a horseradish peroxidase-labeled goat anti-mouse IgG (1:1,000) or goat anti-rabbit IgG in 2% nonfat milk in TBST for 2 hours. The bound antibody was detected by enhanced chemiluminescence on X-ray film or hyperfilm (Amersham, Little Chalfont, Buckinghamshire, England). The membranes were stripped between incubations with different antibodies in a Tris-buffered solution containing 2% sodium dodecyl sulfate and 100 mmol/L β-mercaptoethanol at 50°C.
5. Statistical analysis
The results are presented as means±SEM. The significance of the differences was analyzed by the Student’s t-test of unpaired data.
RESULTS
1. Blood pressure and plasma NO concentration
Body weight, systolic blood pressure and heart rate in SHR and WKY were summarized in Table 1. Systolic blood pressure and heart rate were higher in SHR compared with those in WKY. The plasma NOx (nitrite/nitrate) concentration was significantly higher in SHR compared with that in WKY (Fig. 1).
Table 1.
Body weight (BW), systolic blood pressure (SBP) and heart rate (HR), in spontaneously hypertensive rats (SHR) and Wistar-Kyoto (WKY)
Values are means±SEM. n=6 in each group.
p<0.05,
p<0.01; compared with WKY.
Fig. 1.
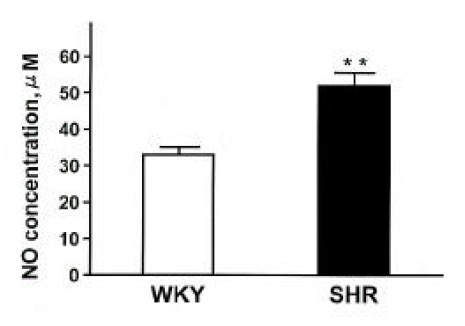
Plasma NOx concentrations (nitrite/nitrate) in SHR and WKY rats. Each column represents mean±SEM from 6 experiments. **p<0.01, compared with control.
2. NOx contents and NOS expression in the kidney
Fig. 2 shows NOx contents in the inner medulla, outer medulla and cortex of the kidney in SHR and WKY. The NOx contents were higher in the outer medulla and cortex of the kidney by about 50% and 60%, respectively, in SHR compared with those in WKY.
Fig. 2.
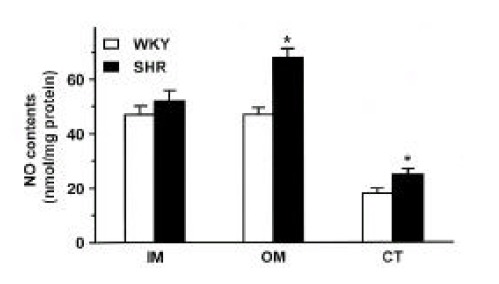
NOx contents in the inner medulla (IM), outer medulla (OM) and cortex (CT) of the kidney in SHR and WKY rats. Eachcolumn represents mean±SEM from 6 experiments. *p<0.05, compared with control.
Three isoforms of NOS (bNOS, iNOS, ecNOS) were determined in the renal cortex and outer and inner medulla of SHR and WKY by Western blot analysis (Fig. 3). Anti-bNOS, anti-iNOS and anti-ecNOS antibodies recognized protein bands with molecular sizes of 155, 130 and 140 kDa, respectively. Fig. 4 shows densitometric analysis of NOS in the kidney. ecNOS protein expression did not significantly differ between SHR and WKY. However, bNOS proteins were expressed higher In the outer medulla and cortex by 80% and 70%, respectively, and iNOS proteins were higher in the inner medulla, outer medulla and cortex by 95%, 135% and 50%, respectively, in SHR.
Fig. 3.
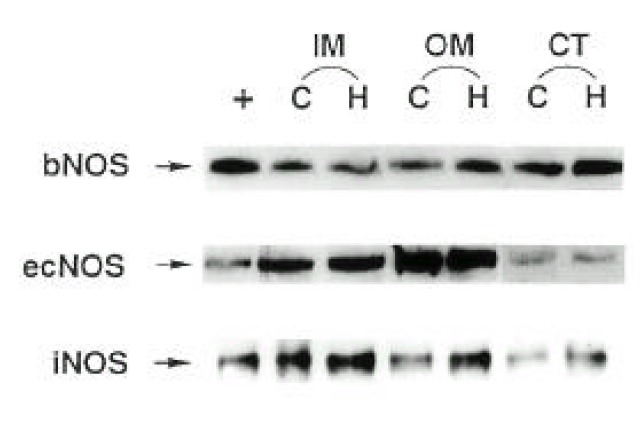
A representative Western blot of total tissue protein (100 μg) extracted from inner medulla (IM), outer medulla (OM) and cortex (CT) of the kidney of SHR (H) and WKY rats (C). Rat pituitary (bNOS), induced macrophage (iNOS) and human endothelial cell (ecNOS) were added as positive control (+) for each NOS isoform.
Fig. 4.
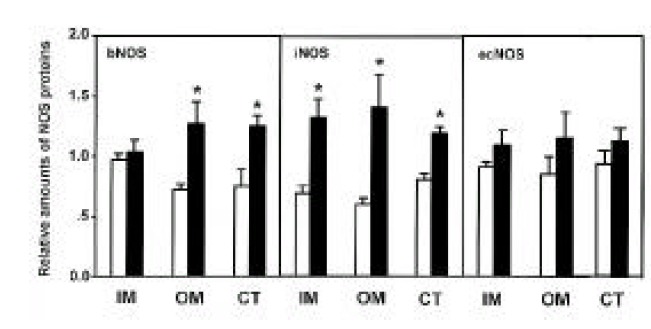
Densitometric analysis of bNOS, iNOS, and ecNOS in inner medulla (IM), outer medulla (OM) and cortex (CT) of the kidney. Open column indicates WKY rats and hatched column indicates SHR. Each point represents mean±SEM from 6 experiments. *p<0.01, compared with control.
DISCUSSION
It is well known that acetylcholine-induced vasodilation is impaired in SHR19–21), however, of which precise mechanisms are not fully understood. A growing number of studies22–25) have demonstrated that NO synthesis may be increased in SHR. It was reported that an inhibition of NOS produced a greater reduction of vasorelaxation to acetylcholine26) and an exaggerated hypertension in SHR27). Furthermore, the present study showed that plasma NOx (nitrite/nitrate) concentration and NOx contents in the kidney were significantly higher in SHR compared with those in WKY. It has been known that the release of NO by endothelial cells can be altered by changes in blood flow28), and their mRNA and protein for ecNOS can be induced by mechanical forces29). The augmented expression of ecNOS may play an important role in the compensatory mechanisms to counteract the hypertension.
The present study showed that renal NOx contents and NOS protein expression in the medulla were higher than in the cortex. These findings are consistent with previous studies revealing the largest signal for constitutive NOS protein expression in the inner medullary collecting duct30), and with the report showing the highest amount of cGMP in the inner medulla, when stimulated with acetylcholine31). These results suggest that the medullary NO system plays a more important role in renal function, such as control of sodium and water excretion.
Tubuloglomerular feedback (TGF) is the critical component of a negative feedback control system that effectively buffers the hemodynamic influences on GFR and stabilizes the amount of NaCl entering the distal nephron32). In the kidney, bNOS protein has been demonstrated exclusively in the macula densa cells, and NO produced in these cells counteract the TGF-mediated vasoconstriction33, 34). This finding indicates that, under normal conditions, NO is one of major determinants of TGF sensitivity. SHR are known to have a sensitive TGF response during development of hypertension35, 36). The exaggerated response could contribute to a lower glomerular filtration and, perhaps, to the development of hypertension37). Thorup and Persson38) compared the effects of intratubular inhibition of NO by [Nω-nitro-L-arginine (L-NNA)] on the TGF between SHR and WKY rats, and showed that L-NNA did not change either sensitivity or the magnitude of the TGF response in SHR, whereas their normotensive control strains responded with a very strong resetting toward higher sensitivity. This finding indicates that NO-mediated vasodilation that counteracts TGF-induced vasoconstriction of the afferent arteriole is less pronounced in SHR than in their normotensive controls. Our study demonstrated that the expression of bNOS protein in renal cortex and outer medulla in SHR was higher than in WKY. Since bNOS in macula densa seems to attenuate the afferent arteriolar constriction induced by the TGF33, 34), enhanced bNOS expression would be expected to increase GFR and sodium excretion which act as a compensatory function opposing the hypertension.
Pressure-natriuresis is associated with significant increase in vasa recta blood flow39). It has been postulated that the mechanism, through which changes in medullary hemodynamics result in pressure-dependent increase in urinary sodium excretion, is due to transmission of pressure from the systemic circulation to the renal interstitium through the renal microcirculation40). In SHR, papillary blood flow in response to increased renal perfusion pressure is reduced, and pressure-natriuresis is blunted41). Hence, the attenuated response of renal medullary hemodynamics to change in renal perfusion pressure has been postulated to be causally linked to the abnormal pressure natriuresis in the SHR41, 42). The restoration of the vasa recta hemodynamic response with L-arginine in SHR supports a role for NO in the regulation of medullary blood flow17). Ikenaga et al43) reported that intravenous administration of L-arginine increase urinary excretion of NO metabolites in normotensive animals, and in SHR to a similiar degree, suggesting that, in the SHR, the ability to synthesize NO is preserved but the renal response to NO is impaired. Our study also demonstrated that the basal production of NO was higher in the kidney in SHR compared with that in WKY. bNOS proteins were expressed higher in the outer medulla and cortex, and iNOS proteins were higher in the inner medulla, outer medulla and cortex in SHR. These results suggest that impaired effect of NO or activation of other vasoconstricting factors may be responsible for the blunted pressure natriuresis in SHR rather than defective NO production.
In conclusion, the NO generation may not be impaired, but rather increased in the kidney of SHR. It is likely that an altered expression of NOS isozymes plays a counter-regulatory role secondary to the hypertension in SHR.
Acknowledgments
This work was supported by research grants from Korea Science and Engineering Foundation(97-0403-09-01-3) and Chonnam University Hospital Research Institute of Clinical Medicine(CUHRI-Y-98003).
REFERENCES
- 1.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 3.Forstermann U, Mulsch A, Bohme E, Busse R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ Res. 1986;58:531–538. doi: 10.1161/01.res.58.4.531. [DOI] [PubMed] [Google Scholar]
- 4.Winquist RJ, Bunting PB, Baskin EP, Wallace AA. Decreased endothelium-dependent relaxation in New Zealand genetic hypertensive rats. J Hypertens. 1984;2:541–545. doi: 10.1097/00004872-198410000-00015. [DOI] [PubMed] [Google Scholar]
- 5.De Mey JG, Gray SD. Endothelium-dependent reactivity in resistance vessels. Prog Appl Microcirc. 1985;8:181–187. [Google Scholar]
- 6.Van De Voorde J, Leusen I. Endothelium-dependent and independent relaxation of aortic rings from hypertensive rats. Am J Physiol. 1986;250:H711–H717. doi: 10.1152/ajpheart.1986.250.5.H711. [DOI] [PubMed] [Google Scholar]
- 7.Otsuka U, Dipiero A, Hirt E, Brennaman B, Lockette W. Vascular relaxation and cGMP in hypertension. Am J Physiol. 1988;254:H163–H169. doi: 10.1152/ajpheart.1988.254.1.H163. [DOI] [PubMed] [Google Scholar]
- 8.Salazar FJ, Alberola A, Pinilla JM, Romero JC, Quesada T. Salt-induced increase in arterial pressure during nitric oxide synthesis inhibition. Hypertension. 1993;22:49–55. doi: 10.1161/01.hyp.22.1.49. [DOI] [PubMed] [Google Scholar]
- 9.Biondi ML, Bolterman RJ, Romero JC. Zonal changes of guanidine 3′,5′-cyclic monophosphate related to endothelium-derived relaxing factor in dog renal medulla. Renal Physiol Biochem. 1992;15:1–7. doi: 10.1159/000173437. [DOI] [PubMed] [Google Scholar]
- 10.Cowley AW, Mattson DL, Lu S, Roman RJ. The renal medulla and hypertension. Hypertension. 1995;25:663–673. doi: 10.1161/01.hyp.25.4.663. [DOI] [PubMed] [Google Scholar]
- 11.Mattson DL, Lu S, Nakanishi K, papanek PE, Cowley AW, Jr : Effect of chronic medullary nitric oxide inhibition on blood pressure. Am J Physiol. 1994;266:H1918–H1926. doi: 10.1152/ajpheart.1994.266.5.H1918. [DOI] [PubMed] [Google Scholar]
- 12.Fenoy FJ, Ferrer P, Carbonell L, Salom MG. Role of nitric oxide on papillary blood flow and pressure natriuresis. Hypertension. 1995;25:408–414. doi: 10.1161/01.hyp.25.3.408. [DOI] [PubMed] [Google Scholar]
- 13.Kaloyanides GJ, DiBona GF, Raskin P. Pressure natriuresis in the isolated kidney. Am J Physiol. 1971;220:1660–1666. doi: 10.1152/ajplegacy.1971.220.6.1660. [DOI] [PubMed] [Google Scholar]
- 14.Mattson DL, Lu SH, Nakanish K, Papanek PE, Cowley AW., Jr Effect of chronic renal medullary nitric oxide inhibition on blood pressure. Am J Physiol. 1994;266:H1918–H1926. doi: 10.1152/ajpheart.1994.266.5.H1918. [DOI] [PubMed] [Google Scholar]
- 15.Roman RJ, Cowley AW. Abnormal pressure-diuresis-natriuresis response in spontaneously hypertensive rats. Am J Physiol. 1985;248:F199–F205. doi: 10.1152/ajprenal.1985.248.2.F199. [DOI] [PubMed] [Google Scholar]
- 16.Roman RJ, Kaldunski ML. Renal cortical and papillary blood flow in spontaneously hypertensive rats. Hypertension. 1988;11:657–663. doi: 10.1161/01.hyp.11.6.657. [DOI] [PubMed] [Google Scholar]
- 17.Larson TS, Lockhart JC. Restoration of vasa recta hemodynamics and pressure natriuresis in SHR by L-arginine. Am J Physiol. 1995;268:F907–912. doi: 10.1152/ajprenal.1995.268.5.F907. [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. 1976. [DOI] [PubMed] [Google Scholar]
- 19.Ito S, Carretero OA. Impaired response to acetylcholine despite intact endothelium-derived relaxing factor/nitric oxide in isolated microperfused afferent arterioles of the spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1992;20(Suppl 12):S187–S189. doi: 10.1097/00005344-199204002-00052. [DOI] [PubMed] [Google Scholar]
- 20.Angus JA, Dyke AC, Jennings GL, Korner PI, Eudhir K, Ward JE, Wright CE. Release of endothelium-derived relaxing factor from resistance arteries in hypertension. Kidney Int Suppl. 1992;37:S73–78. [PubMed] [Google Scholar]
- 21.Tschudi MR, Mesaros S, Uischer TF, Malinski T. Direct in situ measurement of nitric oxide in mesenteric resistance arteries. Increased decomposition by superoxide in hypertension. Hypertension. 1996;27:32–35. doi: 10.1161/01.hyp.27.1.32. [DOI] [PubMed] [Google Scholar]
- 22.Wu CC, Hong HJ, Chou TC, Ding YA, Yen MH. Evidence for inducible nitric oxide synthase in spontaneously hypertensive rats. Biochem Biophys Res Commun. 1996;228:459–466. doi: 10.1006/bbrc.1996.1682. [DOI] [PubMed] [Google Scholar]
- 23.Yen MH, Liu YC, Hong HJ, Sheu JR, Wu CC. Role of nitric oxide in lipopolysaccharide-induced mortality from spontaneously hypertensive rats. Life Sci. 1997;60:1223–1239. doi: 10.1016/s0024-3205(97)00066-0. [DOI] [PubMed] [Google Scholar]
- 24.Junquero DC, Shini VB, Scott Burden T, Vanhoutte PM. Enhanced production of nitric oxide in aortae from spontaneously hypertensive rats by interleukin-1 beta. Am J Hypertens. 1993;6:602–610. doi: 10.1093/ajh/6.7.602. [DOI] [PubMed] [Google Scholar]
- 25.Nava E, Llinas MT, Gonzalez JD, Salazar FJ. Nitric oxide synthase activity in renal cortex and medulla of normotensive and spontaneously hypertensive rats. Am J Hypertension. 1996;9:1236–1239. doi: 10.1016/s0895-7061(96)00325-1. [DOI] [PubMed] [Google Scholar]
- 26.Lee L, Webb RC. Endothelium-dependent relaxation and L-arginine metabolism in genetic hypertension. Hypertension. 1992;19:435–441. doi: 10.1161/01.hyp.19.5.435. [DOI] [PubMed] [Google Scholar]
- 27.Lacolley PJ, Lewis SJ, Brody MJ. L-NG-nitro arginine produces an exaggerated hypertension in anesthetized SHR. Eur J Pharmacol. 1991;197:239–240. doi: 10.1016/0014-2999(91)90533-v. [DOI] [PubMed] [Google Scholar]
- 28.Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991;17:187–193. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- 29.Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- 30.Terada Y, Tomita K, Nonoguchi H, Marumo F. Polymerase chain reaction localization of constitutive nitric oxide synthase and soluble guanylate cyclase messenger RNAs in microdissected rat nephron segments. J Clin Invest. 1992;90:659–665. doi: 10.1172/JCI115908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biondi ML, Bolterman RJ, Romero JC. Zonal changes of guanidine 3′,5′-cyclic monophosphate related to endothelium-derived relaxing factor in dog renal medulla. Renal Physiol Biochem. 1992;15:16–22. doi: 10.1159/000173437. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox CS, Welch WJ. TGF and nitric oxide: effects of salt intake and salt-sensitive hypertension. Kidney Int Suppl. 1996;55:S9–S13. [PubMed] [Google Scholar]
- 33.Braam B, Koomans HA. Nitric oxide antagonizes the actions of angiotensin II to enhance tubuloglomerular feedback responsiveness. Kidney Int. 1995;48:1406–1411. doi: 10.1038/ki.1995.429. [DOI] [PubMed] [Google Scholar]
- 34.Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, Schmidt HH. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci USA. 1992;89:11993–11997. doi: 10.1073/pnas.89.24.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dilley JR, Arendshorst WJ. Enhanced tubuloglomerular feedback activity in rats developing spontaneous hypertension. Am J Physiol. 1984;247:F672–F679. doi: 10.1152/ajprenal.1984.247.4.F672. [DOI] [PubMed] [Google Scholar]
- 36.Dilley JR, Stier CT, Arendshorst WJ. Abnormalities in glomerular function in rats developing spontaneous hypertension. Am J Physiol. 1984;246:F12–20. doi: 10.1152/ajprenal.1984.246.1.F12. [DOI] [PubMed] [Google Scholar]
- 37.Ushiogi Y, Haberle DA. Hyperreactivity of tubuloglomerular feedback in chronically salt-loaded spontaneous hypertensive rats. Kidney Int Suppl. 1991;32:S142–S147. [PubMed] [Google Scholar]
- 38.Thorup C, Persson AE. Impaired effect of nitric oxide synthesis inhibition on tubuloglomerular feedback in hypertensive rats. Am J Physiol. 1996;271:F246–F252. doi: 10.1152/ajprenal.1996.271.2.F246. [DOI] [PubMed] [Google Scholar]
- 39.Farrugia E, Lockhart JC, Larson TS. Relation between vasa recta blood flow and renal interstitial hydrostatic pressure during pressure natriuresis. Circ Res. 1992;71:1153–1158. doi: 10.1161/01.res.71.5.1153. [DOI] [PubMed] [Google Scholar]
- 40.Knox FG, Mertz JI, Burnett JC, Haramati A. Role of hydrostatic and oncotic pressures in renal sodium reabsorption. Circ Res. 1983;52:491–500. doi: 10.1161/01.res.52.5.491. [DOI] [PubMed] [Google Scholar]
- 41.Roman RJ, Cowley AW, Garcia-Estan J, Lombard JH. Pressure diuresis in volume expanded rats. Cortical and medullary hemodynamics. Hypertension. 1988;12:168–176. doi: 10.1161/01.hyp.12.2.168. [DOI] [PubMed] [Google Scholar]
- 42.Gebremedhin D, Fenoy FJ, Harder DR, Roman RJ. Enhanced vascular tone in the renal vascularture of spontaneously hypertensive rats. Hypertension. 1990;16:648–654. doi: 10.1161/01.hyp.16.6.648. [DOI] [PubMed] [Google Scholar]
- 43.Ikenaga H, Suzuki H, Ishii N, Saruta I. Role of NO on pressure-natriuresis in Wista-Kyoto and spontaneously hypertensive rats. Kidney Int. 1993;43:205–211. doi: 10.1038/ki.1993.33. [DOI] [PubMed] [Google Scholar]


