Abstract
Objectives
Clinical presentation of Helicobacter pylori (H. pylori) infection has marked variation mainly due to the strain diversity and host susceptibility. Although H. pylori is identified as a major risk factor for gastric and duodenal ulcers, the ulcerogenic or pathogenic strain has not been documented yet. The objective of this study was to investigate antigenic types of the ulcerogenic strain of H. pylori.
Methods
The sera of 64 patients were tested by Western blot using Helicoblot 2.0 for six major anti-H. pylori antibodies, together with CLO test and histological examination of gastric biopsy tissues. Thirty-five, nine and 20 patients had duodenal ulcer, gastric ulcer and chronic active gastritis, respectively. The antigenic types of H. pylori were analyzed in 54 patients with positive H. pylori infection. In this study, H. pylori was divided into four serotypes according to the presence and absence of CagA and VagA: type I; CagA(+) and VacA(+), type Ia; CagA(+) and VacA(−), type Ib; CagA(−) and VacA(+), and type II; CagA(−) and VacA(−).
Results
There was no difference in the number of bands for six antigens: 3.2 ± 1.4, 3.0±1.2 and 3.1±1.4 in 35 duodenal ulcer, 7 gastric ulcer and 12 chronic gastritis, respectively. The band with 119 kDa was 90.7%, which was the most common band with the order of 35, 30, 26.5, 89 and 19.5 kDa. Type I, Ia and Ib were positive in 22.2, 42.6 and 27.8%, respectively, which were significantly higher than type II (p <0.05). There was no difference in the positive rates of four urease subtypes between the four serotypes.
Conclusions
H. pylori had the marked diversity in antigenic patterns. The strain producing CagA and/or VagA was suggested to be a pathogenic strain.
Keywords: Helicobacter pylori, Western blot, Ulcerogenic strains
INTRODUCTION
A number of studies has revealed that Helicobacter pylori (H. pylori) infection is strongly associated with the pathogenesis of a variety of gastroduodenal diseases, such as chronic active gastritis, gastric atrophy, intestinal metaplasia, gastric ulcer, duodenal ulcer, lymphoreticular hyperplasia, mucosa-associared lymphoid tissue (MALT) lymphoma and adenocarcinoma, etc1–3). It is now well known that clinical presentation of H. pylori infection is diverse within a single population as well as between different populations4, 5). Practically speaking, only a small proportion of infected persons develop peptic ulcer disease or adenocarcinoma, although all infected persons have chronic active gastritis, histologically. Moreover a great portion of persons with chronic gastritis caused by H. pylori infection have no specific subjective symptoms. On the other hand, the regimens used for eradication of H. pylori have two major adverse effects, side effects of drugs and emergence of drug resistant strains. Therefore, all infected persons are not thought to be indicated for eradication of H. pylori6). In consequence, it is of great importance to select persons to be eradicated. In order to select those persons, many attempts have been made to identify pathogenic or ulcerogenic strains of H. pylori7, 8).
Xiang et al.9) divided H. pylori into two categories, type I and II, by the presence and absence of two antigens, CagA and vacuolating cytotoxin (VacA). Type I strain has the gene coding for both cagA and vacA gene, with the consequence of production of CagA and VacA protein. Type II is the strain that does not produce the CagA and VacA. Type I H. pylori was reported to be more prevalent in patients with duodenal ulcer than persons without ulcer7). In addition, the erosive lesions in gastric mucosa were reported to develop by the infection of type I, not type II, in mice10). These results strongly suggest that type I strain is a pathogenic strain. However, clinical presentation is not always consistent with this classification. This study was conducted to investigate the serotypes of H. pylori associated with the development of peptic ulcer. In this study, we examined six antibodies to H. pylori antigens in the sera of patients with peptic ulcer and gastritis by western blot and analyzed the antigenic patterns based on the presence of antibody to VagA and CagA.
MATERIALS AND METHODS
Materials
Gastric mucosal biopsy tissues and sera were obtained from a total of 64 patients (M:F=42:22) with dyspeptic symptoms treated in the Department of Internal Medicine, Asan Medical Center, University of Ulsan. Thirty-five patients had duodenal ulcer, nine had gastric ulcer and 20 had chronic superficial gastritis (Table 1). In 20 patients with chronic superficial gastritis, six had gastric atrophy and two had gastric erosions. There was no difference in age and gender between duodenal ulcer, gastric ulcer and chronic gastritis. Gastric mucosal tissues were obtained by gastroscopy from the two sites, the greater curvature of mid-antrum and mid-body, and tested for H. pylori infection by rapid urease test using CLO test kit (Delta-West, Western Australia) and histological examination with H&E stain.
Table 1.
Age, gender and positive rate for H. pylori in 64 patients
| DU (n=35) | GU (n=9) | CAG (n=20) | |
|---|---|---|---|
| Median age (range) | 45(23–61) | 48(25–65) | 42(29–59) |
| Gender (M:F) | 21:14 | 5:4 | 8:12 |
| Positive rate for H. pylori | |||
| by CLO and histological exam | 35(100)* | 7(77.8) | 12(60.0) |
| by Western blot analysis | 35(100) | 9(100) | 15(75.0) |
DU, duodenal, GU, gastric ulcer, CAG, chronic active gastritis
number of patients (%)
Western blot analysis
Sera obtained from 64 patients were stored at −20°C until experiments. The IgG antibodies to six major antigens of H. pylori were tested using Helicoblot 2.0 (Genelab diagnostics, Singapore). Briefly, the antigens were separated electrophoretically from the lysate of H. pylori isolated from the patients with peptic ulcer. The antigens tested were CagA (119 kDa), VacA (89 kDa) and four urease subunits (35, 30, 26.5, and 19.5 kDa). After reacting sera with the antigens on the nitrocellulose strip, the reactive bands were visualized by adding anti-human IgG antibody conjugated with alkaline phosphatase and substrate, 5-bromo-4-chloro-3-indoyl-phosphate/nitroblue tetrazolium.
The criteria for the presence of H. pylori were any one of bands at 119, 89 and 35 kDa with any two bands among 30, 26.5 and 19.5 kDa. In this study, H. pylori was divided into four types according to the presence and absence of CagA and VagA: Type I and II were the strain positive for both 119 and 89 kDa and the one negative for both antigens, respectively. Type Ia and Ib were the intermediate types: type Ia; 119 (+) and 89 (−), and type Ib; 119 (−) and 89 (+) (Fig. 1).
Fig. 1.
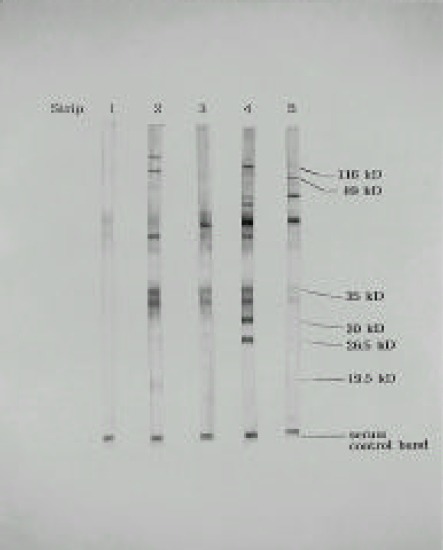
Representative results of Western blot analysis for negative control, type I, II, Ia, and Ib. Type I and II were positive for both 119 and 89 kDa and the one negative for both antigens, respectively. Type Ia and Ib were the intermediate types: type Ia; 119 (+) and 89 (−), and type Ib; 119 (−) and 89 (+).
Statistical analysis
The statistical significance of difference was determined by chi-square test. The difference was considered to be significant when the p value was less than 0.05.
RESULTS
The results of CLO tests, histological examinations and Western blot analysis were summarized in Table 2. The sensitivity and specificity of Western blot analysis were 91.5 and 100%, respectively, when CLO test and histological examination were used as the reference method. Antigenic patterns were analyzed in 54 patients who were confirmed to be positive for H. pylori infection by CLO test and histological examination. The mean number of reactive bands for six antigens were 3.2 ± 1.4, 3.0±1.2 and 3.1±1.4 in 35 duodenal ulcer, seven gastric ulcer and 12 chronic gastritis, respectively, no difference being observed between duodenal ulcer, gastric ulcer and chronic active gastritis (Table 3). The band with 119 kDa was 90.7% which was the most common band with the order of 35, 30, 26.5, 89 and 19.5 kDa. No significant difference in the positive rates for six antibodies was observed between duodenal ulcer, gastric ulcer and chronic active gastritis (Table 4).
Table 2.
Comparison of the results of CLO test and histological examination with those of Western blot analysis in the diagnosis of H. pylori infection
| CLO test and histological examination | |||
|---|---|---|---|
| positive | negative | total | |
| Western blot analysis | |||
| positive | 54* | 5 | 59 |
| negative | 0 | 5 | 5 |
|
| |||
| Total | 54 | 10 | 64 |
number of patients
CLO test and histological examination were used as the reference method in this analysis. Western blot analysis was performed for six major antibodies to H. pylori using Western blot 2.0 as described in Materials and Methods.
Table 3.
The frequency of bands reactive to six major antigens of H. pylori in sera obtained from 54 patients with H. pylori infection
| No. of positive bands | DU (n=35) | GU (n=7) | CAG (n=12) |
|---|---|---|---|
| 1 | 4* | 1 | 0 |
| 2 | 5 | 1 | 3 |
| 3 | 12 | 2 | 4 |
| 4 | 5 | 3 | 2 |
| 5 | 8 | 0 | 2 |
| 6 | 1 | 0 | 1 |
|
| |||
| mean±SD** | 3.2±1.4 | 3.0±1.2 | 3.1±1.4 |
DU, duodenal ulcer, GU, gastric ulcer, CAG, chronic active gastritis
number of patients
mean number of bands±SD
Table 4.
The frequency of bands for six major antibodies to H. pylori proteins in 54 patients with H. pylori infection
| Diseases | molecular weight (Kd) of H. pylori antigens
|
|||||
|---|---|---|---|---|---|---|
| 119 | 89 | 35 | 30 | 26.5 | 19.5 | |
| DU (n=35) | 31* (88.6) | 17 (48.6) | 28 (80.0) | 16 (45.7) | 18 (51.4) | 5 (14.3) |
| GU (n= 7) | 7 (100) | 2 (28.6) | 5 (71.4) | 6 (85.7) | 3 (42.9) | 1 (14.3) |
| CAG (n=12) | 11 (91.7) | 5 (41.7) | 11 (91.7) | 8 (66.7) | 4 (33.3) | 3 (25.0) |
|
| ||||||
| Total (n=54) | 49 (90.7) | 24 (44.4) | 44 (81.5) | 30 (55.6) | 25 (46.3) | 9 (16.7) |
DU, duodenal ulcer, GU, gastric ulcer, CAG, chronic active gastritis Bands were determined by Western blot analysis using Western blot 2.0.
number of patients (%)
The patients were analyzed according to the serotype of H. pylori (Table 5). Type I, Ia and Ib were positive in 22.2, 42.6 and 27.8%, respectively, which were significantly higher than 7.4% in type II (p <0.05, p<0.01 and p<0.01, respectively) (Table 5). There was no statistical difference in the positive rates for these four serotypes among duodenal ulcer, gastric ulcer and chronic active gastritis. To examine the effects of four urease subunits on the virulence of H. pylori, we analyzed the positive rates of the bands with 35, 30, 26.5 and 19.5 kDa according the four serotypes. No difference in the positive rates of the four bands was observed among these four serotypes.
Table 5.
The frequency of H. pylori serotype in 54 patients with H. pylori infection
| Diseases | Serotypes of H. pylori | ||||
|---|---|---|---|---|---|
| Type I | Type II | Type Ia | Type Ib | ||
| DU | n=35 | 11(31.4)* | 2(5.7) | 14(40.0) | 8(22.9) |
| GU | n= 7 | 0(0.0) | 1(14.3) | 5(71.4) | 1(14.3) |
| CAG | n=12 | 1(8.3) | 1(8.3) | 4(33.4) | 6(50.0) |
|
| |||||
| Total | n=54 | 12(22.2) | 4(7.4) | 23(42.6) | 15(27.8) |
DU, duodenal ulcer, GU, gastric ulcer, CAG, chronic active gastritis
number of patients(%)
DISCUSSION
It is a common clinical observation that the diseases produced by H. pylori infection and their natural histories have marked variations even within a single population. Knowledge accumulated on the pathogenic role of H. pylori in the development of gastroduodenal diseases has been greatly advanced by the understanding of different cytotoxicity between H. pylori strains7, 8).
Previous studies have suggested that the factors contributed to the pathogenesis are classified as bacterial factors and host factors11, 12). Bacterial virulent factors reported so far are VacA, CagA, urease activity, motility, adhesins and sensitivity to acid, etc., while the major host factors for susceptibility are the immunity and local acid output. Although the strain diversity and host susceptibility are at present thought to be the main mechanism of the diverse clinical presentation of H. pylori infection13), the exact mechanisms have not yet been clarified. This study was conducted to investigate the ulcerogenic stains of H. pylori. In the present study, we were not able to include the normal control in the analysis for antigenic diversity, because it proved that all infected stomachs had gastritis, histologically. Therefore, we compared the results of peptic ulcer with those of gastritis.
The mechanism by which ulcer develops is, at present, generally accepted to be the imbalance of aggressive factors and defensive factors on the gastric mucosae. The fundamental mucosal pathology of H. pylori-associated duodenal ulcer and gastric ulcer is thought to be active gastritis and infection of gastric metaplasia in duodenal bulb, respectively. Strictly speaking, H. pylori does not directly cause gastric ulcer and duodenal ulcer, but indirectly through inflammation14).
After colonization of H. pylori in the gastric mucosa, the host response, such as local mucosal reaction and systemic immunoglobulins reactive to antigens of H. pylori, develops in nearly all infected persons15). CagA, VacA, urease, adhesin and flagellin are known to be the major antigens of H. pylori16).
Among them, urease, adhesin and flagellin are essential for colonization and are called constant factors for H. pylori infection. On the other hand, some antigens, more frequently detected in H. pylori isolated from the patients with peptic ulcer than persons without ulcer, are called variable factors, which are closely related to the diversity of bacterial virulence17–19).
In this study, we used Western blot analysis to diagnose H. pylori infection serologically using six major antigens of H. pylori. Therefore, the results of the present study also provides the additional information on the serological profiles for six H. pylori proteins. In order to determine the pathogenic or ulcerogenic strains of H. pylori, we compared the antibody patterns between patients with duodenal ulcer, gastric ulcer and chronic gastritis. At first, we classified H. pylori into four types, I, Ia, Ib and II. The results showed that type Ia was 43%, the most common type, with the order of type Ib, I and II, which was a little inconsistent with the results of Xiang et al.9). In this study, the positive rate for type I, Ia and Ib was significantly higher than that of type II, suggesting that the strain producing CagA and/or VagA might be more pathogenic than the strain without CagA and VagA.
The band reactive to CagA was detected in 90.7%, which might be a distinct finding of Korean H. pylori. In an agreement with this result, Miehlke et al.20) reported that all Korean H. pylori expressed CagA, while it was higher than the previous studies demonstrating that only 60% of H. pylori strains isolated had the cagA gene.
Similar to the previous reports21, 22), Western blot analysis has the high sensitivity and specificity for the diagnosis of H. pylori infection, suggesting that the immune system recognizes H. pylori antigens easily and responds to these antigens. In five patients with positive results by Western blot, H. pylori was not detected by both CLO test and histological examination. One of the possible explanation for these cases is that Western blot detects both present and past infection, whereas CLO test and histological examination detect only the present infection. Another possible explanation was the cross reaction with serum antibodies to the antigens commonly existing in other bacteria23).
In this study, we confirmed that H. pylori had marked diversity in antigenic patterns. We have also demonstrated that the incidence of type I and intermediate types, which are positive for CagA and/or VacA, are higher than that of type II, suggesting that the strain producing CagA and/or VagA may be a pathogenic strain, if the opportunity of infection is not different between the serotypes. However, in order to draw a conclusion on the pathogenic or ulcerogenic serotypes, further studies on virulent factors and host response should be conducted concomitantly.
Table 6.
Positive rate (%) for four major antibodies to H. pylori proteins in four serotypes of H. pylori
| Molecular weight (kDa) of H. pylori protein | |||||
|---|---|---|---|---|---|
| 35 | 30 | 26.5 | 19.5 | ||
| Type I | n=12 | 90 | 55 | 45 | 20 |
| Type II | n= 4 | 100 | 25 | 100 | 0 |
| Type Ia | n=23 | 77 | 54 | 42 | 12 |
| Type Ib | n=15 | 60 | 60 | 60 | 40 |
REFERENCES
- 1.Blaser MJ. Helicobacter pylori: Its role in disease. Clin Infec Dis. 1992;15:386–391. doi: 10.1093/clind/15.3.386. [DOI] [PubMed] [Google Scholar]
- 2.Rauws EAJ, Tytgat GNJ. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990;335:1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- 3.Isaacson PG. Gastric lymphoma and Helicobacter pylori. N Engl J Med. 1994;330:1310–1311. doi: 10.1056/NEJM199405053301812. [DOI] [PubMed] [Google Scholar]
- 4.Cellini L, Allocati N, Campli ED, Masulli M, Bartolomeo SD, Dainelli B. Helicobacter pylori isolated from stomach corpus and antrum: comparison of DNA patterns. J infect. 1996;32:219–221. doi: 10.1016/s0163-4453(96)80022-3. [DOI] [PubMed] [Google Scholar]
- 5.Shortridge VD, Stone GG, Flamm RK, Beyer J, Versalovic J, Graham DW, Tanaka SK. Molecular typing of Helicobacter pylori isolates from a multicenter U.S. clinical trial by ureC restriction fragment length polymorphism. J Clin Microbiol. 1996;35:471–473. doi: 10.1128/jcm.35.2.471-473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NIH Consensus Conference: Helicobacter pylori in peptic ulcer disease. NIH Consensus Conference Development Panel on Helicobacter pylori in Peptic ulcer Disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 7.Blaser MJ. Helicobacter pylori phenotypes associated with peptic ulceration. Scand J Gastroenterol. 1994;29(Suppl 205):1–5. [PubMed] [Google Scholar]
- 8.Newell DG. Virulence factors of Helicobacter pylori. Scand J Gastroenterol. 1991;26(Suppl 187):31–38. [PubMed] [Google Scholar]
- 9.Xiang Z, Censini S, Bayeli PF, Telford JL, Figura N, Rappuoli R, Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major. Infect Immun. 1995;63:94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 11.Ito A, Fujioka T, Kubota T, Nasu M. Molecular typing of Helicobacter pylori: Differences in pathogenicity among diverse strains. J Gastroenterol. 1996;31:1–5. doi: 10.1007/BF01211179. [DOI] [PubMed] [Google Scholar]
- 12.Sharma SA, Miller GG, P-P GI, Gupta RS, Blaser MJ. Humoral and cellular immune recognition of Helicobacter pylori proteins are not concordant. Clin Exp Immunol. 1994;97:126–132. doi: 10.1111/j.1365-2249.1994.tb06590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Telford JL, Ghiara P, Dell’Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce MF, Censini S, Covacci A, Xiang Z, Papini E, Montecucco C, Parente L, Rappuoli R. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994;179:1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ando T, Kusugami K, Ohsuga M, Shinoda M, Sakakibara M, Saito H, Fukatsu A, Ichiyama S, Ohta M. Interleukin-8 activity correlates with histological severity in Helicobacter pylori-associated antral gastritis. Am J Gastroenterol. 1996;91:1150–1156. [PubMed] [Google Scholar]
- 15.Engstrand L, Gustavsson S, Schwan A, Scheynius A. Local and systemic immune response in Helicobacter pylori-associated chronic gastritis before and after treatment. Scand J Gastroenterol. 1993;28:1105–1111. doi: 10.3109/00365529309098317. [DOI] [PubMed] [Google Scholar]
- 16.Telford JL, Covacci A, Ghiara P, Montecucco C, Pappuoli R. Unravelling the pathogenic role of Helicobacter pylori in peptic ulcer: potential new therapies and vaccines. Science. 1994;12:420–426. doi: 10.1016/0167-7799(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 17.Cover TL, Dooley CP, Blaser MJ. Characterization of and human serologic reponse to proteins in Helicobacter pylori broth culture supernatants with vacuolizating cytotoxin activity. Infect Immun. 1990;58:603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakita H, Hirai M, Ito S, Azuma T, Kato T, Kohli Y, Kuriyama M. Cytotoxin and urease activities of Helicobacter pylori isolates from Japanese patients with atrophic gastritis or duodenal ulcer. J Gastroenterol & Hepatol. 1996;11:819–824. doi: 10.1111/j.1440-1746.1996.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 19.Crabtree JE, Covacci A, Farmery SM, Xiang Z, Tompkins DS, Perry S, Lindley IJD, Rappuoli R. Helicobacter pylori induced interleukin-8 expression in gastric epithelial cells is associated with CagA positive phenotype. J Clin Pathol. 1995;48:41–45. doi: 10.1136/jcp.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miehlke S, Kibler K, Kim JG, figura N, Small SM, Graham DY, Macg, Go MF. Allelic variation in the cagA gene of Helicobacter pylori obtained from Korea compared to the United States. Am J Gastroenterol. 1996;91:1322–1325. [PubMed] [Google Scholar]
- 21.Nilsson I, Ljungh A, Aleljung P, Wadstrom T. Immunoblot assay for serodiagnosis of Helicobacter pylori infections. J Clin Microbiol. 1997;35:427–432. doi: 10.1128/jcm.35.2.427-432.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cover T, Glupczynski Y, Lage AP, Burette A, Tummuru MKR, P-Perez GI, Blaser MJ. Serologic detection of infection with cagA+ Helicobacter pylori strains. J Clin Microbiol. 1995;33:1496–1500. doi: 10.1128/jcm.33.6.1496-1500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen LP, Espersen F. Immunoglobulin G antibodies to Helicobacter pylori in patients with dyspeptic symptoms investigated by the Western immunoblot technique. J Clin Microbiol. 1992;30:1743–1751. doi: 10.1128/jcm.30.7.1743-1751.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


