Abstract
Objective
To evaluate the patterns of Ro autoantigen recognition in Korean patients with primary Sjogren’s syndrome (SS) and to investigate its clinical significance in SS.
Methods
Sera from primary SS (n=51) and systemic lupus erythematosus (SLE) (n=132) were tested by double immunodiffusion test and immunoblotting for reactivity with 60kDa and 52kDa Ro/SS-A proteins. Clinical manifestations were evaluated on the basis of the presence of anti-Ro/SS-A antibodies and anti-60 kDa/52kDa proteins.
Results
The prevalence of anti-Ro/SS-A antibodies in Korean patients with primary SS was 64.7%. In immunoblotting analysis, the incidence of anti-60kDa without anti-52kDa was lower in patients with SS(3.0% vs. 11.6%, p>0.05), whereas anti-52kDa without anti-60kDa was more common in SS patients than in SLE patients(42.5% vs. 4.3%, p<0.001). Patients with anti-Ro/SS-A antibody were significantly associated with the presence of vasculitis, hyperglobulinemia and rheumatoid factor in primary SS (p<0.05).
Conclusion
The patterns of 52kDa and 60kDa Ro autoantigen recognition were quite different in the SLE and primary SS. Anti-52kDa without anti-60kDa antibody may be used as a diagnostic marker for primary SS. Although the presence of anti-Ro/SS-A antibody was closely associated with certain clinical features in SS, these clinical manifestations were not correlated with the presence of antibodies against each 52kDa and 60kDa proteins. Extended studies with a large population are required to determine the clinical correlation of autoantibodies against each peptides or epitopes of Ro/SS-A proteins.
Keywords: Sjogren syndrome, Systemic lupus erythematosus, anti-Ro antibody, 52kDa and 60kDa Ro proteins
INTROCUCTION
Anti-Ro/SS-A antibodies are found in 35 to 60% of active and/or subacute systemic lupus erythematosus (SLE) and 60–70% of patients with primary Sjogren syndrome (SS)1, 2).
Anti-Ro/SS-A antibody in these diseases has been claimed to be involved in the pathogenesis of certain clinical features3). However, the mechanism by which this antibody lead to tissue injury is unknown. Anti-Ro/SS-A antibodies have classically been identified by immunodiffusion in agarose gel. Using immunoprecipitation and immunoblot analysis, anti-Ro/SS-A antibodies were shown to target 60kDa and 52kDa Ro/SS-A proteins.
Previous studies in enzyme-linked immunosorbent assary (ELISA) and immunoblotting assay have described different patterns of anti-60kDa and anti-52kDa Ro responses between patients with primary SS and patients with SLE. In the present study, we evaluate the antibody responses of 60kDa and 52kDa Ro proteins and their clinical significances in Korean patients with primary SS.
PATIENTS AND METHODS
1. Patients
Fifty-one patients with primary SS and 132 patients with SLE who were seen at the in Rheumatology clinic of Kangnam St. Mary’s Hospital were studied. Patients were classified as having primary SS if they met at least 3 of the Fox criteria8).
The diagnosis of SLE was established by the criteria of the American College of Rheumatology9).
The mean age of SS and SLE patients was 41 years (21–65 years) and 32 years (14–53 years) respectively. Clinical data were obtained by review of hospital records.
2. Double immunodiffusion
Antibodies to Ro/SS-A antigens were identified by double immunodiffusion method (DID) using bovine spleen extract as antigen (Immunovision, Springdale, AR)
3. Immunoblotting analysis
The Hela cell extract was separated on 10% polyacrylamide gels by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane using standard immunoblotting methods10). The nitrocellulose strips were incubated with a 1:500 dilution of serum for 1 hour. Horseradish peroxidase conjugated goat anti-human IgG (Bio-Rad Laboratories, Richmond, CA) was used as the second antibody.
4. Statistical analysis
Chi-square testing with Yates’ correction was used to evaluated the frequency of antibody to 60kDa and 52kDa Ro proteins in patients with primary SS and SLE and to investigate the correaltion between clinical manifestation and autoantibodies. P values less than 0.05 were considered significant.
RESULTS
1. Frequency of antibodies anainst 52kDa and 60kDa Ro/SS-A antigen
The frequency of anti-Ro/SS-A antibody positivity of the primary SS and SLE patients is listed in Table 1. Anti-Ro/SS-A antibody detected by double immunodiffusion method was 64.7% in primary SS and 52.3% in SLE, respectively. Anti-La/SS-B antibodies were noted in 29.4% of sera with primary SS and 12% of sera with SLE, respectively.
Table 1.
Frequencies of Antibodies to Ro Protein and La Protein in Patients with Primary SS and SLE
| primary SS | SLE | |
|---|---|---|
| Immunodiffusion | n=51 | n=132 |
| anti-Ro/SS-A | 33(64.7%) | 69(52.3%) |
| anti-La/SS-B | 15(29.4%) | 16(12.0%) |
|
| ||
| Immunoblotting | n=33 | n=69(%) |
| 60kDa(+)/52kDa(+) | 13(39.5%) | 40(58.0%) |
| 60kDa(+)/52kDa(−) | 1(3.0%) | 8(11.6%) |
| 60kDa(−)/52kDa(+) | 14(42.5%)* | 3(4.3%) |
| 60kDa(−)/52kDa(−) | 5(15.0%) | 18(26.0%) |
p<0.001, ods ratio: 16.21, Fisher exact test was performed
To determine the pattern of antibody response against Ro/SS-A antigen, anti-Ro/SS-A positive sera in double immunodiffusion test were studied by immunoblot analysis. The pattern of reactivity to denatured 60kDa and 52kDa Ro antigen is shown in Table 1. Out of the 33 primary SS patients, 13 (39.5%) patients showed positivity to both proteins, whereas only one (3%) had antibodies to 60kDa antigen only. Fourteen (42.5%) of the patients with primary SS had antibodies to 52kDa protein only, and 5 (15%) had no reactivity to 52kDa or 60kDa proteins. In patients with SLE, forty (58%) sera reacted with both 60kDa and 52kDa proteins, eight (11.6%) reacted with 60kDa protein only.
Antibody to 52-kDa protein only was detected in 3 sera (4.3%), while 18 (26%) were nonreactive to both the proteins. The incidence of anti-52kDa without anti-60kDa was more common in patients with primary SS than in those with SLE (42.5% vs. 4.3%, p<0.001).
2. Correlation between antibody profile and clinical features in patients with primary SS
On the basis of association between anti-Ro/SS-A antibody and clinical manifestations of Primary SS, as shown in Fig. 1, anti-Ro/SS-A antibody positive group was closely associated with the presence hyperglobulinemia (17/33 vs. 2/18, p=0.004), rheumatoid factor positivity (26/33 vs. 8/18, p=0.029) and anti-La/SS-B antibody positivity (13/33 vs. 2/18, p=0.012) and cutaneous vasculitis (7/33 vs. 0/18, p=0.036).
Fig. 1.
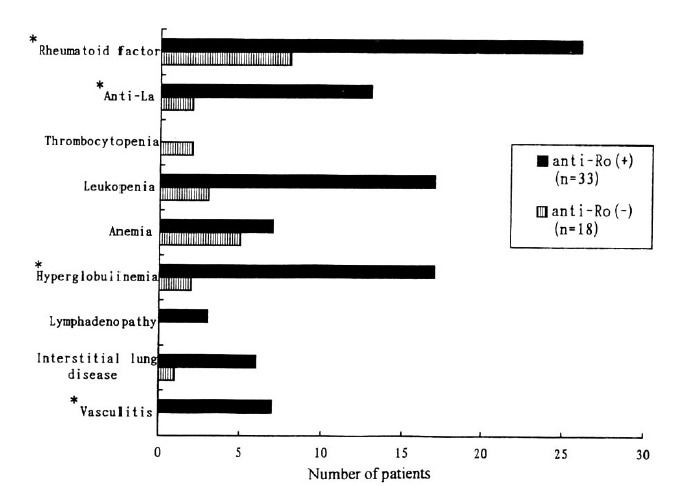
Comparision of the clinical manifestations in anti-Ro positive versus anti-Ro negative primary SS. *p<0.05
However, no significant differences could be demonstrated between anti-52kDa only positive group and both anti-60 and 52kDa positive group, which are the two major antibodies to Ro/SS-A antigen regarding the prevalence of hyperglobulinemia (7/14 vs. 5/13), rheumatoid factor positivity (11/14 vs. 12/13) or cutaneous vasculitis (3/14 vs. 3/13) (Fig. 2). Anti-52kDa antibody only was more commonly associated with the anti-La/SS-B antibodies (7/14 vs. 4/13), but this was not statistically significant (p>0.05).
Fig. 2.
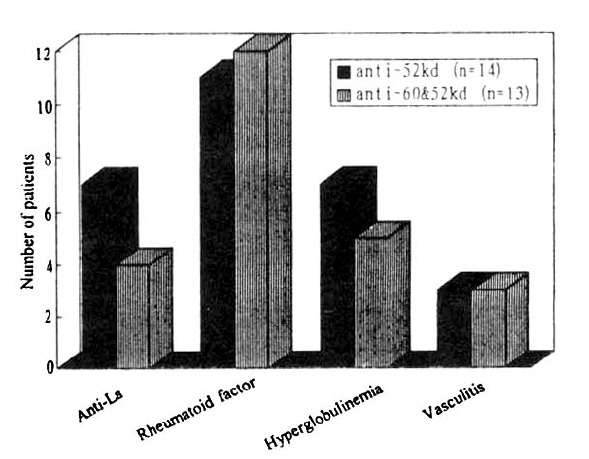
Comparison of clinical manifestations in anti-Ro positive primary SS patients with anti-60kDa/52kDa antibody versus those with only anti-52kDa antibody.
DISCUSSIONS
Anti-Ro/SS-A autoantibodies are found in the sera of patients with SLE and SS. The percentages in differnet studies reported in the literature range from 38% to 96% in primary SS and from 12 to 69% in SLE6, 11). In the present study, using immunodiffusion, the frequency of anti-Ro antibody was 64.7% in primary SS patiens and 52.3% in SLE patients. The frequency of anti-Ro/SS-A antibodies in SS seen in our study may seem low but compares well with recent results reported by Chan et al12). However, the prevalence of anti-Ro antibody in Korean patients with SLE was higher than that of Caucasian or Western countries13–15). Ben-Chetrit et al. reported that prevalence of Ro/SSA antibody was 57% which is in keeping with the prevalence of this study. The prevalences of anti-Ro/SS-A antibodies in Korean patients with SLE were very similar to those of the Ben-Chetrit et al, but different from those of Maddison et al. (20%)15). Pertinent to the reason of different prevalence are selection bias, different genetic background and detection methods including antigen extract. It has been demonstrated that occurrence of anti-Ro antibody are associated with certain HLA alleles although differences exist. Previous study reported that HLA DR3, which was linked to the occurrence of anti-Ro/SS-A antibody, was not common in oriental SLE including Korean16, 17). The relatively high prevalence of anti-Ro/SS-A in our SLE patients deserves further study to determine the possible involvement of genetic factors in the development of anti-Ro/SS-A antibody.
Anti-Ro positive sera contain two sets of antibody populations, directed to the 60kDa and the 52kDa polypeptide component of Ro proteins. It has been repoted that disease state influenced autoantigen recognition from the findings of different serum antibody responses to 60kDa and 52kDa Ro proteins in primary SS and SLE.
Simultaneous detection of antibodies to 60kDa and 52kDa was revealed by immunoblotting analysis with sera whose anti-Ro/SS-A antibody were positive by DID. The frequency of antibodies to 52kDa and 60kDa by immunoblot analysis was shown in Table 1. The prominent patterns associated with primary SS were antibodies to both the 60kDa and 52kDa Ro proteins and 52kDa Ro alone. Anti-52kDa Ro without anti-60kDa Ro antibody was a response that occured more commonly in primary SS than SLE sera. Our results also confirm the reports of Ben-Chetrit et al.4) and Tsuzaka et al.20) that there are differences between primary SS and SLE in their immune responses to the Ro/SS-A proteins. Previous reports demonstrate that anti-52kDa without anti-60kDa antibody was found only in SS and was not detected in SLE18–22). This difference might depend on the method that detects Ro/SS-A antigens. We definded anti-Ro/ SS-A antibodies by immunoblot, which detected antibodies to denatured Ro/SS-A antigens, while the report of Ben-Chetrit et al was performed by the immunoprecipitation method, in which Ro/SS-A antigen is native from.
Anti-SS-A/Ro antibodies have been demonstrated. to be related to subacute cutaneous lupus erythematosus, photosensitive rash, xerophthalmia and congenital heart block in patients with SLE, although controvery exists regarding the association of antibodies against Ro/SS-A antigen and particular features of SLE. In patients with SS, as shown in Fig. 1, anti-Ro/SS-A antibody positive group was correlated with the clinical mainfestations of cutaneous vasculitis, hyperglobulinemia, rheumatoid factor and anti-La/SS-B antibody positivity. These findings are similar to the reports of Alexander et al. and Harley et al.23, 24).
In the patients with anti-Ro/SS-A antibody positive sera, when we compare the clinically significant manifestations in SS patients with anti-Ro/SS-A antibodies and anti 60kDa and anti 52kDa antibody, we could not notice the difference between anti-52kDa antibody only positive group and both anti-60 and 52-kDa positive group which are the major determinants of anti-Ro antibodies. However, the number of patients with extraglandular manifestation observed in our study was not large enough to enable significant conclusions to be drawn.
In summary, the pattern of Ro/SS-A autoantigen recognition was different in SLE and primary SS. Anti-52kDa Ro without anti-60kDa Ro antibody was a response that occurred more commonly in primary SS than SLE, and served to distinguish the two disease entities. Although anti-Ro antibody was associated with the presence of some clinical manifestations in patients with primary SS, these findings were not correlated with the presence of antibodies against 60kDa or 52kDa protein. Additional studies are required to determine the exact correlation betwen antibodies against each peptides of 52kDa or 60kDa Ro/SS-A protein and certain clincial manifestations.
REFERENCES
- 1.Barakar S, Meyer O, Torterotot F, Youinou P, Briand JP, Kahn MF. IgG antibodies from patients with primary Sjogren’s syndrome and systemic lupus erythematosus recognize diffemetepitopes in 60-kD SS-A/Ro protein. Clin Exp Immunol. 1992;89:38–45. doi: 10.1111/j.1365-2249.1992.tb06874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan EKL, Tan EM. Epitopic targets for autoantibodies in systemic lupus erythematosus and Sjogren syndrome. Curr Op Rheumatol. 1980;1:376–381. doi: 10.1097/00002281-198901030-00022. [DOI] [PubMed] [Google Scholar]
- 3.Meilof JF. Autoantibodies against small cytoplasmic ribonucleoproteins: the anti-Ro/SS-A and anti-La/SS-B autoimmune response. Rheumatol Int. 1992;12:129–140. doi: 10.1007/BF00274932. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Chetrit E, Fox RI, Tan EM. Dissociation of immune responses to the SS-A(Ro) 52-kd and 60-kd polypetides in systemic lupus erythematosus and Sjogren’s syndrome. Arthritis Rheum. 1990;33:349–355. doi: 10.1002/art.1780330307. [DOI] [PubMed] [Google Scholar]
- 5.Meilof JF, Bantjes I, De Jong J, Van Dam AP, Smeenk RJT. The detection of anti-Ro/SS-A and anti-Ro/SS-A and anti-La/SS-B antibodies. J Immunol Methods. 1990;133:215–226. doi: 10.1016/0022-1759(90)90362-y. [DOI] [PubMed] [Google Scholar]
- 6.Bozic B, Pruijn G J M, Rozman B, Van Venrooij WJ. Sera from the patients with rheumatic diseases recognize different epitope regions on the 52-kD Ro/SS-A protein. Clin Exp Immunol. 1993;94:227–235. doi: 10.1111/j.1365-2249.1993.tb03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCauliffe DF, Yin H, Wang L, Lucas L. Autoimmune sera react with multiple epitopes on recombinant 52 and 60kDa Ro(SSA) proteins. J Rheumatol. 1994;21:1073–80. [PubMed] [Google Scholar]
- 8.Fox RI, Robinson CA, Curd JG, Kozin F, Howell FV. Sjogren’s syndrome : proposed criteria for classification. Arthritis Rheum. 1986;29:577–585. doi: 10.1002/art.1780290501. [DOI] [PubMed] [Google Scholar]
- 9.Tan EM, Cohen AS, Fries JP, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 10.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichlin M. Significance of the Ro antigen system. J Clin Immunol. 1986;6:339–348. doi: 10.1007/BF00915372. [DOI] [PubMed] [Google Scholar]
- 12.Chan EKL, Andrade LEC. Ahtinuclear antibodies in Sjogren’s syndrome. Rheum Dis Clin North Am. 1992;18:551–570. [PubMed] [Google Scholar]
- 13.Kim HY, Park DJ, Lee KS, Kim BS, Kim DJ. The frequencies and clinical significances of auto-antibodies to extractable nuclear antigens in systemic lupus erythematosus. Korean J Int Med. 1987;32:172–179. [Google Scholar]
- 14.Boey ML, Peebles CL, Tsay G, Feng PH, Tan EM. Clinical and autoantibody correlations in orientals with systemic lupus erythematosus. Ann Pheum Dis. 1988;47:918–923. doi: 10.1136/ard.47.11.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maddison PJ, Mogavero H, Provost TT, Reichlin M. Antibodies to Ro(SSA). The clinical significance of autoantibodies to a soluble cytoplasmic antigen in systemic lupus erythematosus and other connective tissue disease. J Rheumatol. 1979;6:189–195. [PubMed] [Google Scholar]
- 16.Hong KH, Kim HY, Takeuchi F, Nakano K, Yamada H, Matsuta H, Han H, Tokunaga K, Ito K, Park KS. Association of complement C4 and HLA-DR alleles with systemic lupus erythematosus in Korean. J Rheumatol. 1994;21:442–447. [PubMed] [Google Scholar]
- 17.Hartung K, Ehrfeld H, Lakomek HJ, Coldeway R, Lang B, Krapf F, Muller R, Schendel D, Deicher H, Seeling HP. The genetic basis of Ro and La antibody formation in systemic lupus erythematosus. Results of a multicenter study. Rheumatol Int. 1992;11:243–249. doi: 10.1007/BF00301501. [DOI] [PubMed] [Google Scholar]
- 18.Ricchiuti V, Briand JP, Meyer O, Isenberg DA, Pruijn G, Muller S. Epitope mapping with synthetic peptides of 52-kD SS-A/Ro protein reveals hetrogeneous antibody profiles in human autoimmune sera. Clin Exp Immunol. 1994;95:397–407. doi: 10.1111/j.1365-2249.1994.tb07010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boire G, Lopez-Longo F, Lapointe S, Menard H. sera from patients with autoimmune disease recognize conformational determinants on the 60-kd Ro/SS-A protein. Arthritis Rheum. 1991;34:722–730. doi: 10.1002/art.1780340613. [DOI] [PubMed] [Google Scholar]
- 20.Tsuzaka K, Fujii T, Akiuki M, Mimori T, Tojo T, Fujii H, Tsukatani Y, Kubo A, Homma M. Clinical significance of antibodies to native or denatured 60-kd or 52-kd Ro/SS-A protein in Sjogren syndrome. Arthritis Rheum. 1994;37:88–92. doi: 10.1002/art.1780370113. [DOI] [PubMed] [Google Scholar]
- 21.Slobbe RL, Prujin GJM, Damen WGM, Van der Kemp JWCM, Van venrooij WJ. Detection and occurrence of the 60-kd and 52-kd Ro(SS-A) antigens and of autoantibodies against these proteins. Clin Exp Immunol. 1991;86:99–105. doi: 10.1111/j.1365-2249.1991.tb05780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank MB, Itoh K, McCubbin V. Epitope mappong of the 52kDa Ro/SS-A autoantigen. Clin Exp Immunol. 1994;95:390–396. doi: 10.1111/j.1365-2249.1994.tb07009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander EL, Arnett FC, Provost TT, Stevens MB. Sjogren’s syndrome: association of anti-Ro(SS-A) antibodies with vasculitis, hematologic abnormalities and serologic hyperactivity. Ann Intern Med. 1983;98:155–159. doi: 10.7326/0003-4819-98-2-155. [DOI] [PubMed] [Google Scholar]
- 24.Harley JB, Alexander EL, Bias WB, Fox OF, Provost TT, Reichlin M, Yamagata H, Arnett FC. Anti-SS-A(Ro) and anti-SS-B(La) in patients with Sjogren syndrome. Arthritis Rheum. 1986;29:196–206. doi: 10.1002/art.1780290207. [DOI] [PubMed] [Google Scholar]


