Abstract
Amiodarone is an iodinated benzofuran derivative that represents a new and extremely effective therapy for certain life-threatening refractory cardiac arrgythmia. There are numerous side effects associated with amiodarone therapy, including corneal microdeposits. Skin reactions and others. Pulmonary toxicity, however, represents the most serious adverse reaction limiting the clinical efficacy of this antidysrhythmic drug, Recently, we experienced a case of amiodarone-induced pulmonary toxicity confirmed by open lung biopsy with light-and electron-microscopy. So, we report a case of amiodarone-induced pulmonary toxicity with a literature review.
Keywords: Amiodarone, Lung, Toxicity
INTRODUCTION
Amiodarone has emerged as an effective agent in the treatment of cardiac arrhythmias and has now been approved by the FDA for refractory tachycardia and ventricular fibrillation1). Recently, amiodarone has been used increasingly for the control of cardiac arrhythmias in Korea. However, the benefits of amiodarone may be accompanied by adverse effects. The incidence of side effects associated with amiodarone therapy ranges from 40 to 93 percent. The serious complications that may require discontinuation of therapy include pulmonary and neurologic toxicity2). Amiodarone pulmonary toxicity(APT) manifests as acute pneumonitis and chronic fibrosis. The overall incidence of APT has been estimated at 5 to 7 percent of treated patients, and 5 to 10 percent of them will die of the adverse effect3).
APT was first reported in 1980, and a number of similar reports of APT have been published since then, But, only one case of APT has been reported in Korea4). Recently, we experienced a case of APT, confirmed by electron-microscopy (EM), in spite of low-dose, 200mg/day, amiodarone maintenance therapy for 38-month duration. So, we report a case of APT with a literature review.
CASE REPORT
A 77-year-old man was admitted to the hospital because of progressive dyspnea for seven days and fever and chill for two days. Three years prior to admission, he was diagnosed PSVT. He had been prescribed low-dose (200mg/day) amiodarone since then, the family and social historie were not contribytory. He also complained of pleuritic chest pain and non-productive cough.
On admission, blood pressure was 150/90mmHg, temperature 38.4°C, pulse rate 88/min and respiration rate was 28/min.
On physical examination, he was alert, but appeared acutely ill. On auscultation, end inspiratory rales were heard on entire right lung field and left lower lung field, and the heart sound was regular with diastolic murmur on left lower parasternal border. The other physical examinations were normal.
The urine was normal. The hematocrit was 33%; the white-cell count was 36300/mm3, with 83% neutrophils and 1% eosinophils. The ESR was 60mm/hour. The blood chemical findings were normal. Values were normal for urea nitrogen, creatinine, glucose, sGOT, sGPT, alkaline phosphatase, bilirubin and protein(albumin and globulin), the serologic tests for rheumatoid factor, anti-nuclear antibody, LE cell and anti-double strand DNA Ab were negative. The arterial blood gas analysis showed PH; 7.40, PaCo2; 39mmHg, PaO2; 43mmHg, HCO3; 24mmol/L, The electocardiogram showed normal sinus rhythm with complete right bundle branch block pattern. The chest P-A showed confluent increased density in right upper lobe, right middle lobe and both lower lobes with blunting of left costophrenic angle, and also Derlie’s B line in both lower lung fields (Fig. 1).
Fig. 1.
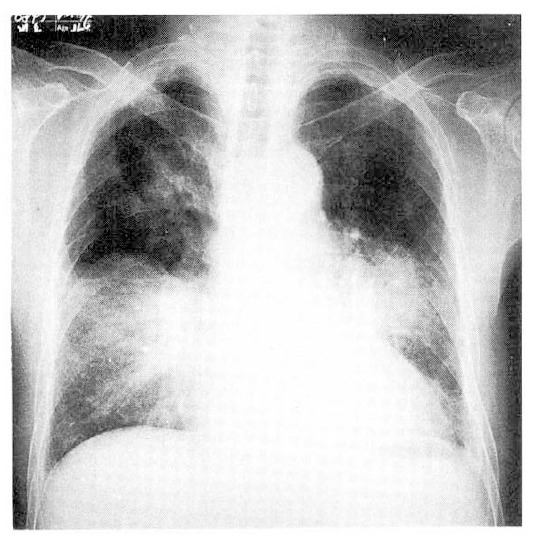
The initial chest radiolograph shows multiple air space consolidations bilaterally.
Under the impression of pneumonia, empirical antibiotics were prescribed and maintenance amiodarone for seven days. But the dyspnea, chest pain, cough and fever aggravated. The follow-up chest X-ray, five days after antibiotics, revealed aggravated and migrating confluent alveolar consolidations (Fig. 2). The high resolution computed tomography revealed patchy or lobar distributed increased opacities with air-bronchograms on both upper lobes, right middle lobe and both lower lobes, thickening of interlobular septums and also noted bilateral minimal pleral effusions (Fig. 3). The hypoxemia aggravated inspite of therapies of antibiotics. The bacteriologic studies showed negative results. On the eighth admission day, we changed amiodarone to verapamil, which is an another alternative drug for control of PSVT, and bronchoalveolar lavage (BAL) was performed under the impression of APT. The BAL showed 40×104/ml of total cell numbers with 45% macrophages, 5% lymphocytes, 50% neutrophils.
Fig. 2.
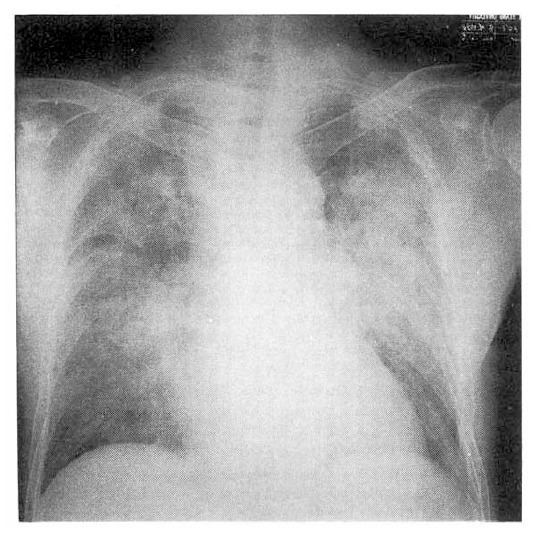
The chest roentgenogram on the 5th hospital day shows aggravated airspace consolidation in both lung fields.
Fig. 3.
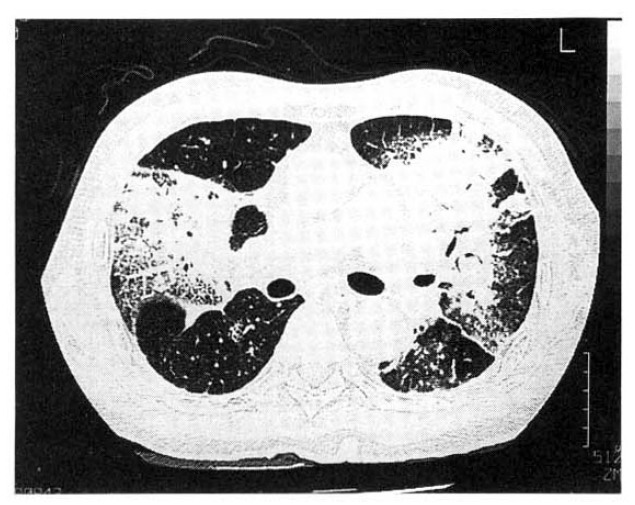
The High-resolution CT of the lung shows ground-grass opacities, airspace consolidation and interlobular septal thickening bilaterally.
For the definitive diagnosis, we carried out open lung biopsy at apical and anterior segment of left upper lobe on the eleventh hospital day. Open lung biopsy disclosed mild intertitial fibrous thickening and infiltration of a few lymphocytes. Most air spaces were filled with a variable number of foamy alveolar macrophages (Fig. 4). These foamy cells were also noted in the alveolar lining cells and within the interstitium. Focal area of firinous exudation and formation of hyaline membrane along the alveoli was present, suggestive of diffuse alveolar dammage. Ultra-structural findings demonstrated multiple electron dense lamellar bodies in the cytoplasm of foam cells (Fig. 5). These structures were surrounded by an unite membrane and lamellae were arranged in concentric fashion. These light-and electron-microscopic findings were compatible with APT.
Fig. 4.
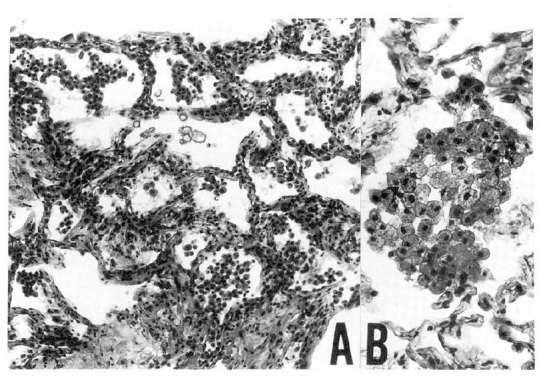
The microphotograph shows mild interstitial thickening (A) and intra-alveolar foamy macrophages (B) (H-E, A: ×100, B: ×400).
Fig. 5.
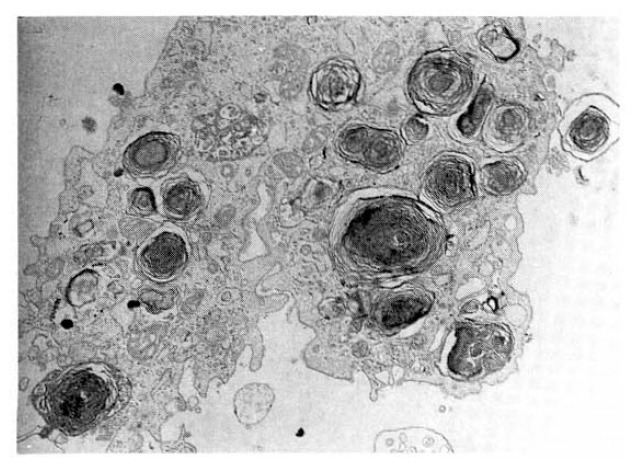
The electron microscopy demonstrates many osmiophilic lamellated bodies in the cytoplasm of foamy alveolar macrophages (uranyl acetate and lead citrate, ×7,000).
Five days after withdrawal of amiodarone, clinical findings and X-ray findings were improved (Fig. 6). The ABGA showed normal. The chest X-ray showed much improved consolidation of both lobes compared with previous films.
Fig. 6.
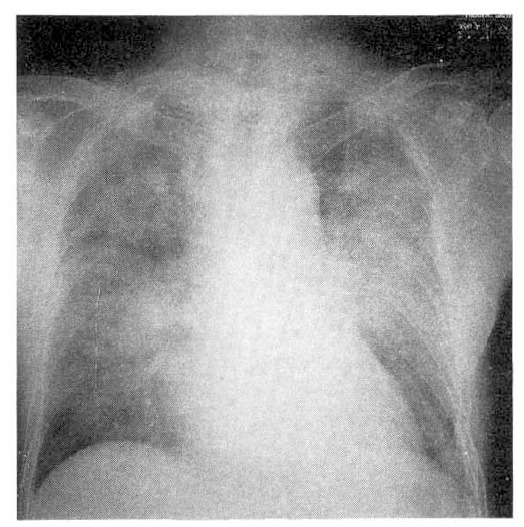
Follow-up chest X-ray at 15th day after withdrawal of amiodarone shows much improved infiltrations compared with those of previous films.
DISCUSSION
Amiodarone is an amhpiphilic compound, that has long elimination half-life, estimated at 45 to 60 days. This long half-life is, in part, related to the liphophilic properties of amiodarone, The lipophilicity of amiocarone is important because amiodarone tends to concentrate in organs with high lipid content, such as fat, lung and liver5).
Aiodarone is metabolized in the liver to its primary derivative, desethylamiodarone. Both the parent drug and its metabolite are concentrated in the lung with estimations of 10 to 1000 fold excess compared with serum levels6). Both computerized tomography (CT) and radionuclide studies, such as gallium-67 lung scanning, have been proposed as useful monitors of APT7,9). This accumulation in the liver may be associated with an increase in tissue density, secondary to the accumulation of iodide8). However, in this case, the hounsefield unites of liver after contrast was only 78 in the abdominal CT scan(not shown). These findings delayed us in approach of APT in the initial periods.
The most characteristic morphologic findings associated with APT is the presence of “foamy” changes in alveolar macrophages on BAL and in lung parenchymal cells on lung biopsy9). By ultra-structural examination, these foamy changes are associated with the presence of lamellar bodies and pathognomic finding of phospholipid accumulation. In this case, light-and electron microscopy also revealed foamy alveolar macrophages and electron dense lamellar bodies in the cytoplasm of foam cells. Unfortunately, we did not find the “foamy” changes of alveolar macrophages on BAL fluid.
The common signs and symptoms of APT are nonproductive cough, exertional dyspnea and pleuritic chest pain, but pleural effusion is unusual10). Weakness and weight loss are also common10). Physical, examination typically reveals bilateral rales. An occasional pleural rub may be heard10).
Peripheral blood of patients with APT may reveal nonspecific elevation of the WBC count, lactate dehydrogenase (LDH) activity and an elevated erythrocyte sedimentation rate (ESR)10). The elevated ESR is of particular interest, although not pathognomic, it woul not be expected in congestive heart failure or pulmonary embolism, the major differential diagnostic considerations in this setting. Serum levels of reverse T3, an inactive metabolite of T4, have also been proposed as a useful test to predict APT as well as its antidysrhythmic effect11).
Pulmonary function test reveals typical changes of restrictive lung disease in most cases of APT12). The diffusing capacity for carbon monoxide (DLCo) is the physiologic test most frequently recommended to assess parenchymal involvement secondary to the drug10). Some authors have included the finding of at least a 15 percent decrease in DLCo or or total lung capacity in the definition of APT13). However, pulmonary function test has its limitations, as many patients with cardiomyopathy and congestive failure often have evidence of a mild decrease in lung volumes and DLCo, possibly secondary to interstitail lung edema. In this case, DLCo was not performed because of severe dyspnea and its weak differentiation of diagnosis. A decline in DLCo during therapy can occur in patients receiving amiodarone without apparent clinical toxicity14).
Chest x-rays represent the primary test for surveillance of patients for evidence of APT9,14). Although most roentgenographic changes will be bilateral and diffuse, localized areas in the periphery of the lungs, such as the apices, may also be involved. The x-ray changes range from those of a pure alveolar pattern to a nearly pure interstitial pattern, with a combination of the two being most common10). The clinician must be aware of the widely variable chest x-ray film appearances and consider the possibility of APT with any new or developing parenchymal or pleural abnormality10).
There are several risk factors that predisposes to APT3,9,14). Kudenchuk et al13) reported that preexisting lung disease appears to increase the risk of clinically evident APT in patients receiving amiodarone. The overall assessment of risk for APT may be increased with higher daily and cummulative doses10). It was well known that APT can occur with as little as 200mg/day, In this case, 200mg/day of amiocarone evokes APT, suggesting the presence of unknown risk factors.
There are no laboratory or clinical data currently available that alone unequivocally establish the diagnosis of APT. To make a clinical diagnosis of APT requires the exclusion of other diagnostic possibilities (especially occult congestive heart failure) together with a reasonable constellation of symptoms or findings consistent with diagnosis10). Kudenchuk et al13). defined APT as any two of the following findings: (1) new or worsening symptoms; (2) new abnormalities on, or worsening of chest roentgenograms; or (3) a decline of at least 15 percent in the DLCo or total lung capacity. Several additional factors should be considered in the establishment of a clinical diagnosis of APT: (1) the absence of phopholipidosis in lung cells makes the diagnosis unlikely, (2) a marked CD8+ lymphocytosis in the lavage is strongly supportive; (3) lung biopsy material that reveals diffuse alveolar damage, interstial pneumonitis, or fibrosis consistent with APT; and (4) drug withdrawal with or without steroid therapy should, in most cases, reverse some or all of the abnormalities in APT10). According to this criteria, our patient was consistent with (1) and (2) kudenchuk’s diagnostic criteria and all additional factors, except for CD8+ lymphocytosis in BAL fluid.
Once the clinical diagnosis of APT has been made, a limited number of therapeutic options are available to the clinician. First, the most frequently used option is simply to discontinue amiodarone. In most cases, symptoms and findings will begin to resolve within a few days, although near-complete resolution may require several months. In our patient, also, symptoms improved only by withdrawal of amiodarone. In general, we use corticosteroids for APT when presenting symptoms or findings are severe and we hope to speed recovery of normal gas exchange. Typically, prednisone should be used at 40 to 60 mg daily with a tapering of dosage over a subsequent two-to six-month period. Withdrawal of steroids too early has induced recurrence in some patients15).
REFERENCES
- 1.Amiodarone The medical letter. 1986;28:49. [Google Scholar]
- 2.Harris L, Mcdenna WJ, Rowland E, Krikler DM. Side effects and possible contraindications of amiodarone use. Am Heart J. 1983;106:916. doi: 10.1016/0002-8703(83)90016-9. [DOI] [PubMed] [Google Scholar]
- 3.Rakita L, Sobol SM, Hostow N, Vrobel J. Amiodarone pulmonary toxicity. Am heart J. 1983;106:906. doi: 10.1016/0002-8703(83)90015-7. [DOI] [PubMed] [Google Scholar]
- 4.Jae Jeong Shim, Sang Hwa Lee, Jae Myung Yoo, Hong Suk Suh, Dong Joo Oh, Jae Youn Joh, Kwang Ho In, Se Hwa Yoo, Kyung Ho Kang, Eun Young Kang, Yang Suk Chae. A case of Amiodarone-induced pulmonary Toxicity. Tuberc Respir Dis. 1994;45:51. [Google Scholar]
- 5.Adams PC, Holt DWs, Storey GC, Morley AR, Callagham J, Campbell RW. Amiodarone and its desethyl metabolite: Tissue distribution and morphologic changes during long-term therapy. Circulation. 1985;72:1064. doi: 10.1161/01.cir.72.5.1064. [DOI] [PubMed] [Google Scholar]
- 6.Camus P, Mehendale HM. Pulmonary sequestration of amiodarone and desethy amiodarone pulmonary toxicity. AJR. 1986;147:607. [PubMed] [Google Scholar]
- 7.Moinuddin M, Rockett J. Gallium scintigraphy in the detection of amiodarone pulmonary toxicity. AJR. 1986;147:607. doi: 10.2214/ajr.147.3.607. [DOI] [PubMed] [Google Scholar]
- 8.Kuhlmans JE, Scatarige JC, Fishman EK, Zerhouni EA, Siegelman SS. CT demonstration of high attenuation pleural-parenchymal lestion due to amiodarone therapy. J Comput assist omogr. 1987;11:160. doi: 10.1097/00004728-198701000-00034. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy JI, Myers JL, Plumb VJ, Fulmer JD. Amiodarone pulmonary toxicity: Clinical, radiologic, and pathologic correlations. Arch Intern Med. 1987;147:50. doi: 10.1001/archinte.147.1.50. [DOI] [PubMed] [Google Scholar]
- 10.Martin WJ, 2d, Rosenow EC., 3d Amiodarone pulmonary toxicity: Recognition and pathogenesis (part I) Chest. 1988;93:1067. doi: 10.1378/chest.93.5.1067. [DOI] [PubMed] [Google Scholar]
- 11.Kerin NZ, Blevins RD, Benaderet D, Faitel K, Jarandilla R, Garfinkel C, Klein S, Rubenfire M. Relationship of serum reverse T3 to amiodarone anti-arrhythmic efficacy and toxicity. Am J Cardiol. 1986;57:128. doi: 10.1016/0002-9149(86)90965-3. [DOI] [PubMed] [Google Scholar]
- 12.Veltri EP, Reid PR. Amiodarone pulmonary toxicity: Early change in pulmonary function tests during amiodarone rechallenge. J Am Coll Cardol. 1985;6:802. doi: 10.1016/s0735-1097(85)80486-1. [DOI] [PubMed] [Google Scholar]
- 13.Kudenchuk PJ, Pierson DJ, Greene HL, Graham EL, Sear GK, Trobaugh GB. Prospective evalution of amiodarone pulmonary toxicity. Chest. 1984;86:541. doi: 10.1378/chest.86.4.541. [DOI] [PubMed] [Google Scholar]
- 14.Adams PC, Gibson GJ, Morley AR, Wright AJ, Corris PA, Reid DS, Campbell RW. Amiodarone pulmonary toxicity: Clinical and subclinical features. Q J Med. 1986;59:449. [PubMed] [Google Scholar]
- 15.Joelson J, Kluger J, Cole S, Conway M. Possible recurrence of amiodarone pulmonary toxicity following corticosteroid therapy. Chest. 1984;85:284. doi: 10.1378/chest.85.2.284. [DOI] [PubMed] [Google Scholar]


