Abstract
Background
After the discovery of type C hepatitis virus, the studies on this virus are extensively progressing. The treatment of this viral infection is also widely progressing. Among many agents, recombinant interferon alpha therapy is generally accepted as an effective single agent. To evaluate the efficacy of interferon and to observe the changes of serum aminotransferase (ALT), antibody to hepatitis C virus (anti-HCV) and HCV ribonucleic acid (HCV-RNA), we treated 10 patients with chronic type C hepatitis for 6 months.
Methods
Patients were randomly divided into 2 groups: 5 patients in group A received interferon and the other 5 in group B received no therapy. Interferon was administered at a dose of 3 million units (MU) daily for the first month and thrice weekly for the following 5 months, and followed up for 2 years.
Results
In group A, serum ALT returned to normal in 4: 3, starting at the first month and one at the 3rd month of therapy and maintained normal throughout the follow-up period. In contrast, serum ALT level persistently fluctuated in 4 patients in group B. In one patient, serum ALT returned to normal one and a half years later. Regardless of therapy, serum anti-HCV titer remained unchanged in all patients. However, HCV-RNA, using polymerized chain reaction (PCR), became undetetable in all responded patients and in one untreated patient whose serum ALT returned to normal spontaneously.
Conclusion
This study suggested that interferon alpha therapy in patients with chronic type C hepatitis may be clinically effective. Our study also indicated that the detection of HCV-RNA by PCR is useful to predict the prognosis of chronic type C hepatitis.
Keywords: Interferon alpha, Chronic type C hepatitis, HCV-RNA, PCR
INTRODUCION
Hepatitis C virus (HCV) is the major cause of non-A, non-B hepatitis world-wide. Immunologic surveys using recombinant viral antigen revealed that 0.5 to 15% of the world population has evidence of infection with HCV1–3). HCV infection is characterized by a marked propensity to chronic liver disease. Although many patients are asymptomatic and mild, an estimated 20% progress to liver cirrhosis and, furthermore may progress to hepatocellular carcinoma2–5). In Korea, the prevalence of type C hepatitis is nearly the same as other Western countries6), while hepatitis B virus infection is noted in 10% of its population7). However, because of the nature of HCV infection, type C hepatitis is one of the leading causes of chronic liver failure. Currently, there is no effective therapy for type C hepatitis; corticosteroid or acyclovir had no effect on this hepatitis8,9). Instead, a potent antiviral agent, interferon alpha, which has recently become available in a highly purified form, is regarded as a single effective agent in the therapy of viral hepatitis. Interferon alpha has been reported have a beneficial effect in chronic hepatitis B10,11) and, more recently, in type C hepatitis12–14). We report here our results of recombinant interferon therapy in 10 patients with chronic type C hepatitis.
MATERIALS AND METHODS
1. Patients
Ten patients who were positive for anti-HCV antibody were included in this study. They were 8 males and 2 females, at the ages between 31 and 56 (mean:45 yrs.), and all of them had chronic fatigue and elevated serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, 72 to 328 (mean: 163)U/L, 67 to 715 (mean: 354)U/L, respectively (Table 1). The source of infection was post-transfusion in 2, intravenous drug abuse in 2, and community acquired in 6. All patients were positive for anti-HCV; 7 by C-100-3 Elisa (Abbott) and 3 by recombinant immunoblot assay (RIBA) test using 5-1-1 and C-33c (Chiron Corp). All of them were tested as negative for serum HBsAg, I gM anti-HBc and I gM anti-HAV. Liver biopsy was performed on 8 patients, and all showed evidence of chronic active hepatitis. The patients were randomly divided into two groups; 5 patients in group A received interferon and the other 5 patients in group B received no therapy. Serum samples were obtained monthly up to 2 yrs and stored at −70°C.
Table 1.
Pretreatment Characteristics of the Patients Studied
| Features | Treated Group | Untreated Group |
|---|---|---|
| No | 5 | 5 |
| Mean Age (yrs) | 45 | 46 |
| Sex (M/F) | 4/1 | 4/1 |
| AST (Mean) U/L | 78–259 (139) | 62–328 (188) |
| SLT (Mean) U/L | 144–564 (356) | 67–715 (350) |
| Albumin g/dl | 3.2–4.7 (4.0) | 3.3–4.3 (3.9) |
2. Assays for Anti-HCV Antibody & HCV-RNA by PCR
Serum anti-HCV was tested using C-100-3 (Abbott Lab), 5-1-1 and C-33c (Chiron Corp). HCV-RNA was tested using a cDNA PCR procedure15). Briefly, RNA was extracted from 100 ul of serum and converted into single stranded cDNA by reverse transcriptase (BRL) using 50 pmoles of primer sequence JHC 51 (5′-CCC AAC ACT ACT CGG CTA-3′). Ten percent of the cDNA was amplified by PCR as described by Saiki et al16), using 50 pmoles each of oligonucleotide JHC 52 (5′-AGT CTT GCG GCC GCA GCG CCA AAT C-3′) and JHC 93 (5′-TTC GCG GCC GCA CTC CAC CAT GAA TCA CTC CCC-3′) (94° C:1.5 min, 60°C: 2 min, 72°C 3 min, 35 cycles). Ten percent of the PCR product was examined in a 2% agarose gel and was analyzed by Southern blot hybridization using a 32P A 89 (5′-CCA TAG TGG TCT GCG GAA CCG GTG AGT ACA-3′) as a probe. HCV-RNA from plasma of an experimentally infected chimpanzee served as positive control; the negative control was serum from a HBV infected patient which was tested as HCV negative by ELISA and PCR. Potential contamination of specimens and reagents with HCV sequences was monitored with a water control for cDNA synthesis and PCR. Data presented here were obtained with duplicate experiments.
RESULTS
Of 5 treated patients in group A, serum ALT levels returned to normal in 4 patients, 3 during the therapy and one during the follow up. However, one patient did not respond to the therapy indicated by persistently fluctuating serum ALT levels. In contrast, 4 of 5 untreated patients in gorup B showed persistently elevated serum ALT level throughout the study period. The level of serum ALT in one patient in group B fluctuated for one and a half years, then returned to normal-spontaneously and maintained thereafter. Clinical symptoms were improved in the responders during and after the therapy and in one patient whose serum ALT returned to normal without therapy. In all patients, anti-HCV profile, tested by ELISA and RIBA, remained unchanged during the study period. However, serum HCV-RNA was undetectable in the 3 treated and in one untreated patients whose serum ALT levels returned to normal spontaneosly (Fig. 1). In all responded patients, serum HCV-RNA became undetectable at the first month of therapy and this was immediately followed by a normalization of serum ALT level. In one treated patient, whose ALT level fluctuated, HCV-RNA was transiently undetectable when the ALT level returned to normal, but it was detectable again when the ALT level was re-elevated (Fig. 2). No HCV-RNA was detectable in one treated patient before and after therapy.
Fig. 1.
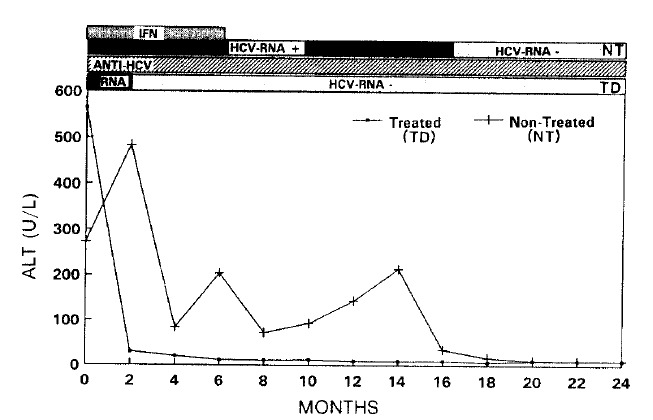
Changes of serum ALT, anti-HCV & HCV-RNA in a responder and a non-responder whose serum ALT returned to normal.
Fig. 2.
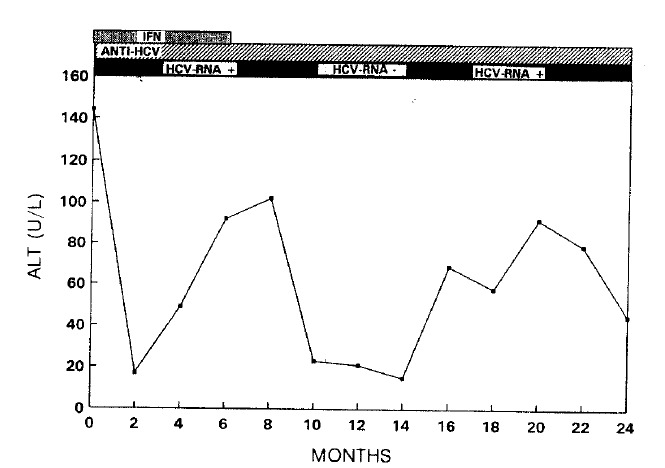
Changes of serum ALT, anti-HCV & HCV-RNA in a non-responder.
DISCUSSION
The recent discovery of HCV as the major cause of non-A, non-B hepatitis1) and the subsequent development of antiboby assay has facilitated the diagnosis of patients with type C hepatitis2). Recombinant interferon alpha has been used to treat patients with chronic hepatitis B and recent data suggest that it may also be effective in patients with type C hepatitis12–14). We have evaluated the efficacy of interferon therapy in 10 Korean patients with type C hepatitis by monitoring changes in the level of serum ALT, anti-HCV antibody and viral RNA. In our study, 4 of 5 treated patients showed a clear response to the therapy while one did not (Table 2). These 4 responders were initially characterized by a rapid conversion in their ALT level which was maintained as normal during the study period. However, the level of circulating antibody in these patients remained high during the therapy and the follow-up period (Table 2). This is consistent with observations made by others17,18), although there have been some conflicting reports indicating that interferon therapy actually lower the antibody titer19–22). In contrast to the anti-HCV response, we no longer detected viremia by PCR in patients whose serum ALT returned to normal23). The loss of HCV-RNA was immediately followed by the normalization of serum ALT. This was true both in responders to interferon therapy and in one patient whose serum ALT returned to normal without therapy. We concluded that, in patients with chronic type C hepatitis interferon therapy may be effective and the viral RNA assay by PCR may be a reliable prognostic marker.
Table 2.
Changes of Serum ALT, Anti-HCV and HCV-RNA During Follow-up
| No. | Anti-HCV | HCV-RNA(PCR) | Serum ALT(U/L) | |||||
|---|---|---|---|---|---|---|---|---|
| Age/Sex | C-100 | 5-1-1 | C-33 | Before | After | Before | After | |
| 1 | TD* M/53 | + | + | −(***) | 144 | 92 | ||
| 2 | TD M/33 | 4+ | + | − | 564 | 11 | ||
| 3 | TD M/48 | 3+ | 4+ | + | − | 163 | 16 | |
| 4 | TD M/36 | 4+ | − | − | 361 | 26 | ||
| 5 | TD F/46 | + | + | − | 552 | 18 | ||
| 6 | UTD**F/42 | + | 4+ | 4+ | + | − | 273 | 93 |
| 7 | UTD M/53 | + | + | + | 216 | 75 | ||
| 8 | UTD M/31 | + | + | + | 715 | 93 | ||
| 9 | UTD M/56 | + | + | + | 402 | 114 | ||
| 10 | UTD M/49 | + | + | + | 67 | 61 | ||
TD: Treated Patient
UTD: Untreated Patient
HCV-RNA in this patient was transiently undetectalbe when the serum ALT returned to normal but again detectale when the ALT was re-elevated (See Fig. 2)
REFERENCES
- 1.Choo QL, Kuo G, Weimer AJ, et al. Isolation of cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 2.Kuo G, Choo QL, Alter HJ, et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- 3.Alter MJ. Hepatitis C and miles to go before we sleep. N Engl J Med. 1989;321:1538. doi: 10.1056/NEJM198911303212208. [DOI] [PubMed] [Google Scholar]
- 4.Govindarajan S, McHutchison JG, Vallinluck B, Redeker AG. Prevalence of anti-HCV in patients with hepato cellular carcinoma (Abstract) 1990;12(Part 2):878. [Google Scholar]
- 5.Liang TJ, Jeffers LJ, Reddy RK, Wands JR, Schiff ER. HBV related and HCV infection in HBsAg negative patients with hepatocellular carcinoma (Abstract) Hepatology. 12(Part 2):881. [Google Scholar]
- 6.Chung KW, Sun HS, Chung WK, Shin HK, Park CK, Yoo JY. A preliminary report on the prevalence of type C hepatitis in Korea. Korean J Intern Med. 1990;38:750. [Google Scholar]
- 7.Chung WK, Sun HS, Park DH, Minuk GY, Hoofnagle JH. Primary hepatocellular carcinoma and hepatitis B virus infection in Korea. J Med Virol. 1983;11:99. doi: 10.1002/jmv.1890110203. [DOI] [PubMed] [Google Scholar]
- 8.Alter HJ, Hoofnagle JH. Non-A, non-B hepatitis: Observations on the first decade. In: Vyas GN, Dienstag JL, Hoofnagle JH, editors. Viral Hepatitis and Liver Disease. Grune & Stratton; Orlando, Fla: 1984. p. 345. [Google Scholar]
- 9.Pappas SC, Hoofnagle JH, Young N, Straus SE, Jones EA. Treatment of chronic non-A, non-B hepatitis with acyclovir: Pilot study. J Med Virol. 1985;1 doi: 10.1002/jmv.1890150102. [DOI] [PubMed] [Google Scholar]
- 10.Hoofnagle JH, Peters M, Mullen KD, et al. Randomized, controlled trial of recombinant human alpha interferon in patients with chronic hepatitis B. Gastroenterology. 1988;95:1318. doi: 10.1016/0016-5085(88)90367-8. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Choi YW, Park SW, et al. Effect of recombinant interferon alpha in patients with chronic hepatitis B. Korean J Intern Med. 1990;38:444. [Google Scholar]
- 12.Davis GL, Balart LA, Schiff ER, et al. Treatment of chronic hepatitis C with recombinant interferon alfa: A multicenter randomized, controlled trial. N Engl J Med. 1989;321:1501. doi: 10.1056/NEJM198911303212203. [DOI] [PubMed] [Google Scholar]
- 13.Di Bisceglie AM, Martin P, Kassianides C, et al. Recombinant interferon alfa therapy for chronic hepatitis C: A Randomized, double-blind, placebo-controlled trial. N Engl J Med. 1989;321:1506. doi: 10.1056/NEJM198911303212204. [DOI] [PubMed] [Google Scholar]
- 14.Marcellin P, Boyer N, Giostra E, et al. Recombinant human alpha interferon in patients with chronic non-A, non-B hepatitis: A multicenter randomized controlled trial from france. hepatology. 1991;13:393. [PubMed] [Google Scholar]
- 15.Thaler MM, Park CK, Landers DV, et al. Vertical transmission of hepatitis C virus. Lancet. 1991;338:17. doi: 10.1016/0140-6736(91)90006-b. [DOI] [PubMed] [Google Scholar]
- 16.Saiki RK, Scharf FF, Mullis GT. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 17.Shindo M, Di Bisceglie AM, Cheung L, et al. Changes in hepatitis C virus RNA in serum associated with alphainterferontherapy (Abstract) 1990;12(Part 2):884. [Google Scholar]
- 18.Farci P, Alter HJ, Wong D, Miller RH, Shih JW, Jett B, Purcell RH. A long-term study of hepatitis C virus replication in non-A, non-B hepatitis. N Engl J Med. 1991;325:98. doi: 10.1056/NEJM199107113250205. [DOI] [PubMed] [Google Scholar]
- 19.Kondo T, Hino K, Fujioka S, et al. Changes in anti-HCV titer and HCV-DNA by IFN therapy in chronic non-A, non-B hepatitis (Abstract) Hepatology. 1990;12:421. [Google Scholar]
- 20.Yoshioka K, kakumu S, Shinogawa T, et al. HCV antibody and chronic hepatitis (Abstract) Hepatology. 1990;12:450. [Google Scholar]
- 21.Hagiwara H, Hayashi N, Mita E, et al. Detection of HCV-RNA in serum by RT-PCR method during treatment of chronic hepatitis C with interferon (Abstract) Hepatology. 1990;12(Part 2):879. [Google Scholar]
- 22.Omata M, Yokosuka O, hosoda K, Kato N, Ohto N. A control study of interferon treatment for acute non-A, non-B hepatitis: Effects on hepatitis C virus (Abstract) Hepatology. 1990;12(Part 2):883. [Google Scholar]


