Abstract
Background
IL-2 production has been measured in several disease including type I diabetes mellitus9), systemic lupus erythematosus10,11), acquired immunodeficiency syndrome12,13) and active pulmonary sarcoidosis14) and its pathogenetic role was suggested. In IgA nephropathy, altered T cell subsets were reported to be associated with increased synthesis of IgA15–19). The altered IL-2 production and the expression of IL-2 receptor might be involved in the pathogenesis of IgA nephropathy20–23).
Methods
To investigate the role of T cell mediated immunity in the pathogenesis of IgA nephropathy, the immune parameters such as T cell subsets, NK cell activity, interleukih-2 (IL-2) production and IL-2 receptor expression on peripheral blood mononuclear cells (PBMC) were measured before and/or after phytohemagglutinin (PHA) stimulation in 15 patients with IgA nephropathy. Age and sex matched 15 healthy controls and the correlations between the IL-2 production and immune parameters were evaluated.
Results
The mean percentages of T helper/inducer cells (CD4), T suppressor/cytotoxic cells (CD8) end the CD4/CD8 ratio of the patients were not different from those of controls and the proportions of CD8 CD11b cell in the patients (21.0±3.6%) were significantly lower than those in controls (30.5±5.3%) (p<0.005). The production of IL-2 by fresh PBMC of both patients and controls was in undetectable ranges. The production of IL-2 by PHA stimulated PBMC of patients was significantly higher than that of controls (140.03±43.2 U/ml vs 106.5±42.1 U/ml, p<0.05). The proportions of lymphocytes expressing the IL-2 receptor (CD25) before the stimulation with PHA in patients were 1.22±1.00 percents and were not different from those in controls (1.12±0.78 percents). The correlations between the production of IL-2 and the concentrations of serum IgA, the degrees of histologic alterations and the proportions of CD8 and CD8CD11b cells were not significant. There was a weak tendency of a positive correlation (p<0.1) between the production of IL-2 and the proportions of CD4 cells, and the CD4/CD8 ratio showed a significant correlation with the production of IL-2 (p<0.05). After PHA stimulation, the mean percentages of lymphocytes expressing the IL-2 receptors in patients were increased to 47.6±8.9 percents which is higher than those (40.4±9.9%) in controls (p<0.05). The NK cell activity of the patients was higher than that of controls (75.6±19.6% vs 56.1±16.2%, p<0.005), and was well correlated with the production of IL-2 by PBMC (r = 0.89, p<0.05).
Conclusions
It seemed that patients with IgA nephropathy have an 'latent' cellular immunoregulatory dysfunciton that becomes apparent on the stimulation of extrinsic antigens or mitogens.
Keywords: Interleukin-2, Interleukin-2 receptor, IgA nephropathy
INTRODUCTION
Although the immunopathogenesis of IgA nephropathy is not completely defined, it is suggested that immunoregulatory dysfunction plays an important role in the pathogenesis of IgA nephropathy1–5). Recent studies also suggest the association between the disordered immunoregulation of T cell and the disease activity of IgA nephropathy6–8).
IL-2 production has been measured in several diseases including type I diabetes mellitus9), systemic lupus erythematosus10,11), acquired immunodeficiency syndrome12,13) and active pulmonary sarcoidosis14) and its pathogenetic role was suggested. In IgA nephropathy, altered T cell subsets were reported to be associated with increased synthesis of IgA15–19). The altered IL-2 production and the expression of IL-2 receptor might be involved in the pathogenesis of IgA nephropathy20–23).
To investigate the role of T cell mediated immunity in the pathogenesis of IgA nephropathy, the immune parameters such as T cell subsets, NK cell activity, interleukin-2 (IL-2) production and IL-2 receptor expression on PBMC were measured before and/or after PHA stimulation in patients with IgA nephropathy.
MATERIALS AND METHODS
1. Patients
Fifteen patients with primary IgA nephropathy were studied. The clinical and laboratory characteristics of the patients were shown in Table 1. The diagnosis of IgA nephropathy was based on the presence of predominant mesangial IgA deposits and mesangial and paramesangial electron-dense deposits in renal biopsy specimens51). Patients with the clinical and laboratory evidences of hepatic disease, systemic lupus erythematosus, Henoch-Schoenlein nephritis and other systemic diseases were excluded in this study. No patient had renal insufficiency or nephrotic syndrome and all patients were in clinically quiescent state without fever, gross hematuria and upper respiratory or gastrointestinal illness. No patient had infection and was on corticosteroid or immunosuppressive treatment within at least one month of entry into the study.
Table 1.
Clinical and Laboratory Characteristics of Patients with IgA Nephropathy
| Sex (M/F) | 6/9 | |
| Age (years) | 34.6±13.4 | 20–61 |
| Serum creatinine (mg/dl) | 0.9±0.3 | 0.5–1.3 |
| Serum albumin (g/dl) | 4.0±0.5 | 3.3–4.7 |
| Serum IgA (mg/dl) | 332±109 | 270–565 |
| Pathology score | 4.2±2.6 | 1–9 |
Medical doctors and nurses, matched for age and sex with the patients with IgA nephropathy, were used as healthy controls.
Pathologic changes of renal biopsy specimen were calculated by the semiquantitative method proposed by Kobayashi et al24). Seven parameters of mesangial hypercellularity, mesangial sclerosis, interstitial fibrosis, tubular atrophy, small crescent, tuft adhesion and global sclerosis were graded from 0 to 3. Each grade of the first 4 parameters was determined according to the intensity of the lesion and that of the other 3 parameters was determined as follows; Grade 3 was defined as the presence of more than 3 altered glomeruli per 10 glomeruli and grade 2, 1, and 0 were defined as the presence of 2, 1, and no altered glomeruli respectively.
2. Separation and Culture of Lymphocytes
Separation of lymphocytes was done by Ficoll-Hypaque gradient separation method of Boyum25). Heparinized venous blood was diluted with an equal volume of phosphate buffered saline (PBS) pH 7.4 and layered over a Ficoll-Hypaque density gradient. After centrifugation, the interface layer containing lymphocytes was washed thrice in PBS and suspended at a concentration of 5×106 cells/ml in complete culture medium consisting of RPMI 1640 (GIBCO, NY, USA) supplemented with 2% human AB type serum, 2 mM L-glutamine, 100 units/ml penicillin, 100 ug/ml streptomycin and 0. 25 ug/ml fungizone.
Lymphocytes culture was done by the microculture technique26). Briefly, the lymphocytes were cultured with purified phytohemagglutinin (CSL, Melbourne, Australia) 10 ug/ml at 37°C in 5% CO2 in air for 18 hours.
3. T Lymphocyte Subsets
Assay for the lymphocyte subsets was done by the method of indirect immunofluorescence (IF) using monoclonal antibodies (Becton dickinson, CA, USA)27). 100 ul of the lymphocyte suspension adjusted to 5×106 cells/ml were placed in plastic tubes (Costar 2022, MA, USA) and incubated with 20 ul of anti-leu-2a, anti-leu-3a, and anti-leu-4 for 30 minutes at 4°C. After incubation, the cells were washed thrice in PBS and labeled with 100 ul of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Becton Dickinson, CA, USA). The cells were then incubated for 30 minutes at 4°C and washed in PBS. 100 ul of mounting medium (PBS, pH 7.4, containing 30% glycerol) were added to cell suspension and one drop was examined by a fluorescence microscope (Olympus, type BH-2, Japan).
Subsets of CD8CD11b cells were determined by direct IF after double IF staining. 100 ul of lymphocyte suspension was incubated for 30 minutes at 4°C with 10 ul of FITC-conjugated anti-leu-2a (Becton Dickinson, CA, USA) and washed thrice in PBS. The cells were then incubated for 30 minutes at 4°C with 20 ul of phycoerythrin-conjugated anti-leu-15 (Becton Dickinson, CA, USA) and washed thrice in PBS. After that, the cells were resuspended in mounting medium and one drop was examined by a fluorescence microscope.
4. IL-2 Receptor on Lymphocyte
Lymphocytes expressing IL-2 receptor were identified by indirect IF using monoclonal antibody of anti-CD25 (Becton Dickinson, CA, USA) with the similar methods of analyzing T lymphocytes subsets.
5. Natural Killer Cell (NK cell) Activity
NK cell activity was assayed by a chromium-release method of Jondal et al28). K562 cells derived from a patient with chronic myeloid leukemia were used as target cells.
Target cells were suspended at a concentration of 5×106 cells/ml in complete culture medium and labeled with 100 uCi of Na2 51Cr O4 (New England Nuclear, MA, USA) added to the 0.5 ml of cell suspension. After incubation for 90 minutes at 37°C in 5% CO2 in air and washing thrice, 10 ul of cell suspension adjusted to 5×106 cells/ml in complete culture medium were placed into 96-well micro-plate (Costar 3799, MA, USA). 20 ul of effector peripheral blood lymphocytes adjusted to the concentration of 1×106 cells/ml were added to target cells in triplicate with the ratio of lymphocyte to target cell of 40. Target cells were incubated in complete culture medium as controls for spontaneous release (SR) and maximum release (MR) was measured after the addition of 1% Triton X-100 solutions. Samples were counted in a gamma-counter (Gamma 5500; Beckman, CA, USA). Percent cytotoxicity was calculated with the following formula;
6. IL-2 Production
Peripheral blood lymphocytes suspended at the concentration of 5×106 cells/ml in complete culture medium were stimulated with 10 ul/ml of PHA (CSL, Melbourne, Australia) at 37°C in 5% CO2 in air for 18 hours, when supernatants were harvested after centrifugation for 10 minutes at 400×G. IL-2 activity in supernatants was assayed by its ability to stimulate the incorporation of 3[H] thymidine into IL-2 dependent cells29).
100 ul of test samples were placed in 96-well microplates followed by serial twofold dilutions with complete culture medium supplemented with heat inactivated 5% fetal calf serum. To these, 100 ul/well of 1×105 CTLL cells (an IL-2 dependent murine line of cytotoxic T lymphocytes; Osaka, Japan) were added and incubated for 24 hours at 37°C in 5% CO2 in air. 0.5 uCi 3[H] thymidine (New England Nuclear, MA, USA) was added to each well and cultured for 4 hours. The cells were harvested with a cell culture harvester (Adaps, MA, USA) onto glass wool filters (Gelman 61638, MI USA). 3[H]-thymidine incorporation into DNA of CTLL cells was counted on a β-scintillation counter (Beckman 8000, Irvine, CA, USA).
In every assay, the uptake of 3[H] thymidine by CTLL cells in response to the tested samples was compared to their response to standard IL-2 preparations (Collaborative Research Co, MA, USA) by probit analysis. Results are expressed as units of IL-2/ml (U/ml). 1 unit was defined as the amount of IL-2 to exhibit the 50% of maximum amount of 3[H] thymidine uptake.
7. Statistical Analysis
The results are expressed as mean±standard deviation. Students t-test and regression analysis were used to compare the means of two groups. AP-value less 0.05 is interpreted to be significant.
RESULTS
1. T lymphocyte Subsets (Table 2)
Table 2.
T cell Subsets in Patients with IgA Nephropathy and Healthy Controls
| IgA N (n = 15) | Control (n = 15) | Significance | |
|---|---|---|---|
| Lymphocyte (No/ul) | 3038±440 | 3125±623 | NS |
| CD3 (%) | 75.9±10.8 | 75.4±7.0 | NS |
| CD4 (%) | 43.3±7.6 | 40.3±4.7 | NS |
| CD8 (%) | 29.5±5.9 | 27.9±5.6 | NS |
| CD8 CD11 (%) | 21.0±3.6 | 30.5±5.3 | p <0.005 |
| CD4/CD8 | 1.46±0.32 | 1.43±0.30 | NS |
IgA N = IgA nephropathy Control = Healthy control NS = Not significant
Values are expressed as mean±S.D.
The absolute lymphocyte counts of the patients with IgA nephropathy were 3038±440/ul and were not different from those of healthy controls (3125±623/ul). The mean percentages of CD3 cell, CD4 cells, CD8 cell in peripheral blood lymphocytes of the patients were not different from those of healthy controls. The ratio of CD4/CD8 of the patients was 1.46±0.32 and was not different from those of healthy controls (1.43±0.30). However, the proportions of CD8 CD11b cells with the suppressive activity on B cell in patients was significantly lower than those in healthy controls (p<0.005).
2. IL-2 Production
The production of IL-2 by fresh peripheral blood mononuclear cells (PBMC) before PHA stimulation in the patients and the healthy controls was in undetectable ranges. After PHA stimulation, IL-2 production was increased to the level of 140.3±43.2 U/ml in patients, which was significantly higher than that in controls of 106.5±42.1 U/ml (p<0.05, Table 3). When the patients were divided into 2 groups, according to the level of mean CD4/CD8 ratio of the patients (=1.46), the group of patients with higher CD4/CD8 ratio (≥ 1.46) produced more IL-2 compared with that with lower CD4/CD8 ratio (<1.46) and healthy controls (p<0.05, Table 3).
Table 3.
IL-2 Production by PHA-Stimulated PBMC According to the Level of CD4/CD8 Ratio in Patients with IgA Nephropathy
| IL-2 production (U/ml) | |
|---|---|
| IgA nephropathy (n = 15) | 140.3±43.2* |
| CD4/CD8≥1.46 (n = 9) | 177.5±48.9** |
| <1.46 (n = 6) | 126.8±29.2 |
| Healthy controls (n=15) | 106.5±42.1 |
p<0.05 vs healthy controls
p<0.005 vs healthy controls and p<0.05 vs IgA nephropathy with lower (<1.46) CD4/CD8 ratio
Values are expressed as mean±S.D.
3. IL-2 Receptor Expression
The proportions of lymphocytes expressing the IL-2 receptor in patients were 1.22±1.00 percents before PHA stimulation and were not different from those in healthy controls of 1.12±0.78 percents. After PHA stimulation, the mean percentages of lymphocytes expressing the IL-2 receptors were increased to the level of 47.6±8.9%, which were significantly higher than those of healthy controls of 40.4±9.9% (p<0.05, Table 4).
Table 4.
IL-2 Receptor Expression on the Surface of Fresh and PHA-Stimulated PBMC in Patients with IgA Nephropathy
| IL-2 receptor expression (%) | ||
|---|---|---|
| Fresh | IgA nephropathy(n= 15) | 1.22±1.00 |
| PBMC | Healthy controls (n= 15) | 1.12±0.78 |
| PHA-stimulated | IgA nephropathy (n= 15) | 47.6±8.9* |
| PBMC | Healthy controls (n= 15) | 40.4±9.9 |
p<0.05
Values are expressed as mean±S.D.
4. NK Cell Activity
The NK cell activity of the patients was significantly higher than that of controls (75.6±19.6% vs 56.1±16.2%, p<0.005, Table 5). It was well correlated with the production of IL-2 by PHA-stimulated PBMC (r = 0.89, p<0.05, Fig. 1).
Table 5.
NK Cell Activity in Patients with IgA Nephropathy and Healthy Controls
| NK cell activity (%) | |
|---|---|
| IgA nephropathy (n= 15) | 75.6±19.6* |
| Healthy controls (n= 15) | 56.1±16.2 |
p <0.005
Values are expressed as mean±S.D.
Fig. 1.
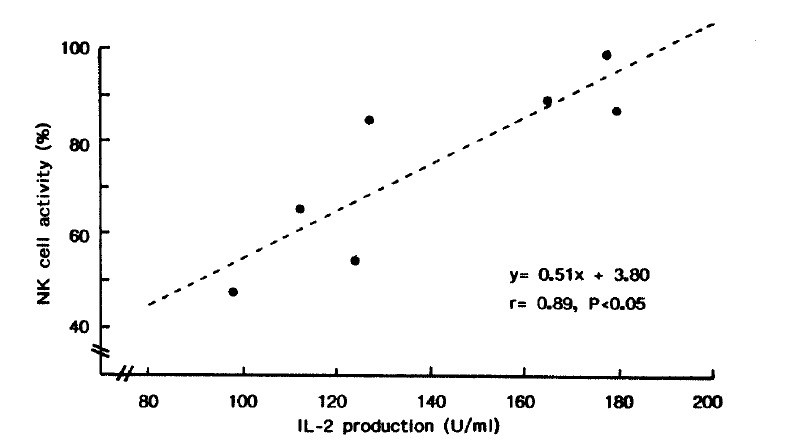
Correlation between PHA-stimulated IL-2 production and NK cell activity
5. Correlation between IL-2 production and immune parameters (Table 6)
Table 6.
Correlation Coefficient between PHA-Stimulated IL-2 Production and other Parameters
| r | Significance | |
|---|---|---|
| Serum IgA (mg/dl) | 0.271 | NS |
| Pathology score | −0.405 | NS |
| CD4 (%) | 0.620 | p<0.1 |
| CDS (%) | −0.218 | NS |
| CD4/CD8 | 0.690 | p<0.05 |
| CD8CD11b (%) | 0.243 | NS |
| NK cell activity (%) | 0.860 | p<0.05 |
NS = Not significant
The correlation between the production of IL-2 and the concentration of serum IgA, the degrees of histologic alterations, as well as the proportions of CD8 and CD8CD11b cells was not significant. The relationship between the production of IL-2 and the proportions of CD4 cells showed a weak tendency of a positive correlation (p<0.1) and the CD4/CD8 ratio showed a significant correlation with the production of IL-2 (p<0.05).
DISCUSSION
Although the pathogenesis of IgA nephropathy is not well defined, it is suggested that immunoregulatory dysfunciton plays a major role1–5). In IgA nephropathy, many evidences of immunological involvements such as the elevated level of serum IgA30) and the circulating IgA immune complexes31–34), the deposition of IgA in dermal capillaries, liver, lung, and gut35,52), and the recurrence of original disease in renal allograft36) were demonstrated.
Studies on the T cell subsets in peripheral blood of the patients with IgA nephropathy so far have contradictory results. Some of them showed the increased percentages of CD4 cells and CD4/CD8 ratio as well as the reduced percentages of CD8 cells2,16,19). However no abnormalities in the percentages of CD4 cells, CD8 cell and the CD4/CD8 ratio were observed by others8,37). In this study, the mean percentages of CD4 cells, CD8 cells and the CD4/CD8 ratio in patients with IgA nephropathy were not different from those in healthy controls. Because our patients were all in a clinically silent phase of the disease, these results are consistent with the previous reports that an activation of the T cell subsets is not observed in the quiescent phase of the disease but becomes apparent during clinical exacerbation6,7,38).
In this study, the proportions of CD8CD11b cells in patients with IgA nephropathy are significantly lower than those in healthy controls. This result is in accordance with that of Schena et al39), and raises the possibility that defective immune suppression caused by the decreased activity of CD8CD11b cells, which is known to have the suppressive activity on B cells, may be important in the pathogenesis of IgA nephropathy. However the reason why the suppressive activity of CD8CD11b cells is confined to the production of IgA is not certain in this study.
In IgA nephropathy, there is a close temporal relationship between its onset and upper respiratory tract infection or gastroenteritis and a significant rise in antibody titer to specific infectious agents such as herpes virus, influenza virus, mycoplasma and gut flora was found40). In this aspect, the measurement of the activity of natural killer (NK) cells that are known to play an important role in immune surveillance mechanisms to provide resistance against viral infections41) in IgA nephropathy may be somewhat interesting to us. In this study, the NK cell activity of the patients with IgA nephropathy was significantly higher than that of healthy controls and was well correlated with the production of IL-2 by PBMC. Because it is known that IL-2 can enhance the proliferation42,43) and the cytolytic activity of NK cells44–46), increased production of IL-2 may be associated with elevation of NK cell activity. However our patients were in a clinically quiescent phase with undetectable IL-2 production without PHA stimulation. The causative factors of the increased NK cell activity in our patients may be explained by the remnant effect of previously elevated IL-2 production in the phase of clinical exacerbation, the residual effects of previously elevated interferon, or other unknown substances that can increase the NK cell activity in IgA nephritic patients. The clinical significance of elevated NK cell activity in IgA nephropathy is uncertain and further study may be needed.
IL-2 is a protein with a molecular weight of 15,000 which has an autocrine function for the T cells and the various immunoregulatory functions47,48). The function of IL-2 as a T cell growth factor20) is performed by induction of and interaction with IL-2 receptors22). Interaction of IL-2 with its receptor leads to the proliferation and differentiation of activated T lymphocytes into effector cells23). IL-2 also stimulates the synthesis of immunoglobulin by B cells with the involvement in the early stage of T cell dependent B cell activation and the late stage of B cell differentiation49,50). In these ways, IL-2 is involved iin the immunoregulatory function and may play some roles in the pathogenesis of immunological disorders.
IL-2 production by PBMC has been measured in several immune-mediated diseases including type 1 diabetes mellitus9), systemic lupus erythematosus10,11), acquired immunodeficiency syndrome12,13) and active pulmonary sarcoidosis14). In IgA nephropathy, only Schena et al39) have investigated the production of IL-2 by PBMC. They observed the spontaneous production of IL-2 by PBMCs and increased expression of IL-2 receptor on the surface of PBMCs and proposed that these might be responsible for the increased activity of helper T cells. They also showed the significant IL-2 production in patients with active phase of the disease and with renal insufficiency. As the production of IL-2 by fresh PBMC in our patients with IgA nephropathy was in undetectable ranges, we didn't confirm the spontaneous production of IL-2 in IgA nephritic patients. The state of clinical quiescence in our patients may account for the result. The results of the undetectable production of IL-2 before PHA stimulation in both IgA nephritic patients and healthy controls and more marked production of IL-2 after PHA stimulation in patients than in healthy controls in this study suggest that immunoregulatory dysfunction may become evident only after the stimulation with mitogens and/or extrinsic antigens.
Lai et al8) observed that the percentages of CD4 and CD8 lymphocytes, CD4/CD8 ratio did not differ between IgA nephritic patients and healthy controls in fresh isolated, unstimulated lymphocytes. Following pokeweed mitogen stimulation, individual T cell subsets bearing IL-2 receptors were distinctly different between IgA nephritic patients and healthy controls, that is, IgA nephritic patients had increased activated CD4 lymphocytes (with IL-2 receptor) and reduced activated CD8 lymphocytes. They proposed a defective immunoregulation in IgA nephropathy with enhanced T-helper/inducer and reduced T-suppressor/cytotoxic activity when stimulated with mitogen.
In this study, the proportions of lymphocytes expressing the IL-2 receptor before PHA stimulation were low in both the patients with IgA nephropathy and the healthy controls. After PHA stimulation, lymphocytes expressing the IL-2 receptor were more increased in IgA nephritic patients than in healthy controls. Although subsets of lymphocytes expressing IL-2 receptor were not analyzed in this study, the results that lymphocytes expressing the IL-2 receptors after PHA stimulation in patients with IgA nephropathy were more than those in healthy controls, as was the results of IL-2 production, potentiate the suggestion that immunoregulatory dysfunction may become apparent only after stimulation with mitogens. Altered immune parameters such as IL-2 production and IL-2 receptor expression after mitogen stimulation may play important roles in the pathogenesis of IgA nephropathy. Finally, our results that the mitogen stimulated production of IL-2 was correlated well with the ratio of CD4/CD8 and that the proportions of CD8 CD11b cells with the suppressive activity on B cells was significantly decreased in patients with IgA nephropathy suggest that altered activity of T cell subsets may be associated with the mitogen stimulated overproduction of IL-2 in IgA nephropathy.
REFERENCES
- 1.Woodroffe AJ, Gormly AA, Mckenzie PE, Wooton AM, Thompson AJ, Seymour AE, Clarkson AR. Immunologic studies in IgA nephropathy. Kidney Int. 1980;18:366. doi: 10.1038/ki.1980.147. [DOI] [PubMed] [Google Scholar]
- 2.Bannister KM, Drew PA, Clarkson AR, Woodroffe AJ. Immunoregulation in glomerulonephritis, Henoch-Schonlein purpura and lupus nephritis. Clin Exp Immunol. 1983;53:384. [PMC free article] [PubMed] [Google Scholar]
- 3.Egido J, Blasco R, Sancho J, Lozano L. T-cell dysfunctions in IgA nephropathy; specific abnormalities in the regulation of IgA synthesis. Clin Immunol Immunopathol. 1983;26:201. doi: 10.1016/0090-1229(83)90138-1. [DOI] [PubMed] [Google Scholar]
- 4.Egido J, Blasco RA, Sancho J. Immunological abnormalities in healthy relatives of patients with IgA nephropathy. Am J Nephrol. 1985;5:14. doi: 10.1159/000166897. [DOI] [PubMed] [Google Scholar]
- 5.Ihm CG, Woo JT, Chang YW, Kwon OS, Kim MJ. Immunological abnormalities in patient with IgA nephropathy. J Kor Med Sci. 1986;1:43. doi: 10.3346/jkms.1986.1.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linne T, Wasserman J. Lymphocyte subpopulations and immunoglobulin production in IgA nephropathy. Clin Nephrol. 1985;23:109. [PubMed] [Google Scholar]
- 7.Lai KN, Leung CK, Lai FM. In vitro study of expression of interleukin-2 receptors in T-lymphocytes from patients with IgA nephropathy. Clin Nephrol. 1988;30:330. [PubMed] [Google Scholar]
- 8.Lai KN, Leung CK, Lai FM. In vitro study of expression of interleukin-2 receptors in T-lymphocytes from patients with IgA nephropathy. Clin Nephrol. 1988;30:330. [PubMed] [Google Scholar]
- 9.Zier KS, Leo MM, Spielman RS, Baker L. Decreased synthesis of interleukin-2 (IL-2) in inusulin-dependent diabetes mellitus. Diabetes. 1984;33:552. doi: 10.2337/diab.33.6.552. [DOI] [PubMed] [Google Scholar]
- 10.Alcover-Varela J, Alarcon-Segovia D. Decreased production of and response to interleukin-2 by cultured lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 1982;69:1388. doi: 10.1172/JCI110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linker-Israeli M, Bakke AC, Kitridon RC, Gendler S, Gillis S, Horwitz DA. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE) J Immunol. 1983;130:2651. [PubMed] [Google Scholar]
- 12.Borzy MS. Interleukin 2 production and responsiveness in individuals with acquired immunodeficiency syndrome and the generalized lymphadenopathy syndrome. Cell Immunol. 1987;104:142. doi: 10.1016/0008-8749(87)90015-3. [DOI] [PubMed] [Google Scholar]
- 13.Prince HE, John JK. Abnormalities of interleukin 2 receptor expression associated with decreased antigen induced lymphocytes proliferation in patients with AIDS and related disorders. Clin Exp Immunol. 1987;67:59. [PMC free article] [PubMed] [Google Scholar]
- 14.Pinkston P, Bitterman PB, Crystal RG. Spontaneous release of interleukin 2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med. 1983;308:793. doi: 10.1056/NEJM198304073081401. [DOI] [PubMed] [Google Scholar]
- 15.Sakai H, Endoh M, Tomino Y, Nomoto Y. Increase of IgA specific helper Tα cells in patients with IgA nephropathy. Clin Exp Immunol. 1983;50:77. [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JK, Kim PK. Role of T-cells and B-cells in childhood glomerulopathies. Kor J Nephrol. 1987;6:64. [Google Scholar]
- 17.Endoh H, Sakai H, Nomoto Y, Tomino Y, Kaneshige H. IgA-specific helper activity of Tα cells in human peripherl blood. J Immunol. 1981;127:2612. [PubMed] [Google Scholar]
- 18.Sakai H, Nomoto Y, arimori S. Decrease of IgA-specific suppressor T cell activity in patients with IgA nephropathy. Clin Exp Immunol. 1979;38:243. [PMC free article] [PubMed] [Google Scholar]
- 19.Chatenoud L, Bach M. Abnormalities of T-cell subsets in glomerulonephritis and systemic lupus erythematosus. Kidney Int. 1981;20:267. doi: 10.1038/ki.1981.130. [DOI] [PubMed] [Google Scholar]
- 20.Morgan DA, Ruscetti FW, Gallo RC. Selective in vitro growth of T lymphocytes from normal human bone marrow. Science. 1976;193:1007. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 21.Mier JW, Gallo RC. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc Natl Acad Sci USA. 1980;77:6134. doi: 10.1073/pnas.77.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robb RJ, Munck A, Smith KA. T cell growth factor receptors: Quantitation, specificity and biological relevance. J Exp Med. 1981;154:1455. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Depper JM, Leonard WJ, Robb RJ, Waldmann WA, Greene WA. Blockade of the interleukin-2 receptor by anti-Tac antibody: Inhibition of human lymphocyte activation. J Immunol. 1983;131:690. [PubMed] [Google Scholar]
- 24.Kobayashi Y, Tateno S, Hiki Y, Shigematsu H. IgA nephropathy: Prognostic significance of proteinuria and histological alteration. Nephron. 1983;34:146. doi: 10.1159/000183000. [DOI] [PubMed] [Google Scholar]
- 25.Boyum A. Separation of leukocytes from blood and bone marrow with special reference to factors which influence and modify sedimentation properties of hematopoietic cells. Scand J Clin Lab Invest. 1968;21(Suppl 97):7. [PubMed] [Google Scholar]
- 26.Lotze MT, Rosenberg SA. In vitro growth of cytotoxic human lymphocytes The preparation of Lectin-free T cell growth factor (TCGS) and analysis of its activity. J Immunol. 1981;126:2215. [PubMed] [Google Scholar]
- 27.Evans RL, Wall DW, Platsoucas CD, Siegal FP, Fikrig SM, Testa CM, Good RA. Thymus-dependent membrane antigens in man: Inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci USA. 1981;78:544. doi: 10.1073/pnas.78.1.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jondal H, Pross H. Surface markers on human B and T lymphocytes VI Cytotoxicity against cell lines as a functional marker for lymphocyte subpopulations. Int J Cancer. 1975;15:596. doi: 10.1002/ijc.2910150409. [DOI] [PubMed] [Google Scholar]
- 29.Gillis S, Ferm MM, Du W, Smith KA. T cell growth factor: Parameters of production and a quantitative microassay for activity. J Immunol. 1978;120:2027. [PubMed] [Google Scholar]
- 30.Egido J, Blasco R, Sancho J, Lozano L, Sanchez-Crespo M, hernando L. Increased rates of polymeric IgA synthesis by circulating lymphoid cells in IgA mesangial glomerulonephritis. Clin Exp Immunol. 1982;47:309. [PMC free article] [PubMed] [Google Scholar]
- 31.Kincaid-Smith P, Nicholls P. Mesangial IgA nephropathy. Am J Kidney Dis. 1983;3:90. doi: 10.1016/s0272-6386(83)80020-1. [DOI] [PubMed] [Google Scholar]
- 32.Clarkson AR, Woodroffe AJ, Bannister KM, Lomax-Smith JD, Aarons I. The syndrome of IgA nephropathy. Clin Nephol. 1984;21:7. [PubMed] [Google Scholar]
- 33.Sissons JGP, Woodrow DF, Curtis JR, Evans DJ, Gower PE, Sloper JC, Peters DK. Isolated glomerulonephritis with IgA deposits. Br Med J. 1975;3:611. doi: 10.1136/bmj.3.5984.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coxio FG, Lam S, Folami AO, Conley ME, Michael AF. Immune regulation of immunoglobulin production in IgA nephropathy. Clin Immunol Immunopathol. 1982;23:430. doi: 10.1016/0090-1229(82)90127-1. [DOI] [PubMed] [Google Scholar]
- 35.Navas-Palacios JJ, Gutierrez-Millet V, Usera-Sarrage G. IgA nephropathy: An ultrastructural study. Ultrastruct Pahtol. 1981;2:151. doi: 10.3109/01913128109064244. [DOI] [PubMed] [Google Scholar]
- 36.Berger J, Yaneva H, Nabana B, Barbancel C. Recurrence of mesangial deposition of IgA after renal transplantation. Kidney Int. 1975;7:232. doi: 10.1038/ki.1975.35. [DOI] [PubMed] [Google Scholar]
- 37.Lai KN, Lai FM, Chui SH, Leung KN, Lam CWK. Effect of ciclosporin on lymphocyte subpopulations and immunoglobulin productiion in IgA nephropathy. Nephron. 1989;52:307. doi: 10.1159/000185668. [DOI] [PubMed] [Google Scholar]
- 38.Feehally J, Beattie T, Brenchley P, Coupes B, Mallick N, Postlethwaite R. Sequential study of the IgA system in relapsing IgA nephropathy. Kidney Int. 1986;30:924. doi: 10.1038/ki.1986.274. [DOI] [PubMed] [Google Scholar]
- 39.Schena FP, Mastrolitti G, Jirillo E, Munno I, Pellegrino N, Fracasso AR, Aventaggiato L. Increased production of interleukin-2 and IL-2 receptor in primary IgA nephropathy. Kidney Int. 1989;35:875. doi: 10.1038/ki.1989.67. [DOI] [PubMed] [Google Scholar]
- 40.Wolldroffe AJ, Gormly AA, Mckenzie PE, Wootton AJ, Thompson AJ, Seymour AE, Clarkson AR. Immunologic studies in IgA nephropathy. Kidney Int. 1980;18:366. doi: 10.1038/ki.1980.147. [DOI] [PubMed] [Google Scholar]
- 41.Djeu JY, Stocks N, Zoon K, Stanton GJ, Timonen T, Herberman RB. Positive self regulation of cytotoxicity in human natural killer cells by production of interferon upon exposure to influenza and herpes viruses. J Exp Med. 1982;156:1222. doi: 10.1084/jem.156.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki R, Handa K, Itoh K, Kumagai K. Natural killer (NK) cells as a responder to interleukin-2 (IL-2) Proliferative response and establishment of cloned cells. J Immunol. 1983;130:981. [PubMed] [Google Scholar]
- 43.Vose BM, Bonnard GD. Limiting dilution analysis of the frequency of human T cells and large granular lymphocytes proliferating in response to interieukin 21 The effect of lectin on the proliferative frequency and cytotoxic activity of cultured lymphoid cells. J Immunol. 1983;130:687. [PubMed] [Google Scholar]
- 44.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine activated killer cell phenomenon; lysis of natural killer-resistant fresh solid tumor cells by interieukin 2 activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155:1823. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henny CS, Kuribayashi K, Kern DE, Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981;291:335. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- 46.Kuribayashi K, Gillis S, Kern DE, Henny CS. Murine NK cell cultures: Effects of interleukin-2 and interferon on cell growth and cytotoxic reactivity. J Immunol. 1981;126:2321. [PubMed] [Google Scholar]
- 47.Luger TA, Smolen JS, Chused TM, Steinberg AD, Oppenheim JJ. Human lymphocytes with higher OKT4+and OKT8+ phenotype produced IL-2 in culture. J Clin Invest. 1982;70:470. doi: 10.1172/JCI110637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meuer SC, Hussey RE, Penta AC, Fitzgerald KA, Stadler BM, Scholossman SF, Reinherz EL. Cellular origin of IL-2 in man: Evidence for stimulus restricted IL-2 production by OKT4+ and OKT8+ T lymphocytes. J Immunol. 1982;129:1076. [PubMed] [Google Scholar]
- 49.Kehrl JH, Muraguchi A, Goldsmith PK, Fauci AS. The direct effects of interleukin 1, interleukin 2, interferon-α, interferon-r, B-cell growth factor, and a B-cell differentiation factor on resting and activated human B cells. Cell Immunol. 1985;96:83. doi: 10.1016/0008-8749(85)90338-7. [DOI] [PubMed] [Google Scholar]
- 50.Nakagawa T, Nakagawa N, Goldstein H, Volkman DJ, Fauci AS. Demonstration that human B cells respond differently to interleukin 2 and B cell differentiation factor based on their stages of maturation. J Immunol. 1986;137:3175. [PubMed] [Google Scholar]
- 51.Kim MJ, Ihm CG, Cho BS, Kim YW, Yang MH. A Histopathological study of IgA nephropathy. Kor J Nephrol. 1985;4:197. [Google Scholar]
- 52.Lee JW, Woo JT, Bae JG, Kwon OS, Ihm CG, Kim MJ. Clinical investigation and follow-up study of IgA nephropathy. Kor J Int Med. 1986;30:242. [Google Scholar]


