Abstract
Basal plasma tissue type plasminogen activator (t-PA) and plasminogen activator inhibitor type 1 (PAI-1) antigen levels were studied in 49 non-insulin dependent diabetic patients (23 men, 26 women: ages 51.3±14.9 years) and 16 age matched non-diabetic subjects (9 men, 7 women: ages 49.8±12.2 years) as a control group. Compared to a control group, the diabetic patients had a significantly higher mean t-PA antigen (5.15±3.02 vs 3.20±2.30 ng/ml) and PAI-1 antigen (35.89±18.59 vs 17.60±15.36 ng/ml) levels (p<0.05). Plasma t-PA antigen level was not influenced by each treatment modality. There was a significant decrease of plasma PAI-1 antigen level after Metformin administration compared to that of before Metformin administration (39.74±19.39 vs 25.14±16.18 ng/ml) (p<0.05), and the insulin-treated group showed a tendency for a decrease of plasma PAI-1 antigen levels after insulin administration but this did not reach statistical significance (29.93±15.37 vs 17.32±10.60 ng/ml). Sulfonylurea did not change both plasma t-PA and PAI-1 antigen levels.
In conclusion, diabetic patients have high t-PA and PAI-1 antigen levels. Biguanide reduced plasma PAI-1 antigen levels, which might play some helpful role in the improvement of chronic complications in NIDDM.
Keywords: t-PA, PAI-1, NIDDM, Treatment Modality
INTRODUCTION
Diabetic patients are predisposed to micro and macro-angiopathy which occurs earlier and more frequently than in comparable non-diabetic patients1). Thromboembolic occlusion of the microvasculature might be caused by a disturbance of the delicate equibrium between coagulation and fibrinolysis. The endothelial cells produce the t-PA and also an inhibitor (PAI) of this activator2). After vascular occlusion, t-PA is immediately released and activates plasminogen to plasmin, which starts to digest the fibrin mesh work of the thrombus3). Recently Auwerx et al. reported that the low fibrinolytic activity presented by NIDDM patients was related to elevated concentration of t-PA and PAI activity4). Defective fibrinolysis in diabetic patients might constitute a factor of pathogenetic significance for the development of the vascular manifestations of diabetes5–8). Implicit in such a concept of the cause and effect of an impaired fibrinolysis is the possibility that stimulation of fibrinolysis by the administration of drugs might delay the development of angiopathy in diabetes. Sulfonylurea has been reported to enhance fibrinolysis and to improve the microcirculation in diabetes9–12). Insulin stimulates the synthesis of PAI-1 by the human hepatocelluar cell line13) and metformin decreases the PAI14). The aim of this study is, in addition to evaluating the fibrinolytic system compared with non-diabetic controls, to know what effects conventional treatment modalities (insulin, sulfonylurea and biguanide) have on plasma t-PA and PAI antigen levels in diabetic patients.
MATERIALS AND METHODS
49 NIDDM patients (23 men, 26 women), mean age (SD) 51.3 years (14.9), were studied. The mean duration of diabetes was 7.8±6.1 years. They were treated with insulin (Humulin-N, Eli Lilly, mean 28. 2±9.4 U/day) in 17 cases, sulfonylurea (Gliclazide, 80–240 mg/day) in 10 cases, and biguanide (Metformin, 500–1500 mg/day) in 22 cases. Their mean follow up period was 36.1 days (range 16–62 days). During the trial of each treatment, subjects were kept on weight maintaining diet, and there were no significant blood sugar and body weight changes. Clinico-biochemical profiles of diabetic patients were listed on Table 1. 16 age-matched subjects (9 men, 7 women), mean age (SD) 49.8 years (12.2), served as controls.
Table 1.
Clinico-biochemical Profiles of Diabetic Patients Studied (mean±SD)
| Profiles | Total diabetics (n=49) | Insulin-treated group (n=17) | Sulfonylurea-treated group (n=10) | Biguanide-treated group (n=22) |
|---|---|---|---|---|
| Men: Women | 23:26 | 10:7 | 7:3 | 6:16 |
| Age (year) | 51.2±14.9 | 50.6±30.7 | 49.9±16.6 | 52.2±13.2 |
| Duration (year) | 7.8±6.1 | 9.2±6.2 | 8.4±7.6 | 7.5±5.5 |
| BMI (kg/m2) | 23.2±3.4 | 20.8±1.7 | 21.3±3.6 | 26.0±2.3≠ |
| HbAlc (%) | 8.2±1.5 | 8.4±1.9 | 7.4±1.2 | 8.4±1.1 |
| Blood sugar (mg/dl) | ||||
| Fasting 1* | 160.9±69.5 | 175.0±90.8 | 156.1±59.2 | 152.2±57.8 |
| Fasting 2+ | 156.0±53.6 | 153.8±69.3 | 150.0±83.4 | 151.5±27.9 |
| C-peptide (ng/ml) | ||||
| Basal | 2.01±1.62 | 1.77±1.87 | 1.67±1.04 | 2.56±1.46 |
| Serum lipid (mg/dl) | ||||
| Total cholesterol | 187.3±65.3 | 171.5±72.7 | 173.6±52.1 | 204.7±62.7 |
| HDL cholesterol | 45.2±10.9 | 43.1±9.9 | 45.3±17.1 | 46.6±9.2 |
| LDL cholesterol | 114.6±49.5 | 100.4±65.4 | 103.1±35.7 | 128.5±37.7 |
| Triglyceride | 168.8±78.2 | 142.9±73.9 | 172.7±117.4 | 186.5±62.1 |
| Proteinuria (mg/24 hr) | 239.5±555.4 | 417.4±803.21 | 169.2±164.1 | 134.1±228.7 |
| Follow-up perio (day) | 36.1±16.7 | 33.8±18.5 | 35.7±16.6 | 38.0±15.4 |
Fasting blood sugar (FBS) before treatment,
FBS after treatment
p<0.05 compared with insulin and sulfonylurea-treated groups
Blood samples for blood sugar, HbAlc, serum total cholesterol, HDL cholesterol, LDL cholesterol and triglyceride were obtained at a fasting state. Glycosylated hemoglobin (HbAlc) was measured by cation-exchange chromatography. Serum C-peptide was assayed by radioimmunoassay and serum samples were extracted with polyethylene glycol. Proteinuria was assessed by estimating the 24 hour urinary protein with the trichloroacetic acid method. For t-PA and PAI-1 antigen determination, blood from the antecubital vein was collected from resting state at 8:00 a.m., after at least 8 hours fasting, and was collected into trisodium citrate (final concentration 0.011 M). Platelet poor plasma (PPP) was obtained by centrifuging at 4000 g (for 15 min) within 60 min after blood collection. The PPP was aliquoted and stored at −70°C t-PA and PAI-1 antigen was measured by an enzyme linked immunosorbent assay (ELISA) using a commercially available kit (Asserachrom t-PA, Imubind PAI-1, respectively), and each assay was run in one session in order to eliminate interassay variation.
Statistical analysis was achieved by the student’s t-test on paired or unpaired value results, expressed as the mean±SD, were considered as significantly different when p value was lower than 0.05. Simple linear regression analysis was used to evaluate the univariate relationships between fibrinolytic indices (t-PA and PAI-1) and various parameters of diabetes.
RESULTS
Table 1 shows the means and standard deviations of various parameters in diabetic patients. No significant differences between men and women were observed. There was no difference between each group of diabetic patients, except body mass index which is higher in biguanide-treated group than other groups.
In diabetic patients, the basal plasma concentration of t-PA (5.15±3.02 ng/ml) and PAI-1 antigen (35.89±18.59 ng/ml) were significantly higher than in the controls (3.20±2.30, 17.60±15.36 ng/ml), respectively (p<0.05) (Table 2, Fig. 1).
Table 2.
Comparison of Plasma t-PA and PAI-1 Antigen Levels between Diabetic and Non-diabetic Subjects
| Subject | t-PA (ng/ml) | PAI-1 (ng/ml) |
|---|---|---|
| Diabetics (n=49) | 5.15±3.02* | 35.89±18.59* |
| Non-diabetics (n=16) | 3.20±2.30 | 17.60±15.36 |
p<0.05 compared with non-diabetic subjects
Fig. 1.
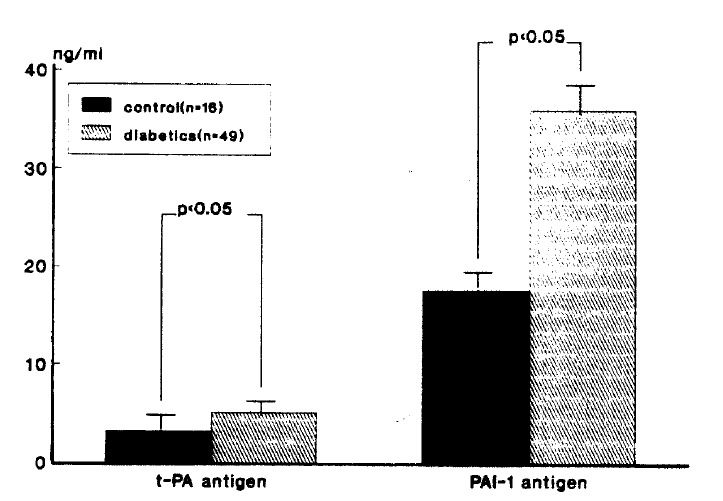
Comparison of t-PA and PAI-1 antigen levels diabetic and non-diabetic subjects.
There was no difference of plasma t-PA antigen levels between before and after each treatment modality and plasma t-PA showed an increase in the insulin-treated group, but this did not reach statisitcal significance (p=0.19) (Table 3). Metformin treated group exhibited a significant decrease in PAI-1 antigen level (p<0.05) while other treatment groups showed no alteration in PAI-1 antigen level (Table 4, Fig. 2).
Table 3.
Changes of Plasma t-PA Antigen Levels According to Treatment
| Treatment | t-PA (ng/ml)
|
|
|---|---|---|
| Before | After | |
| Insulin (n=10) | 4.07±3.28 | 6.13±3.00 |
| Sulfonylurea(n=8) | 4.07±1.90 | 3.93±2.91 |
| Biguanide (n=18) | 6.47±2.80 | 7.10±6.97 |
Table 4.
Changes of Plasma PAI-1 Antigen Levels According to Treatment
| Treatment | PAI-1 (ng/ml)
|
|
|---|---|---|
| Before | After | |
| Insulin (n=10) | 29.93±15.37 | 17.32±10.60 |
| Sulfonylurea(n=8) | 30.70±23.17 | 33.73±20.55 |
| Biguanide (n=18) | 39.74±19.39 | 25.14±16.18* |
p<0.05 compared with before treatment
Fig. 2.
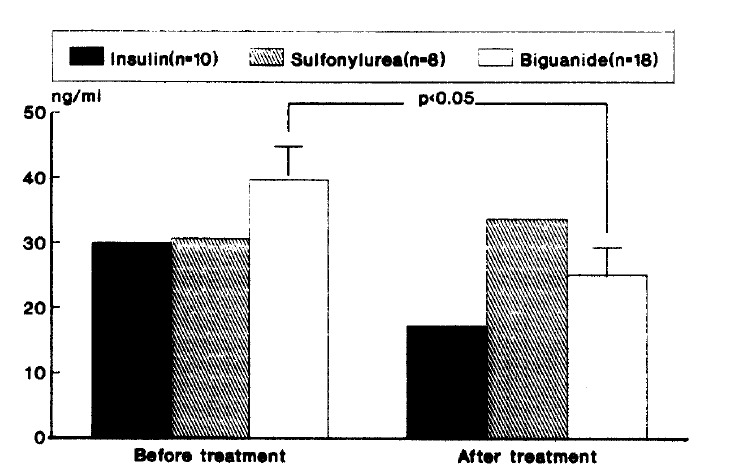
Changes of plasma PAI-1 antigen level according to treatment modality.
DISCUSSION
The pathogenesis of the micro-vascular complications in diabetes is still obscure. Among the possible etiologic factors in the development of diabetic angiopathy are the disturbances of the fibrinolytic system.
Almer et al reported that a poor defense mechanism against deposits in the vessel walls in diabetes might contribute to the development of diabetic microangiopathy3). Final outcome of fibrinolytic activity is determined by balance in the blood between t-PA and PAI, and it is difficult to differentiate free t-PA and/or their complexes in current methods. And there are no indices that reflect the exact fibrinolytic activities of the blood. Cash et al reported that the mean basal levels of t-PA in the diabetic population appeared to be higher than those of the controls and high level of t-PA is a physiological response to this relative hypercoagulativity15). Deficient fibrinolysis is mostly due to the increase in plasma PAI-1 level16) and several reports have pointed that the reduced plasma fibrinolytic activity showed a negative correlation with PAI activity4,6,17,18). The elevated level of PAI activity causes inhibition of t-PA18). Garcia Frade et al reported that elevated PAI activity and decreased fibrinolytic response to stimulus may contribute to vascular disease in diabetic patients6). In this study, diabetic patients showed an elevation of t-PA and PAI antigen levels. These findings are similar to the results reported by others4,6,18). Increased t-PA antigen levels in the presence of decreased fibrinolytic activity can be explained on the basis of the occurrence of inactive t-PA-PAI complexes in blood, resulting in reduced free t-PA levels4,20). But it is impossible to decide if the elevation of PAI in diabetics is secondary to angiopathy, or if it is primarily a metabolic defect of the endothelial synthesis and release of the inhibitor3).
Several studies reported that sulfonylurea induced a significant increase in the t-PA activity9–12), while another study reported that treatment of newly diagnosed maturity-onset diabetic patients with gliclazide did not raise the fibrinolytic activity inspite of a significant improvement of the glycemic control21). In this study, sulfonylurea did not alter both t-PA and PAI-1 antigen levels. Discrepancy between other and our result is not clear and we speculated that insufficient subjects, and difference in patient selection and/or treatment protocol, may contribute to this discrepancy. Long-term prospective and longitudinal studies should be required.
A reduction of the plasma insulin levels was associated with a decrease in PAI and high insulin levels might stimulate the synthesis of PAI13,22). The existence of a significant correlation between plasma PAI and insulin concentration and the simultaneous variation of these two parameters in intervention studies aimed at resulting in the insulin level suggest that insulin may act directly and indirectly on the cells which synthesize PAI-123,24). Another mechanism by which insulin influences the release of PAI-1 may be either direct or indirect through modification in plasma lipoproteins. Very low density lipoprotein stimulates PAI-1 secretion by endothelial cells25). Recently, Modrescek et al reported that PAI-1 was significantly decreased after introduction of insulin26), while another study reported acute hyperinsulinism does not affect the fibrinolytic activity in control and obese subjects27). In our results, the insulin-treated group showed an increase of plasma t-PA and PAI-1, but this did not reach statistical significance.
In agreement with our results, there are some previous reports that biguanide stimulate fibrinolysis28–30). Chakrabarti et al reported Metformin-ameliorated fibrinolysis in some patients with occlusive vascular disorders28) and Hocking et al reported that biguanide had a beneficial effect on euglobulin fibrinolytic activity30).
The mechanism by which Metformin decreases PAI is not clear. Primarly inhibitory effect of Metformin on PAI production at the liver and endothelial cell should be considered. The stimulating effect of insulin on PAI-1 synthesis by HepG2 cell line was abolished when Metformin was added to the cell cultures at the same time as insulin, and this inhibitory effect of Metformin was dose dependent31).
In conclusion, diabetic patients are hypofibrinolytic compared with non-diabetic patients. Administration of Metformin could lead to an enhancement of fibrinolysis in NIDDM patients by reduction of a decrease in plasma PAI levels, which might contribute as a useful role to reduce the progression of dibetic microangiopathy, but long-term prospective studies should be required in order to determine if the change of the fibrinolytic system induced by Metformin is favourably associated with a delayed development of the vascular complications of diabetes.
REFERENCES
- 1.Crofford OB. The long-range plan to combined diabetes. Vol. 1. US Department of Health, Education and Welfare, Public Health Service, National Institute of Health; 1976. Report of the National Commission on Diabetes. Publication NO. (NIH) 76-1018. [Google Scholar]
- 2.Sprengers ED, Kluft C. Plasminogen activator inhibitors. Blood. 1987;69:381. [PubMed] [Google Scholar]
- 3.Almer LO. Fibrinolytic disorders in diabetes mellitus. Diabete & Metabolism. 1988;14:519. [Google Scholar]
- 4.Auwerx J, Bouillon R, Collen D, Goboers J. Tissue-type plasminogen activator antigen and plasminogen activator inhibitor in diabetes mellitus. Arteriosclerosis. 1988;8:68. doi: 10.1161/01.atv.8.1.68. [DOI] [PubMed] [Google Scholar]
- 5.Fearnley GR, Chakrabarti R, Avis PR. Blood fibrinolytic activity in diabetes mellitus and its bearing on ischemic heart disease and obesity. Brit Med J. 1963;1:921. doi: 10.1136/bmj.1.5335.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia Frade LJ, be la Calle H, Torrado MC, Lara JI. Hypofibrinolysis associated with vasculopathy in non-insulin dependent diabetes mellitus. Thromb Res. 1990;59:51. doi: 10.1016/0049-3848(90)90270-m. [DOI] [PubMed] [Google Scholar]
- 7.Almer LO, Pandolfi M, Nilsson IM. Diabetic retinopathy and the fibrinolytic system. Diabetes. 1975;24:529. [PubMed] [Google Scholar]
- 8.Cho YW, Oh DY, Kim SJ, Hong SY, Lee HC, Huh KB. Euglobulin fibrinolytic activity in NIDDM patients. Diab Res Clin Prac. 1991;13:139. doi: 10.1016/0168-8227(91)90057-k. [DOI] [PubMed] [Google Scholar]
- 9.Desnoyers P, Saint-Disier D. Gliclazide: Haemobiological properties. A synopsis with emphasis on inhibition of platelet coagulation factors. R Soc Med. 1980;20:19. [Google Scholar]
- 10.Almer LO. Vascular fibrinolytic activity in long-term treatment with second generation sulfonylurea compounds. Acta Endocrinol. 1990;239(Suppl):53. [PubMed] [Google Scholar]
- 11.Duhault J, Boulanges M, Lonchampt M. Gliclazide and the microvascular system. R Soc Med. 1980;20:9. [Google Scholar]
- 12.Almer LO. Effect of chloropropramide and gliclazide on plasminogen activator activity in vascular wall in patients with maturity-onset diabetes. Throm Res. 1984;35:19. doi: 10.1016/0049-3848(84)90309-8. [DOI] [PubMed] [Google Scholar]
- 13.Alessi MC, Juhan-Vague I, Kooistra T, Declerck PJ, Collen D. Insulin stimulates the synthesis of plasminogen activator inhibitor 1 by the human hepatocellular cell line Hep G2. Thromb Haemost. 1988;60:491. [PubMed] [Google Scholar]
- 14.Vague P, Juhan-Vague I, Alessi MC, Badier C, Valadier J. Metformin decreases the high plasminogen activator inhibition capacity, plasma insulin and triglyceride levels in non-diabetic obese subjects. Thromb Haemost. 1987;57:326. [PubMed] [Google Scholar]
- 15.Cash JD, McGill RC. Fibrinolytic response to moderate exercise in young male diabetes mellitus and non-diabetics. J Clin Pathol. 1969;22:32. doi: 10.1136/jcp.22.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiman B, Hamsten A. The fibrinolytic enzyme system and its role in the etiology of thromboembolic disease. Semin Thromb Haemostasis. 1990;26:207. doi: 10.1055/s-2007-1002671. [DOI] [PubMed] [Google Scholar]
- 17.Vague P, Juhan-Vague I, Aillaud MF, Badier C, Vired R, Alessi MC, Collen D. Correlation between blood fibrinolytic activity, plasminogen activator inhibitor level, plasma insulin level and relative body weight in normal and obese subjects. Metabolism. 1986;35:250. doi: 10.1016/0026-0495(86)90209-x. [DOI] [PubMed] [Google Scholar]
- 18.Juhan-Vague I, Collen D. Hypofibrinolysis and atherothrombosis. Diabete & Metabolism. 1988;14:479. [Google Scholar]
- 19.Suenson E, Peterson LC. Fibrin and plasminogen structures essential to stimulation of plasmin formation by tissue-type plasminogen activator. Biophys Acta. 1986;870:510. doi: 10.1016/0167-4838(86)90260-8. [DOI] [PubMed] [Google Scholar]
- 20.Collen D. Mechanisms of inhibition of tissue-type plasminogen activator in blood. Thromb Haemost. 1983;50:678. [PubMed] [Google Scholar]
- 21.Panton RC, Kernoff PBA, Wales JK, McNicol GP. Effect of diet and gliclazide on the haemostatic system of non-insulin dependent diabetics. Br Med J. 1981;283:1018. doi: 10.1136/bmj.283.6298.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juhan-Vague I, Roul C, Alessi MC, Ardissone JP, Heim M, Vague P. Increased plasminogen activator activity in non-insulin dependent diabetic patients-relationship with plasma insulin. Thromb Haemost. 1989;61:370. [PubMed] [Google Scholar]
- 23.Loskutoff DJ, van Mourik JA, Erickson LA, Lawrence D. Detection of an unusually stable fibrinolytic inhibitor produced by bovine endothelial cells. Proc Natl Acad Sci USA. 1983;80:2956. doi: 10.1073/pnas.80.10.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sprenger ED, Princen HMG, Kooistra T, van Hinsberg VWM. Inhibition of plasminogen activators by conditioned medium of human hepatocytes and hepatoma cell line. Hep G2. J Lab Med. 1985;105:751. [PubMed] [Google Scholar]
- 25.Stiko-Rahm A, Wiman B, Hamsten A, Nilsson IM. Secretion of plasminogen activator inhibitor 1 from cultured human umbilical vein endothelial cells is induced by very low density lipoprotein. Arteriousclerosis. 1990;10:1067. doi: 10.1161/01.atv.10.6.1067. [DOI] [PubMed] [Google Scholar]
- 26.Medvescek M, Keber D. Depressed fibrinolysis following introduction of insulin therapy in type 2 diabetics. Diabetes. 1991;40(Suppl 1):465A. [Google Scholar]
- 27.Lormeau B, Debussche X, Gross S, Puy H, Fendri S, Delobel J, Oqichaud J. Acute insulinism has no effect on fibrinolysis in lean and obese subjects. Diabetes. 1991;(Suppl 1):494A. [Google Scholar]
- 28.Chakrabartic R, Hocking ED, Fearnley GR. Fibrinolytic effect of Metformin in coronary artery disease. Lancet. 1963;1:352. doi: 10.1016/s0140-6736(65)92383-4. [DOI] [PubMed] [Google Scholar]
- 29.Bouisson H, Thiers JC, Douste-Blazy L, Pieraggi MT, Julian M. Chronic lathyrism and atheromatosis in the rat. Protective effect of Metformin. Gerontology. 1980;26:188. doi: 10.1159/000212414. [DOI] [PubMed] [Google Scholar]
- 30.Hocking ED, Chakrabarti JE, Fearnley GR. Effect of biguanide and atromid on fibrinolysis. J Atheroscl Res. 1967;7:121. doi: 10.1016/s0368-1319(67)80074-7. [DOI] [PubMed] [Google Scholar]
- 31.Juhan Vague I, Alessi MC, Vague P. Increased plasma plasminogen activator inhibitor 1 levels. A possible link between insulin resistance and atherothrombosis. Diabetologia. 1991;34:457. doi: 10.1007/BF00403280. [DOI] [PubMed] [Google Scholar]


