Abstract
Neurofibromatosis (von Recklinghausen’s disease) is a neuroectodermal disorder characterized by pigmentary changes of the skin (café-au-lait spots), cutaneous and visceral tumors (neurofibromas) and systemic abnormalities. The involvement of gastrointestinal tract in neurofibromatosis is not common. The most common symptoms, refer able to lesions in the gut, are hematemesis, melena and abdominal pain.
We experienced a case of intestinal neurofibroma in von Recklinghausen’s disease. The patient was a 39 year-old female who had suffered from chronic iron deficiency anemia and recurrent gastrointestinal hemorrhage due to two neurofibromas of jejunum for 3 years, which was diagnosed by superior mesenteric and ileal arteriogram and 99mTc pertechnate-labelled RBC scan, and treated by segmental resection of jejunum with end to end anastomosis.
Keywords: Neurofibromatosis, Anemia, Intestinal hemorrhage
INTRODUCTION
Neurofibromatosis, first clearly described by von Recklinghausen, is a dominantly inherited neuroectodermal dysplasia, with the frequency of 1 out of every 3,000 births1) and occurs slightly more often in male than in female. Characteristic lesions are café-au-lait spots and neurofibromata along the path of peripheral nerves.
Multiple neurofibromas have been often found in almost all parts of the body, including the gastrointestinal tract2). Although neurofibromatosis is always a generalized disease, the involvement of the gastrointestinal tract is not common, Therefore, when intestinal hemorrhage or chronic anemia occurs in a case with neurofibromatosis, gastrointestinal involvement should be suspected3).
Here, we reported a case of intestinal neurofibromatosis with chronic iron deficiency anemia due to recurrent gastrointestinal hemorrhage.
CASE REPORT
A 39-year old woman was admitted to Severance Hospital via the emergency room with symptoms of palpitation, dyspnea on exertion and tarry stool for 20 days.
There was no history of pulmonary tuberculosis, hypertension and diabetes mellitus. There was no family history of neurofibromatosis. Five years prior to admission, she visited a private clinic for multiple skin nodules, which she had had since birth, and biopsy from the lesion of the thigh revealed neurofibromatosis. Three years before admission, she had palpitation and dizziness and was hospitalized in another general hospital, where she was discharged with the diagnosis of anemia of unknown origin, in spite of all kinds of examinations. Later she had experienced the above events three times for two years before admission.
Laboratory findings showed iron deficiency anemia at the first admission (October 1988). Simultaneously, UGI series showed no abnormality. Abdominal ultrasonography showed gall bladder dilatation with cystic duct stone and splenomegaly. Endoscopic retrograde cholangiopancreatography showed cystic duct stone. On sigmoidoscopy, internal hemorrhoid with some blood was noticed. After hemorrhoidectomy, she had taken iron pills. For twenty days before readmission, she had had tarry stool with pale face.
On admission, the temperature was 36.9°C, the pulse 70/min and the respirations 14/min. The blood pressure was 100/70 mmHg. The height was 151 cm and the body weight 53 kg. She had a chronically ill-looking appearance. The conjunctivae were pale and the sclerae were not icteric. There were multiple 1–2 cm sized neurofibromas on the whole body, especially on the back (Fig. 1). The chest, heart and abdomen were normal.
Fig. 1.
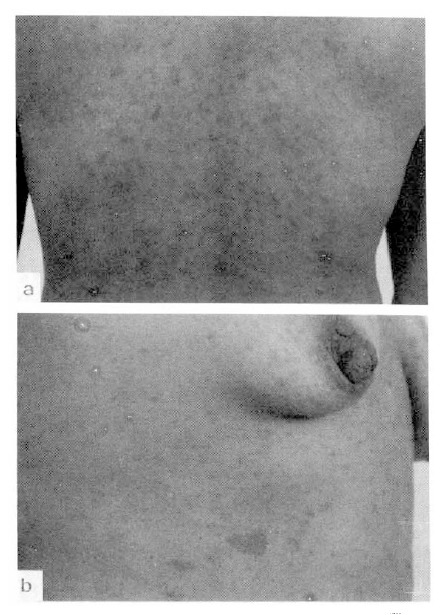
Multiple, 1–2 cm sized, flesh colored neurofibromas on the back (a) and café-au-lait spots on the chest wall (b).
The hemoglobin was 5.1 g/dl, the hematocrit 17.4 percent, the white cell was 6,200/mm3 with 66 percent neutrophil. The platelet was 339,000/mm3. The reticulocyte count was 1.6 percent. The corrected erythrocyte sedimentation rate was 0 mm/hr. The urine was normal. The prothrombin time was 12.3 seconds. The calcium was 8.5 mg/dl, the phosphorus 3.1 mg/dl, the glucose 95 mg/dl, the blood urea nitrogen 9.0 mg/dl, the creatinine 0.7 mg/dl, the uric acid 4.4 mg/dl, the total bilirubin 1.2 mg/dl, the alkaline phosphatase 53 IU/l, the SGOT 13 IU/l and the SGPT was 5 IU/L. The electrolyte was within normal limit. The serum iron was 12 mg/dl, the total iron binding capacity was 355 mg/dl and the iron saturation 3.4 percent. The occult blood examination of stool was negative. The antinuclear antibody titration was 1:20 positive and 1:80 negative, and the anti-DNA was 1:10 negative. The electrocardiography was normal.
After transfusion of 5 units of packed red cell, the hemoglobin was 9.9 g/dl, the hematocrit 31.1 percent. The fiberoptic gastroscopy showed mild diffuse chronic superficial gastritis. The UGI series and small bowel series (Fig. 2) revealed no specific abnormal findings. The radionuclide scanning, using 99mTc pertechnate-labelled RBC, revealed abnormal radioactivity in the right lower abdomen and increased activity by the time sequence, which was suggestive of lower gastrointestinal bleeding from the right hypogastric arterial branch (Fig. 3). On the flexible sigmoidoscopy, internal hemorrhoid without evidence of recent active bleeding was found. The superior mesenteric and ileal arteriogram showed bleeding from the left ileal arterial branches (Fig. 4). On the 15th hospital day, operation was performed under the diagnosis of small bowel tumor. There were two masses on jejunum located 60 and 120 cm from the ligament of Treitz, respectively. The distal mass was irregulary lobulated with some ulcerations on its surface, and tumor bleeding into the lumen was noted.
Fig. 2.
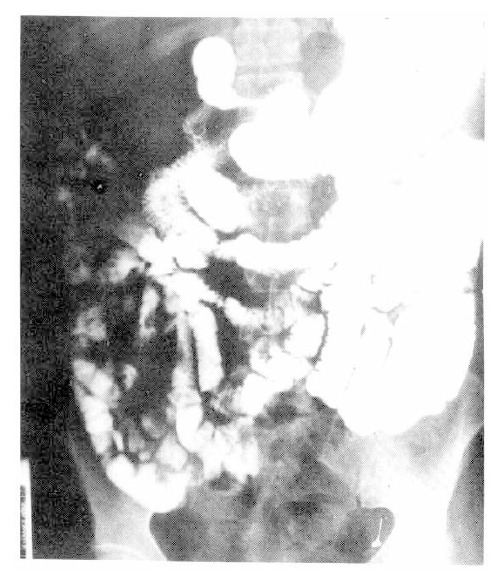
Small bowel series showing no abnormal mucosal changes or filling defect.
Fig. 3.
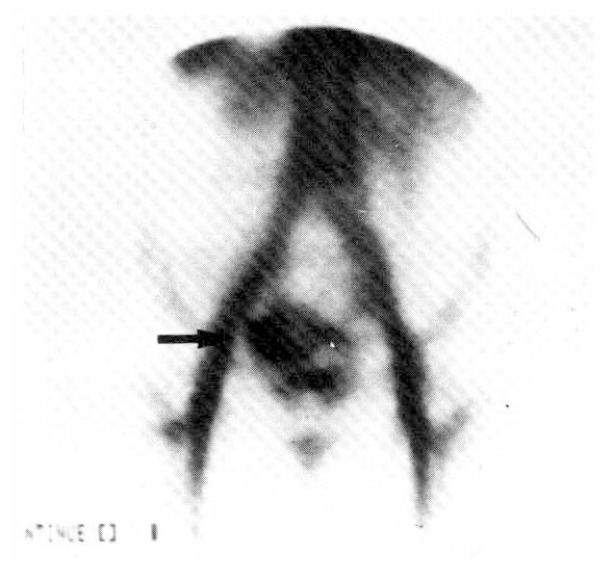
Radionuclide angiogram using 99mTc pertechnate-labelled RBC 20 mCi intravenously and taken 30 minutes after injecion showing an abnormal accumulation of radioactivity in the pelvic cavity (arrow).
Fig. 4.
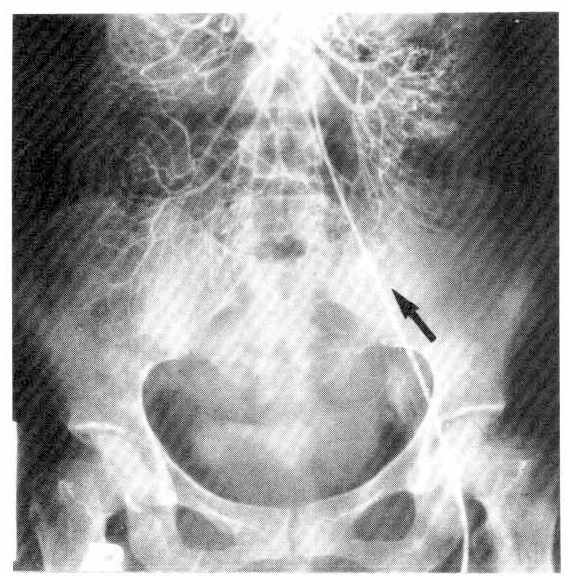
Superior mesenteric angiogram showing abnormal contrast media extravasation from ileal artery (arrow).
Segmental resection of jejunum with end to end anastomosis and cholecystectomy were performed. Also the skin biopsy on the left breast was performed during the operation. The pathologic diagnosis was neurofibromas of the jejunum (Fig. 5–, 8) and the skin (Fig. 9). The patient has been in good health without intestinal bleeding until now.
Fig. 5.

A polypoid mass of the jejunum is protruded into the serosa and bowel lumen. It is covered by the serosa and intact mucosa.
Fig. 6.
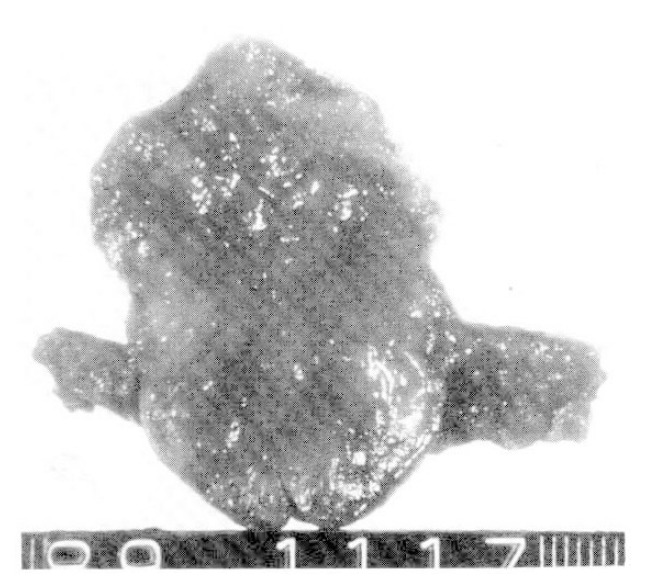
The cut surface of the jejunal mass showing a gray-white myxoid appearance.
Fig. 7.

The well circumscribed but not encapsulated mass is located in the muscle proper. The mucosa of jejunum shows inflammatory infiltrates in the lamina propria (H & E, ×40).
Fig. 8.
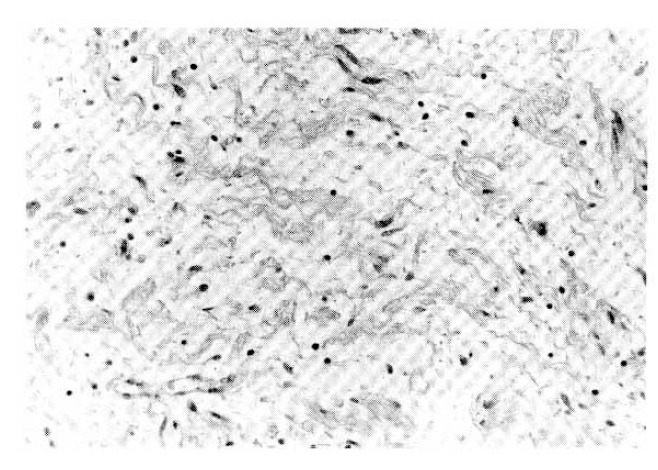
The neurofibroma of the jejunal mass showing dark staining axons and Schwann cell cylinders separated by mucinous matrix. The stroma is dotted with occasional mast cells and lymphocytes (H & E, ×400).
Fig. 9.
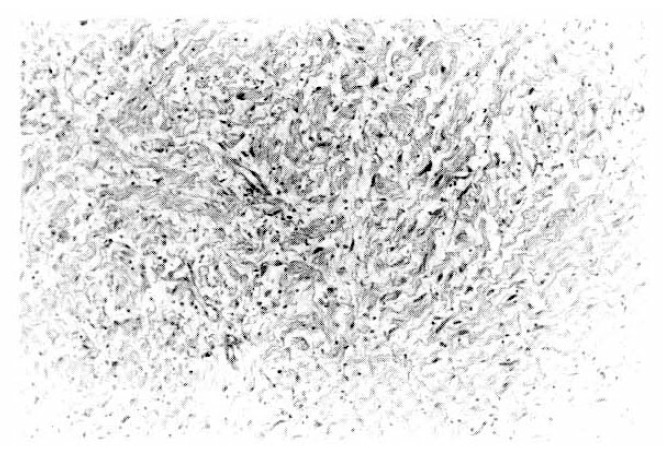
The neurofibroma excised from the left breast showing Schwann cells associated with wirelike collagen fibrils and modest amount of stromal mucosubstance (H & E, ×200).
DISCUSSION
Neurofibromatosis is characterized by tumors and pigmentary change of the skin4), and six or more café-au-lait spots are present in 78% of neurofibromatosis1). Multiple neurofibromas have been found to involve almost all parts of the body, including the gastrointestinal tract2). River et al5) reported that out of 90 benign gastrointestinal tumors of neurogenic origin, including neurofibromas, neurinomas and ganglioneuromas, only 14 of them were associated with neurofibromatosis. Davis and Berk6) estimated that gastrointestinal neurofibromas occurred in 25 percent of patients with multiple neurofibomatosis, but only 15 percent of patients with gastrointestinal neurofibromas had neurofibromatosis, and Christ7) reported that only three of 12 cases with neurofibromatosis showed gastrointestinal involvement out of the total number of 27,149 autopsy cases.
Intestinal neurofibromas begin in the submucosa, possibly in Auerbach’s plexus8). In River’s series of 57 cases, in which the location was specificed, 25 tumors were extraluminal, 18 intraluminal, six intramural and seven combined5). The actual number of tumors of bowel lesions may vary from one to a hundred or more9,10). Sarcomatous degeneration occurs in 15 percent of patients, usually in those over 40 years of age11). In benign neurofibromas, ulcers are single, small and well defined, compared with neurofibrosarcomas in which the ulcers tend to be bizzare, large and irregular12). In the case of this study, two well defined neurofibromas without capsule were found in submucosa of the jejunum with ulcerations on the covering mucosa.
All ages are affected, but the highest incidence is ranged from 40 to 60 years of age5). The most common locations of involvement are the jejunum, followed by stomach, ileum, colon and mensentery of the small intestine13). However, some authors reported that the ileum is most frequently involved6). In our case, the patient was slightly younger (39 years old). The involved site was jejunum. There were two well defined masses without capsule, in the submucosa of the jejunum with small ulcers on the covering mucosa (Fig. 5–6).
When the gastrointestinal tract is involved, symptoms produced are very vague. The tumors are often asymptomatic unless ulceration leads to bleeding and anemia11,14–16). The most common gastrointestinal symptoms were melena, pain and hematemesis, and less common symptoms of constipation or peptic-ulcer disease. The tumor arises somewhere in the mesenchymal tissues and gradually grows in size until the overlying mucosa becomes ulcerated. Ulceration of the mucosa is always caused by the presence of a growing tumor underlying it and not by direct involvement. This is characteristic of all mesenchymal gastrointestinal tumors. Since ulceration and subsequent hemorrhage are functions of the mucosal pressure produced by the tumor, it becomes obvious that bleeding will occur at different times in the various tumors. A small, submucosal nodule growing into the bowel lumen will produce ulceration much sooner than a larger, subserous nodule growing outward into the peritoneal cavity. The bleeding in the patient with intestinal neurogenic tumors is usually chronic, leading to symptoms such as weakness, fatigue and exertional dyspnea, but occasionally massive hemorrhage will occur17). As the tumors enlarge, there may be a palpable mass, bowel obstruction, volvulus or perforation6,17). Bowel obstruction is seldom produced and pain is minimal.
It has been reproted that 50 percent of cases of von Recklinghausen’s disease may show hyperplasia of the Auerbach’s plexus throughout the entire gastrointestinal tract9). These, showing elevated levels of nerve-growth factor in the sera of von Recklinghausen’s disease, suggest a basic abnormality in neuronal proliferation18).
The small bowel tumor is not easy to detect and is most often discovered at the time of operation for intestinal obstruction, which frequently appears to be intussusception, occasionally volvulus or obstruction of bowel by the tumor itself. Less commonly, intestinal bleeding may occur and, in one large series, was present in 30% of patients with small bowel tumors5). In a third of these patients, the bleeding was massive. In the group of patients which had only occult bleeding and anemia, a surprisingly large number of them were followed for many years without a correct diagnosis of the cause of bleeding being made. Also, in this case, the patient had suffered from chronic iron deficiency anemia and recurrent intestinal hemorrhage for 3 years until a correct diagnosis was made.
Patients with cutaneous neurofibromata who have vague gastrointestinal complaints should be carefully examined for gastrointestinal tumors. Radiologic examination of the small bowel is seldom helpful in establishing a diagnosis. Since the tumors are hypervascular, abdominal arteriogrophy is helpful in identifying the number and location of tumors13). And the persistence of symptoms is an indication for early surgical exploration14). Most authors favor surgical removal of intestinal neurofibroma if possible, because of the danger of malignant degeneration, as well as obstruction, intussusception and bleeding19). In this case, bowel barium study did not show any abnormality of the small bowel. However, arteriography and 99mTc pertechnate RBC scan were helpful to detect the bleeding source and in diagnosis of intestinal neurofibromata.
The technique of resection of intestinal neurofibroma should include an appreciation of the activity of these tumor. Since they seldom metastasis to lymph nodes, a block dissection as applied for carcinoma is not indicated unless lymphatic involvement is demonstrated. Local excision with the sufficient distance from the tumor is usually adequate. Segmental resection of the bowel is indicated if the nodes are so closely scattered that bowel with antibiotics and correcting the anemia before operation, should not be neglected. In this case, segmental resection of jejunum with the end to end anastomosis were performed.
It is suggested that, if gastrointestinal dysfunction, particularly iron deficiency anemia, is found in the patient with von Recklinghausen’s disease, the radiologic evaluation of gastrointestinal tract, including barium study as well as abdominal arteriography, is necessary for the detection of neurofibroma of the bowel. Surgical resection is the cornerstone of treatment, and during operation a careful examination is demanded to find out unrecognized neurofibroma.
REFERENCES
- 1.Crowe FW, Schull WJ, Neil JW. A clinical, pathological and genetic study of multiple neurofibromatosis. Springfield, IL: Charles C Thomas, Publisher; 1956. pp. 176–198. [Google Scholar]
- 2.Canale DJ, Bebin J. Von Recklinghausen’s disease of the nervous system. In: Vinken PF, Bruyn GW, editors. Handbook of Clinical Neurology. Vol. 14. Amsterdam: North-Holland Publishing Company; 1972. pp. 132–162. [Google Scholar]
- 3.Kleitsch WP, Kehne JH, Gutch DF. Gastrointestinal hemorrhage due to neurofibromatosis. JAMA. 1951;147:1434. doi: 10.1001/jama.1951.03670320034012. [DOI] [PubMed] [Google Scholar]
- 4.Cowie RL. An unusual case of gastrointestinal bleeding. S Afr MJ. 1967;41:286. [PubMed] [Google Scholar]
- 5.River L, Silverstein J, Tope JW. Benign neoplasms of the small intestine: A critical comprehensive review with reports of 20 new cases. Int Abstr Surg. 1965;102:1. [PubMed] [Google Scholar]
- 6.Davis GB, Berk RN. Intestinal neurofibromas in von Recklinghausen’s disease. Am J Gastroenterol. 1973;60:410. [PubMed] [Google Scholar]
- 7.Christ TD. Gastrointestinal involvement in neurofibromatosis. Arch Intern Med. 1963;112:111. doi: 10.1001/archinte.1963.03860030111011. [DOI] [PubMed] [Google Scholar]
- 8.Lukash WM, Morgan RI, Sennett CO. Gastrointestinal neoplasms in von Recklinghausen’s disease. Arch Surg. 1966;92:905. doi: 10.1001/archsurg.1966.01320240093020. [DOI] [PubMed] [Google Scholar]
- 9.Grill J, Kuzzman JF. Recklinghausen’s disease with unusual symptoms from intestinal neurofibroma. Arch Path. 1942;34:902. [Google Scholar]
- 10.Shaw R, Cunliffe M. Von Recklinghausen’s disease of the small intestine with associated skin lesion. Am J Surg. 1950;80:360. doi: 10.1016/0002-9610(50)90513-7. [DOI] [PubMed] [Google Scholar]
- 11.Levy D, Khatib R. Intestinal neurofibromatosis with malignant degeneration. Dis Colon Rectum. 1960;3:140. doi: 10.1007/BF02616545. [DOI] [PubMed] [Google Scholar]
- 12.Marshak RH, Freund S, Maklansky D. Neurofibromatosis of the small bowel. Am J Dig Dis. 1963;8:478. [Google Scholar]
- 13.Hochberh FH, Dasilva AB, Galdabini J, Richardson EP. Gastrointestinal involvement in von Recklinghausen’s neurofibromatosis. Neurology. 1974;24:1144. doi: 10.1212/wnl.24.12.1144. [DOI] [PubMed] [Google Scholar]
- 14.Farrar T, Chappell FW. Severe gastrointestinal hemorrhage resulting from von Recklinghausen’s disease. Arch Surg. 1959;79:106. doi: 10.1001/archsurg.1959.04320070110018. [DOI] [PubMed] [Google Scholar]
- 15.Shaiken J. Tumors of the small bowel. Am Pract. 1952;3:388. [PubMed] [Google Scholar]
- 16.Wilson JS, Anderson AA. Cutaneous and intestinal neurofibromatosis. Am J Surg. 1960;100:761. doi: 10.1016/0002-9610(60)90424-4. [DOI] [PubMed] [Google Scholar]
- 17.Collins NP. Intestinal hemorrhage in von Recklinghausen’s disease: Neurogenic tumors as cause. Arch Surh. 1963;87:374. doi: 10.1001/archsurg.1963.01310150010003. [DOI] [PubMed] [Google Scholar]
- 18.Schenkein I, Bueker ED, Helson L. Increased nerve-growth stimulating activity in disseminated neurofibromatosis. N Engl J Med. 1974;290:613. doi: 10.1056/NEJM197403142901108. [DOI] [PubMed] [Google Scholar]
- 19.Christ TD. Gastrointestinal involvement in neurofibromatosis. Arch Intern Med. 1963;112:357. doi: 10.1001/archinte.1963.03860030111011. [DOI] [PubMed] [Google Scholar]


