Abstract
One hundred nineteen patients with inoperable esophageal cancer who had been treated with radiotherapy and/or chemotherapy from Jan. 1982 to Dec. 1986 at the Korea Cancer Center Hospital were retrospectively analyzed. Of 119 patients, 111 were male, and eight were female. Ten (8%) had a lesion in the cervical esophagus; 71 (60%), in the upper and midthoracic esophagus; and 38 (32%), in the lower esophagus. One hundred ten (92%) patients had squamous cell carcinoma, and nine (8%) had adenocarcinoma. In 40 patients receiving radiotherapy alone, the response rate was 50% (complete response, 10%; partial response, 40%) with a median survival of 9 months. The 1-, 2-, and 3-year survival rates were 35%, 10%, and 5%, respectively. In 36 patients receiving radiochemotherapy, the response rate was 61% (complete response. 20%; partial response, 41%) with a median survival of 15 months. The 1-, 2-, and 3-year survival rates were 45%, 31%, and 12%, respectively. Six patients who had received chemotherapy alone had no objective response and died within one year. None of the 37 patients who had not received a specific treatment for underlying cancer survived two years. T1 stage, a lesion in the cervical esophagus, and good performance status (0–2) were considered to be favorable prognostic factors. There was no significant difference in the response rate and the actuarial survival between the radiotherapy and radiochemotherapy groups. These results suggest that both radiotherapy and radiochemotherapy are effective treatment modalities for inoperable esophageal cancer.
Keywords: Esophageal cancer, Radiotherapy, Radiochemotherapy
INTRODUCTION
Esophageal cancer has been the fifth most common cancer in Korean males. 1) Recently, many methodological advances in surgery, radiotherapy, and chemotherapy have improved the response rate, however, the duration of survival has not been prolonged. Most previous studies have demonstrated that the 5-year survival rates after surgery or radiotherapy were less than 15%, 2–6) although some authors have reported 5-year survival rates of 25–44% after curative surgery, 7) which were the results for cases of highly selected patients. Because of the discouraging results with single modality treatment, various combined modality treatments have been tried to improve patient survival. 5, 7–9)
We treated 119 patients with radiotherapy and/or chemotherapy, who refused surgery or were considered inoperable because of unresectability or high operative risk. In this study, the response rate and length of survival were assessed in patients receiving radiotherapy and radiochemotherapy. We have also analyzed the effect of some factors which may influence survival.
PATIENTS AND METHODS
1. Patient Characteristics
Between Jan. 1982 and Dec. 1986, 119 patients with inoperable esophageal cancer were managed at the Korea Cancer Center Hospital. The main reasons for inoperability were unresectability, high operative risk, and refusal of surgery. The mean age was 57.6 years. One hundred eleven patients (93%) were male and eight (7%) were female. The age, sex, and performance status were not significantly different between the treatment groups (Table 1). The locations of the lesions were the cervical esophagus, 10 cases (8%); the upper and mid-thoracic esophagus, 71 (60%); and the lower esophagus, 38 (32%). One hundred ten patients (92%) had squamous cell carcinoma and nine (8%) had adenocarcinoma. The extent of the primary tumor was classified according to American Joint Committee on Cancer classification: T1, 13%, T2, 73%; and T3, 14%. Tumor location, histology, and extent of primary tumor were not significantly different between the treatment groups (Table 2). The sites of distant metastasis were lung (6 cases), liver (5 cases), supraclavicular lymph nodes (3 cases), and celiac lymph nodes (1 case).
Table 1.
Age, Sex, and Performance Status in Each Treatment Group.
| RT | TR + CT × | CT× | No treatment | Total | |
|---|---|---|---|---|---|
| No. of patients | 40 | 36 | 6 | 37 | 119 |
| Age (yrs)* | 59.1 | 54.9 | 61 3 | 57.1 | 57.6 |
| Sex | |||||
| Male | 35(88)** | 35(97) | 6(100) | 35(95) | 111(93) |
| Female | 5(12) | 1(3) | 0(0) | 2(05) | 8(7) |
| P.S. (ECOG)‡ | |||||
| 0–1 | 17(43) | 14(39) | 1(17) | 12(32) | 44(37) |
| 2 | 18(45) | 19(53) | 5(83) | 20(54) | 62(52) |
| 3–4 | 5(12) | 3(8) | 0(0) | 5(14) | 13(11) |
RT: radiotherapy, CT ×: chemotherapy.
Mean age
No. of patient (%)
Performance status according to Eastern Cooperative Oncology Group. (%)
Table 2.
Site of Lesion, Histologic Diagnosis and Extent of Primary Tumor in Each Treatment Group.
| RT | RT + CT × | CT × | No treatment | Total | |
|---|---|---|---|---|---|
| Lesion | |||||
| Cervical | 3(8)* | 3(8) | 1(17) | 3(8) | 10(8) |
| Upper & midthoracic | 25(63) | 24(67) | 2(33) | 20(56) | 71(60) |
| Lower | 12(29) | 9(25) | 3(50) | 14(36) | 38(32) |
| Histology | |||||
| Sq. cell ca.‡ | 0(98) | 33(92) | 6(100) | 32(86) | 110(92) |
| Adenocarcinoma | 1(2) | 3(8) | 0(0) | 5(14) | 9(8) |
| Extent of primary tumor (T) | |||||
| T1 | 6(15) | 8(22) | 0(0) | 2(5) | 16(13) |
| T2 | 31(78) | 26(72) | 6(100) | 24(65) | 87(73) |
| T3 | 3(7) | 2(6) | 0(0) | 11(30) | 16(14) |
No. of patients (%)
Squamous cell carcinoma.
2. Treatment Schedule
Forty patients were treated with radiotherapy alone; 36, radiochemotherapy; and six, chemotherapy alone. Thirty seven patients did not receive any specific treatment for underlying cancer. Radiotherapy was administered five times per week with a total does of 5000–6000 rads. Chemotherapy consisted of 5-fluorouracil (5-FU), adriamycin, and cisplatin (FAP) in 13 cases; cisplatin, bleomycin, and mitomycin C (DBM) in 12; 5-FU and cisplatin (FP) in five; cisplatin alone in nine; bleomycin in one; 5-FU in one; and adriamycin in one. In the radiochemotherapy group, chemotherapy was given four to five weeks after the completion of radiotherapy.
3. Evaluation of Treatment Results
Treatment results were evaluated by esophagogram and/or esophagoscopy. Complete disappearence of any evidence of the tumor for four weeks was defined as complete response. A reduction of more than 50% of the longitudinal diameter of the tumor as compared with that of the pretreatment esophagogram was defined as partial response.
For statistical analysis, Student’s t-test was used to evaluate significance. Estimate of actuarial survival was performed by the method of Kaplan and Meier. For comparison of survival, log-rank test was used.
RESULTS
The complete and partial response rates in the radiotherapy group were 10% and 40%, respectively. Those of the radiochemotherapy groups were 19% and 42%, respectively. No objective response was observed in the chemotherapy alone group. There was no significant difference in the response rates between the radiotherapy group and the radiochemotherapy group (Table 3).
Table 3.
Response Rates in Each Treatment Group.
RT: radiotherapy; CT×: chemotherapy.
No. of patients.
Percentage.
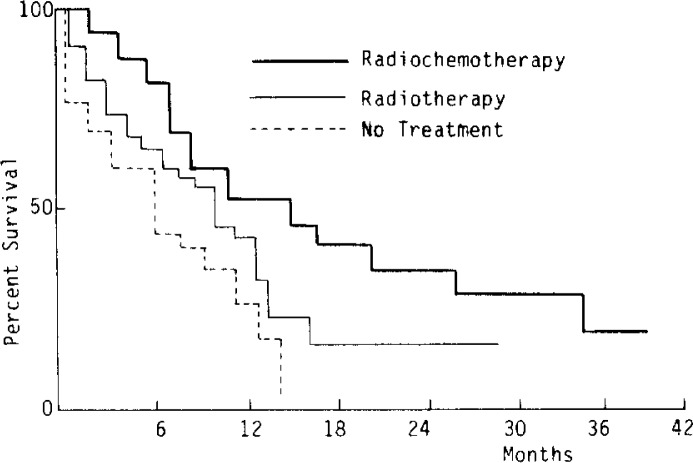
Fig. 1 showed percent survival in the three treatment groups with better survival being observed in the order of the radiochemotherapy, radiotherapy, and no treatment groups, but a significant difference was only found between the radiochemotherapy group and the no treatment group (p<0.01), and between the radiotherapy group and the no treatment group (p<0.05). There was no significant difference between the radiotherapy group and the radiochemotherapy group (p = 0.06). The 1-, 2-, and 3-year survival rates in the radiotherapy group were 35%, 10%, and 5%, respectively. Those in the radiotherapy group were 45%, 31% and 12%, respectively. No patient survived one year in the chemotherapy alone group. Among the 37 patients who had not received any specific treatment, the 1-years survival rate was 22%, but no patient survived two years (Table 4). The median lengths of survival in the radiotherapy group and the radiochemotherapy group were 9 months and 15 months, respectively.
Fig. 1.
Percentage of Survival in Patients with Esophageal Cancer by Treatment Group.
Table 4.
Survival Rates in Each Treatment Group.
| RT | RT + CT × | CT × | No treatment | Total | |
|---|---|---|---|---|---|
| 1 year | 35* | 45 | 0 | 22 | 34 |
| 2 year | 10 | 31 | 0 | 0 | 13 |
| 3 year | 5 | 12 | 0 | 0 | 5 |
RT: radiotherapy; CT×: chemotherapy.
Percentage.
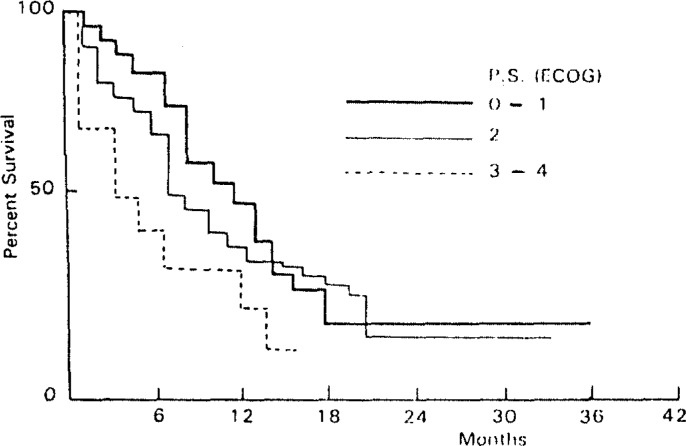
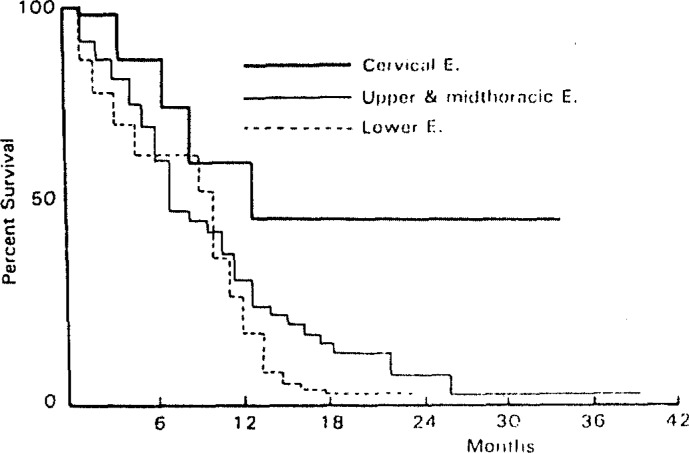
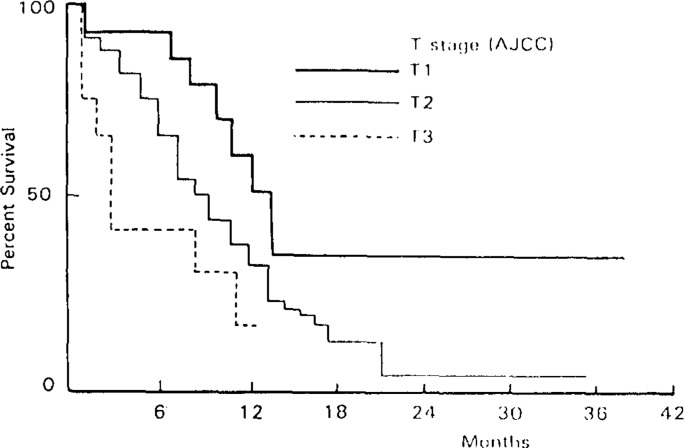
Survival in the performance status 3–4 group was poorer than that in the performance status 0–1 (p<0.001) and performance status 2 groups (p<0.01) (Fig. 2). Although 1-year survival rates were similar in patients with three locations, patients with a cervical location demonstrated a better 3-year survival rate compared with upper and midthoracic or lower esophagus location (Fig. 3). Patients in the T1 stage had better survival rates than those in the T2 and T3 stages (p<0.01 and p<0.001, respectively) (Fig. 4).
Fig. 2.
Percentage of Survival in Patients with Esophageal Cancer by performance Status (ECOG).
Fig. 3.
Percentage of Survival in Patients with Esophageal Cancer by Location of Lesion.
Fig. 4.
Percentage of Survival in Patients with Esophageal Cancer by Extent of Primary Tumor (T).
DISCUSSION
The prolongation of long-term survival in esophageal cancer has not been achieved despite numerous trials with various new therapeutic approaches. In general, a 5-year survival rate of less than 15% had been reported with single modality treatment with surgery, radiotherapy, or chemotherapy. Although Wu et al. have reported 5-year survival rates of 24–44% after curative surgery, most of the patients in their studies had an early carcinoma of the esophagus discovered by cytologic examination during a group survey for the detection of early cancer.7)
The role of radiotherapy and chemotherapy in patients with esophageal cancer has been investigated for a long time by many investigators. Beatty et al.4) reported a 3-year survival rate of 10% with radiotherapy and no significant difference in longterm survival between radiotherapy and surgery. Gisselbrecht et al.9) have reported that the objective response rate in 21 patients receiving combination chemotherapy with 5-FU, adriamycin, and cisplatin was 33%, but the median length of survival was only 8 months. Various combinations of treatment modalities, such as surgery with pre- or postoperative radiotherapy, 5, 10, 11) preoperative chemotherapy followed by surgery,12) and combinations of radiotherapy and chemotherapy,8,12) have been tried to improve the response rate and longterm survival, demonstrating that combined modality treatment has not been always advantageous compared with single modality treatment.10) Some reports have shown that the combination of chemotherapy with radiotherapy was much more efficient than single modality with chemotherapy or radiotherapy for inoperable esophageal cancer.8,9) However, others have failed to demonstrate the benefit of combination of chemotherapy and radiotherapy.13) In our results, the tendency of longer survival was observed in the radiochemotherapy group, compared with the radiotherapy alone group, although there was no statistical significance (p = 0.06). Although the beneficial effect of radiochemotherapy over radiotherapy alone failed to be demonstrated in this study, it might be difficult to evaluate the valid effect of different treatments in a retrospective, nonrandomized study. We are currently conducting a prospective, randomized study on the therapeutic effect of radiotherapy and radiochemotherapy in inoperable esophageal cancer.
The sequence of treatment modalities may be the important factor influencing clinical outcome. Most previous studies on combined modality treatment with radiotherapy and chemotherapy have been performed with initial chemotherapy followed by radiotherapy. This schedule was based on the idea that if pre-radiotherapeutic chemotherapy could eradicate the microscopic metastasis, subsequent radiotherapy could be expected to improve survival. Our patients received chemotherapy four or five weeks after radiotherapy for the purpose of adjuvant or salvage treatment. Therefore, we think that a study including both sequences should be performed before conclusions of the benefit of combined modality treatment with radiotherapy and chemotherapy are made.
In this study, most patients were treated with four regimens of chemotherapy (FAP, DBM, FP, and cisplatin). We have compared the effect of radiotherapy with chemotherapy between FAP, DBM, FP, and cisplatin resulting in no significant difference in the response rate (data not shown). No objective response was observed in our chemotherapy alone group. The response rates with chemotherapy in other series were 19–33%, although the long-term survival was very poor.13, 14) We think that the poor response in our chemotherapy alone group was probably due to the small number of patients and the advanced state of the disease, because patients who had distant metastasis were mainly considered to be candidates for the chemotherapy alone group.
The long term survival rate after radiotherapy and/or chemotherapy in cervical location was better, which was similar to other investigators’ results.15, 16) Although the reason for this result was unclear, we have confirmed that a patient with a cervical lesion was a good candidate for radiochemotherapy and/or chemotherapy.
In this study, we achieved a meaningful response with radiotherapy and radiochemotherapy in patients with inoperable esophageal cancer and have demonstrated that long-term survival with radiochemotherapy and radiotherapy was better than the no-treatment group. With these results, we suggest that both radiotherapy and radiochemotherapy are effective treatment modalities for inoperable esophageal cancer.
REFERENCES
- 1.National Bureau of Statistics Economic Planning Board, Republic of Korea. Annual report on the cause of death statistics. 1986:25. [Google Scholar]
- 2.Parker EF, Gregorie HB, Prioleau WH, Marks RD, Bartles DM. Carcinoma of the esophagus. Ann Surg. 1982;195:618. doi: 10.1097/00000658-198205000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilui R, Gignoux M. Treatment of carcinoma of the esophagus. Ann Surg. 1980;192:44. doi: 10.1097/00000658-198007000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beatty JD, Deboer G, Rider WD. Carcinoma of the esophagus. Cancer. 1979;43:2254. doi: 10.1002/1097-0142(197906)43:6<2254::aid-cncr2820430616>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Langer M, Choi NC, Orlow E, Grillo H, Wilkins EW. Radiation therapy alone or in combination with surgery in the treatment of carcinoma of the esophagus. Cancer. 1986;58:1208. doi: 10.1002/1097-0142(19860915)58:6<1208::aid-cncr2820580606>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Oh WY, Suh Co, Kim GE. Radiation treatment of esophageal cancer. J Korean Soc Ther Radiol. 1985;3:41. [Google Scholar]
- 7.Wu Ying-kai, Chen Pao-tien, Fang Jung-pao, Lin Shun-sheng. Surgical treatment of esophageal carcinoma. Am J Surg. 1980;139:805. doi: 10.1016/0002-9610(80)90386-4. [DOI] [PubMed] [Google Scholar]
- 8.Kolaric K, Maricic Z, Roth A, Dujmovic I. Combination of bleomycin and adriamycin with and without radiation in the treatment of inoperable esophageal cancer. Cancer. 1980;45:2265. doi: 10.1002/1097-0142(19800501)45:9<2265::aid-cncr2820450908>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Asakawa H, Kowada K, Watarai J. Radiotherapy in conjunction with bleomycin therapy for esophageal carcinoma. Tokyo: Documentation of the Nippon-Kayaku Co; 1973. [Google Scholar]
- 10.Kelsen DP, Ahuja R, Hopfan S, Bains MS, Kosloff C, Martini N, Mccormack P, Golbey RB. Combined modality therapy of esophageal carcinoma. Cancer. 1981;48:31. doi: 10.1002/1097-0142(19810701)48:1<31::aid-cncr2820480108>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Marks RD, Scruggs HJ, Wallace KM. Preoperative radiation therapy for carcinoma of the esophagus. Cancer. 1976;38:84. doi: 10.1002/1097-0142(197607)38:1<84::aid-cncr2820380115>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Kelsen DP, Hilaris B, Coonley C, Chapman R, Lesser M, Dukeman M, Heelan R, Bains MS. Cisplatin, vindesine, and bleomycin chemotherapy of local-regional and advanced esophageal carcinoma. Am J Med. 1983;75:645. doi: 10.1016/0002-9343(83)90451-5. [DOI] [PubMed] [Google Scholar]
- 13.Earle JD, Gelber RD, Moertel CG, Hahn RG. A controlled evaluation of combined radiation and bleomycin therapy for squamous cell carcinoma of the esophagus. Int J Radiation Oncology Biol Phys. 1980;6:821. doi: 10.1016/0360-3016(80)90318-1. [DOI] [PubMed] [Google Scholar]
- 14.Gisselbrecht C, Calvo F, Mignot L, Pujade E, Bouvry M, Danne O, Belpomme D, Marty M. Fluorouracil, adriamycin, and cisplatin combination chemotherapy of advanced esophageal carcinoma. Cancer. 1983;52:974. doi: 10.1002/1097-0142(19830915)52:6<974::aid-cncr2820520607>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Kelsen DP, Cvitkovic E, Bains M, Shils M, Howard J, Hopfen S, Gelbey RB. cis-Dichlorodiammineplatinum (II) and bleomycin in the treatment of esophageal cancer. Cancer Treat Rep. 1978;62:1041. [PubMed] [Google Scholar]
- 16.Skinner DB, Dowlatshahi KD, Demeester TR. Potentially curable cancer of the esophagus. Cancer. 1982;50:2751. [PubMed] [Google Scholar]