Abstract
Background:
In 2007, an Asthma/chronic obstructive pulmonary disease (COPD) (AC) service was implemented in the North of the Netherlands to support General Practitioners (GPs) by providing advice from pulmonologists on a systematic basis.
Aims:
To evaluate the feasibility and effectiveness of this service on patient-related outcomes.
Methods:
We report baseline data on 11,401 patients and follow-up data from 2,556 patients. GPs can refer all patients with possible obstructive airway disease (OAD) to the service, which is conducted by the local laboratory. Patients are assessed in the laboratory using questionnaires and spirometry. Pulmonologists inspect the data through the internet and send the GP diagnosis and management advice.
Results:
A total of 11,401 patients were assessed by the service, covering almost 60% of all adult patients with projected asthma or COPD in the area. In all, 46% (n=5,268) of the patients were diagnosed with asthma, 18% (n=2,019) with COPD and 7% (n=788) with the overlap syndrome. A total of 740 (7%) patients were followed up after 3 months because the GP advised them to change medication. In this group, the proportion of unstable COPD patients (Clinical COPD Questionnaire (CCQ)⩾1) decreased from 63% (n=92) at baseline to 49% (n=72). The proportion of patients with uncontrolled asthma (Asthma Control Questionnaire (ACQ)⩾1.5) decreased from 41% (n=204) to 23% (n=115). In all, 938 (8%) patients were followed up after 12 months. From these patients, the proportion of unstable COPD patients (CCQ⩾1) decreased from 47% (n=115) to 44% (n=107). The proportion of patients with uncontrolled asthma (ACQ⩾1.5) decreased from 16% (n=95) to 14% (n=85).
Conclusion:
The AC service assessed a considerable proportion of patients with OAD in the area, improved patients’ outcomes, and is considered to be feasible and effective.
Introduction
Asthma and chronic obstructive pulmonary disease(COPD) are prevalent chronic diseases in the community.1 In the Netherlands, 60 to 80% of all asthma and COPD patients are treated by their general practitioner (GP), and patients are only referred to the pulmonologist in case of severe uncontrolled asthma or severe COPD.2 Misdiagnosis and underdiagnosis are common,3–5 as asthma and COPD overlap in symptoms, whereas their treatments are different.6 Some patients have both asthma and COPD, the so-called overlap syndrome, which can be described as (partly) reversible but progressive deterioration in lung function, often combined with a history of smoking and previous diagnosis of asthma and/or allergies. These diagnostic problems may lead to suboptimal treatment, whereas early correct treatment can reduce costs, morbidity and mortality, can improve symptoms and enhance patient outcomes.3–5,7
In daily clinical practice, many GPs often lack the knowledge, time and enthusiasm to perform all tasks that are recommended by guidelines. For example, Dutch GPs are obliged to follow 96 guidelines for common diseases, of which only three are concerned with asthma, asthma in children, and COPD.8–10 GPs therefore could benefit from the knowledge and experience of pulmonologists by obtaining advice for each patient with pulmonary symptoms. Cooperation between GPs and other caregivers in integrated care projects for COPD patients has been proven to be effective in improving the quality of life and health status of patients, thus reducing costs and the number of hospitalisations.11,12 Asthma/COPD services in which GPs are supported by pulmonologists in interpreting spirometry results are feasible and might improve diagnostic accuracy.13 GPs and pulmonologists in the North of the Netherlands collaborated and implemented the Asthma/COPD (AC) service in 2007 as a support service for GPs. The aim of this service is to improve the management of asthma and COPD patients in primary care. Although the service was not developed for scientific reasons, data from included patients are available for research. This paper describes the development and feasibility of this service, the patient population and its effect on patient-related outcomes.
Materials and Methods
Design and feasibility
Meetings with local physicians were organised, resulting in four starting principles: (1) the service should optimise diagnosis, treatment and management; (2) the GP is in lead; (3) the service should be easily accessible for patients and physicians; (4) allocation of tasks between primary and secondary care have to be defined clearly.14 Yearly meetings are organised to inform physicians about current developments, discuss casuistry and enhance commitment.
The role of the GP
GPs can refer individual patients (⩾8 years of age) who are suspected to have asthma, COPD, overlap syndrome or present with pulmonary symptoms of unknown origin further referred to as obstructive airway disease (OAD). The GP can also choose to refer all OAD patients in his/her practice on the basis of inhaled medication use and/or courses of prednisolone. When preferred by the GP, referral may also include follow-up assessments by the AC service. Finally, the GP decides what to do with the advice of the pulmonologist and is responsible for the disease management.
Self-reported information by patients
Patients complete the ‘Asthma Control Questionnaire (ACQ),’ the ‘Clinical COPD Questionnaire (CCQ)’ and a medical history questionnaire assessing gender, age, age of onset, family history, symptoms, exacerbations (having used oral corticosteroids or antibiotics for lung problems), allergy and other stimuli-provoking symptoms, medication, occupation and smoking history as part of the regular assessment procedure.
The ACQ is used to measure asthma control and contains six questions (range 0–6), and the total score can be divided into ‘controlled’ (<0.75), ‘partially controlled’ (0.75–1.50) and ‘uncontrolled’ (⩾1.50).14 The CCQ is used to measure COPD health status, and it contains 10 questions. The total score (range 0–6) can be distributed between ‘stable’ (<1), ‘not entirely stable’,1,2 ‘unstable’2,3 and ‘very unstable’ (⩾3). This questionnaire contains three subdomains with four questions about functional status, four questions about symptoms and two questions about mental status.14,15
Assessment by the trained lung function technician
The assessments take place in local laboratories according to a strict protocol. The following measurements are taken:
Body mass index.
Evaluation and, if needed, instruction of the inhalation technique according to the Dutch ‘Inhalation Medication Instruction School’ guidelines.
Spirometry according to international guidelines.16
All data, including the scores on the questionnaires, are inserted in an Electronic Diagnostic Support (EDS) system.
The role of the pulmonologist
Pulmonologists are trained in using the service by following at least two training sessions. In this paper, we included pulmonologists who had assessed ⩾300 patients to avoid the influence of learning effects. Pulmonologists assess the quality of the spirometry, inspect all outcomes in the Electronic Diagnostic Support system and make a report (based on current international guidelines2,17) with diagnosis, follow-up and treatment advice. Treatment advice can include lifestyle advice (e.g., dietician, smoking cessation or physical activity) or medication change. Pulmonologists were not provided with strict diagnostic rules. The GPs receive the report from the pulmonologist within 5 working days through the internet directly in their patient information system.
Statistical data analyses
IBM-SPSS version 22 was used for statistical analysis. The baseline population was described by age, gender, body mass index, exacerbation history, smoking history, lung function performances, diagnosis and GOLD 2013 category (A, B, C and D).17 A positive bronchodilator response was defined as an increase in forced expiratory volume in 1 s (FEV1) of ⩾12% and ⩾200 ml. The median scores of GOLD A, B, C and D patients on the CCQ subscales are presented.
Feasibility analyses
Feasibility was assessed by analysing the following: (1) the proportion of GPs in the target area who used the AC service between 2007 and 2012; (2) the proportion of patients with asthma or COPD who were assessed by the service in the target area since 2007;18 (3) the quality of the spirometry; (4) the number of patients that could be diagnosed, and the variation in diagnostic pattern between the different pulmonologists by using chi-square.
Follow-up visits
Patients for whom medication change was advised by the pulmonologist were automatically scheduled for an additional follow-up assessment after 3 months (range 2–4 months, n=740). If the GP requested follow-up visits and no medication change was advised, patients were assessed after 12 months (range 10–14, n=938). Baseline data of adult patients on exacerbations/year, smoking status, inhalation technique, ACQ and CCQ scores were compared with follow-up data. Nonparametric paired tests were used to compare baseline data with follow-up data. Paired t-tests were used for the longitudinal evaluation of FEV1(in litres). Follow-up data of baseline GOLD stages are presented to show the distribution of these patients to other GOLD stages.17 To prevent overlap in our results, we excluded patients with >1 follow-up assessment in 1 year (n=79).
Results
Baseline patient characteristics are presented in Tables 1 and 2.
Table 1. Baseline characteristics of the total patient population in the AC tele-medicine service.
| Variable | Total group, n=11,401 |
|---|---|
| Diagnosis of | n (%) |
| COPD | 2,031 (17.8) |
| Very unstable (CCQ⩾3) | 161 (8.0) |
| Asthma | 5,223 (45.8) |
| Uncontrolled (ACQ⩾1.50) | 2,049 (39.3) |
| Asthma/COPD overlap syndrome | 787 (6.9) |
| Very unstable (CCQ⩾3) | 77 (9.9) |
| Uncontrolled (ACQ⩾1.50) | 308 (39.4) |
| Indication for restriction | 159 (1.4) |
| No lung disease | 796 (7.0) |
| Unclear diagnosis | 2,354 (20.6) |
| Missing at random | 15 (0.1) |
| Diagnosis is unclear because of | n (%) |
| Incorrect lung function test | 297 (12.6) |
| Unknown | 2,057 (87.4) |
| Referral to pulmonologist because of | n (%) |
| Unclear diagnosis | 1,966 (54.7) |
| Indication of restriction | 18 (0.005) |
| Unable to perform lung function test | 18 (0.005) |
| COPD | 558 (15.5) |
| FEV1 <50% predicted | 302 (54.3) |
| Asthma/COPD overlap syndrome | 214 (6.0) |
| FEV1 <50% predicted | 27 (12.7) |
| Asthma | 690 (19.2) |
| Unstable (ACQ⩾1.50) | 469 (68.2) |
| Total | 3,593 (31.5) |
| Quality lung function test | n (%) |
| Sufficient | 10,670 (93.6) |
| Insufficient | 730 (6.4) |
In 31.5% of the assessments, the pulmonologist advised the GP to refer their patient to secondary care mostly because of unclear diagnosis (54.7%). Most patients with unclear diagnosis (age, 51±19; age of onset, 39±23; 42% male) had no obstruction (FEV1/FVC⩾70%: 90%), no positive bronchodilator response (93%) and no allergy (76%). However, these patients were high in symptoms (CCQ⩾1: 68%).
Abbreviations: AC, asthma/COPD; ACQ, Asthma Control Questionnaire; CCQ, Clinical COPD Questionnaire; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GP, general practitioner.
Table 2. Baseline characteristics of the patient population per diagnosis.
| Variable | COPD, n=2,031 | Overlap syndrome, n=787 | Asthma, n=5,223 |
|---|---|---|---|
| Gender and age | n (%) | n (%) | n (%) |
| Male | 1,190 (58.6) | 388 (49.3) | 2,007 (38.4) |
| Self-reported ⩾1 allergy | 243 (12.0) | 312 (39.6) | 2,658 (50.9) |
| Mean (s.d.) | Mean (s.d.) | Mean (s.d.) | |
| Age, years | 66.6 (10.8) | 60.6 (12.3) | 43.6 (18.7) |
| Age of onset, years | 52.2 (19.6) | 33.6 (23.1) | 22.3 (19.6) |
| Body mass index (kg/m2) | 26.6 (4.8) | 27.1 (4.9) | 26.7 (6.0) |
| Exacerbations in the last 12 months | 0.7 (1.1) | 0.9 (1.2) | 0.8 (1.2) |
|
Lung function post bronchodilator, mean (s.d.)
| |||
| FEV1 (L) | 2.0 (0.7) | 2.3 (0.7) | 3.1 (0.9) |
| FEV1% predicted | 69.0 (18.1) | 76.0 (15.5) | 94.6 (15.1) |
| FVC (L) | 3.6 (1.0) | 3.7 (1.1) | 4.0 (1.1) |
| FVC % predicted | 98.6 (18.4) | 102.1 (17.2) | 101.1 (16.3) |
| FEV1/FVC | 56.0 (11.2) | 61.0 (9.5) | 79.3 (8.8) |
| Reversibilitya | 6.4 (7.9) | 11.2 (10.0) | 6.7 (7.6) |
|
Positive BDT
b
adults, n (%)
| |||
| Post FEV1/FVC<70% | 198 (99.0) | 236 (91.5) | 204 (25.0)c |
| Post FEV1/FVC=70–80% | 2 (1.0) | 22 (8.5) | 358 (43.8) |
| Post FEV1/FVC=80–90% | 214(26.2) | ||
| Post FEV1/FVC⩾90% | 40 (4.9) | ||
| Total | 200 (10.7) | 258 (34.8) | 817 (16.6) |
|
Inhalation technique
a
, n (%)
| |||
| Correct | 325 (36.1) | 205 (36.5) | 1,434 (38.0) |
| Incorrect | 576 (63.9) | 357 (63.5) | 2,339 (62.0) |
|
Smoking history (age⩾18 years), n (%)
| |||
| Smoking history missing | 8 (0.4) | 0 (0.0) | 17 (0.4) |
| Never smoked | 70 (3.4) | 54 (6.9) | 2,041 (43.4) |
| Quit ⩾12 months ago | 948 (46.7) | 378 (48.0) | 1,628 (34.6) |
| Current smoker | 1,005 (49.5) | 355 (45.1) | 1,014 (21.6) |
| Males | 517 (51.4) | 151 (42.5) | 383 (37.8) |
| Males motivated to quit | 304 (58.8) | 89 (58.9) | 231 (60.3) |
| Females | 475 (47.3) | 197 (55.5) | 614 (60.6) |
| Females motivated to quit | 295 (62.1) | 112 (56.8) | 407 (66.3) |
Abbreviations: BDT, bronchodilator test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
Increase in FEV1 pre bronchodilator compared with FEV1 post bronchodilator.
Positive bronchodilator response test defined as a reversible lung function of ⩾200 ml and ⩾12% increase in FEV1 pre bronchodilator compared with FEV1 post bronchodilator.
Adult asthma patients (male: 59%, mean age: 52 years, 19% current smokers) with obstruction before and after bronchodilator.
Feasibility
The service included around 2,000 (range: 1,813–2,109) new patients yearly from 79.3% of the GPs in the target area. Approximately 50% of patients were included by practice screening. In all, 60% of all adult asthma and COPD patients in the target area were assessed at least once by the AC service.18,19 Pulmonologists considered the quality of 93.6% of the spirometry graphs to be usable for diagnosis and could diagnose 79.4% of the patients (asthma: 45.8%, COPD: 17.8%, overlap syndrome: 6.9%). See Supplementary 1 for an overview of COPD GOLD A, B, C and D patients at baseline. Baseline diagnosis was compared with follow-up diagnosis, and it did not change during the follow-up in 91.2% (confidence interval: 89.4–92.7%) of baseline asthma patients, in 87.7% (confidence interval: 84.4–90.4%) of baseline COPD patients and in 74.2% (confidence interval: 68.0–79.5%) of baseline overlap syndrome patients. There was variation in diagnostic pattern in adult patients between the different pulmonologists (n=10, P<0.000); see Figure 1.
Figure 1.
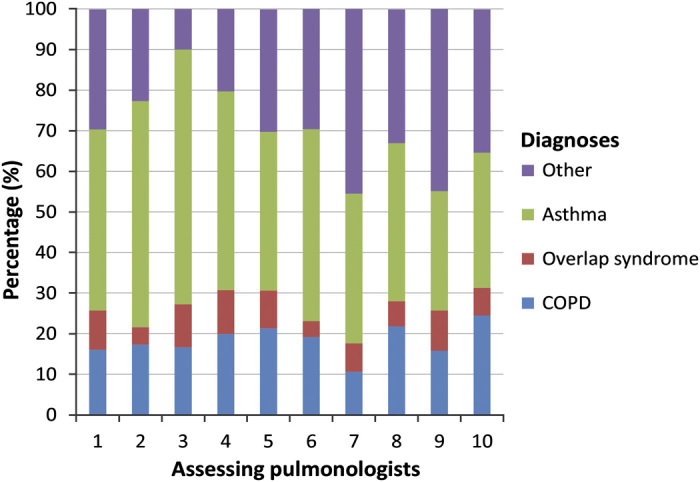
This picture shows the variation in diagnoses in adult patients between the assessing pulmonologists. Most variation is seen in the diagnoses of asthma and other (unclear diagnoses, indication for restriction or no disease). The variation in diagnoses between the pulmonologists was significant (Chi-square=580, n=10,656, P<0.000).
Follow-up visits
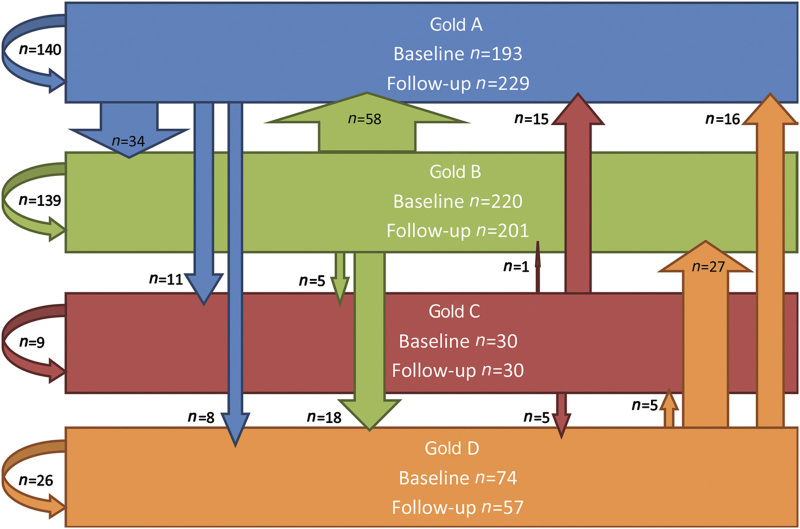
Patients frequently changed from GOLD (2013) category, and many GOLD D patients (n=74) moved to other GOLD categories at follow-up (Follow-up category: GOLD A: 21.6%, B: 36.5%, C: 6.8%, D: 35.1%); see Figure 2.
Figure 2.
Distribution of COPD patients at baseline (n (total)=2,004) according to the new GOLD classification of airflow limitation. Apparent is the large proportion of patients at risk: 22% of the COPD patients were classified as GOLD D. GOLD D patients have a high risk of exacerbations combined with a high burden of disease.
Patients advised to change medication (Patients followed-up after 3 months, total=740)
Inhalation technique improved significantly (correct at baseline 35.1% to 52.5% after 3 months, n total=459, P<0.000). The proportion of well-controlled asthma patients increased from 23.9% (baseline) to 49.5% (3 months, n total=487, P<0.000), and the proportion of stable COPD patients increased from 27.4% (baseline) to 48.9% (3 months, n total=145, P=0.004). The proportion of GOLD D patients decreased from 12.8% at baseline to 6.7% after 3 months; see Table 3 (n total=145, P<0.000).
Table 3. Longitudinal differences in lung function, exacerbations, health status and asthma control of patients assessed at baseline and after 3 months (range: 2–4 months, n total=740).
| Diagnosis | n | Baseline | After 3 months | P value a |
|---|---|---|---|---|
| Asthma (n=503) | n (%) | n (%) | ||
| ⩾1 exacerbation last yearb | 483 | 188 (38.9) | ||
| Current smoker | 497 | 92 (18.5) | 87 (17.5) | NS |
| Sufficient inhalation techniquec | 320 | 120 (37.5) | 173 (54.1) | <0.000 |
| Well controlled (ACQ<0.75) | 497 | 119 (23.9) | 246 (49.5) | <0.000 |
| Partially controlled (ACQ⩾0.75 and <1.50) | 174 (35.0) | 136 (27.4) | ||
| Uncontrolled (ACQ⩾1.50) | 204 (41.0) | 115 (23.1) | ||
| Mean (s.d.) | Mean (s.d.) | |||
| FEV1 pre (L) | 455 | 3.0 (0.9) | 2.9 (0.9) | <0.000 |
| COPD (n=148) | n (%) | n (%) | ||
| ⩾1 exacerbation | 148 | 60 (40.5) | ||
| Current smoker or quit <12 months ago | 147 | 63 (42.9) | 62 (42.2) | NS |
| Sufficient inhalation techniquec | 79 | 19 (24.1) | 38 (48.1) | NS |
| Stable (CCQ<1) | 147 | 55 (37.4) | 75 (51.0) | 0.004 |
| Not entirely stable (CCQ⩾1 &<2) | 64 (43.5) | 48 (32.7) | ||
| Unstable (CCQ⩾2 and <3) | 19 (12.9) | 18 (12.2) | ||
| Very unstable (CCQ⩾3) | 9 (6.1) | 6 (4.1) | ||
| Mean (s.d.) | Mean (s.d.) | |||
| FEV1 pre (L) | 148 | 2.1 (0.6) | 2.1 (0.6) | NS |
| Overlap syndrome (n=82) | n (%) | n (%) | ||
| ⩾1 exacerbation | 82 | 41 (50.0) | ||
| Current smoker or quit <12 months ago | 82 | 33 (40.2) | 34 (41.5) | NS |
| Sufficient inhalation techniquec | 58 | 21 (36.2) | 29 (50.0) | NS |
| Well controlled (ACQ<0.75) | 82 | 35 (42.7) | 48 (58.5) | NS |
| Partially controlled (ACQ⩾0.75 and <1.50) | 30 (36.6) | 19 (23.2) | ||
| Uncontrolled (ACQ⩾1.50) | 17 (20.7) | 15 (18.3) | ||
| Stable (CCQ<1) | 31 | 8 (25.8) | 16 (51.6) | 0.073 |
| Not entirely stable (CCQ⩾1 &<2) | 16 (51.6) | 11 (35.5) | ||
| Unstable (CCQ⩾2 and <3) | 7 (22.6) | 2 (6.5) | ||
| Very unstable (CCQ⩾3) | 0 (0.0) | 2 (6.5) | ||
| Mean (s.d.) | Mean (s.d.) | |||
| FEV1 pre (L) | 81 | 2.3 (0.6) | 2.3 (0.7) | NS |
Patients were referred to the 3-month follow-up assessment if change in medication was advised by the pulmonologist.
Abbreviations: ACQ, Asthma Control Questionnaire; CCQ, Clinical COPD Questionnaire; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; NS, not significant.
P values are two-sided and P values ⩾0.10 are reported as ‘NS’.
Exacerbations are defined as having used oral corticosteroids or antibiotics for lung problems last year.
Inhalation technique in patients who use medication at baseline.
Patients advised to continue current medication (Patients followed-up after 12 months, n=938)
Inhalation technique did improve significantly (correct baseline 37.4 to 49.9% after 12 months, n=741, P<0.000). The proportion of current smokers decreased from 25.2% at baseline to 23.2% after 12 months (n=984, P=0.013). The proportion of asthma and COPD patients with ⩾1 exacerbation last year decreased (asthma: 35.0% at baseline, 25.2% at 12 months, P>0.000; COPD: 34.8% at baseline, 25.4% at 12 months, P=0.010); see Table 4.
Table 4. Longitudinal differences in lung function, exacerbations, health status and asthma control of patients referred by their GP to single or yearly follow-up assessment after 12 months (range 10–14 months, n total=991). Patients in this table were not assessed after 3 months.
| Diagnosis | n | Baseline | After 12 months | P value a |
|---|---|---|---|---|
| Asthma (n=598) | n (%) | n (%) | ||
| ⩾1 exacerbationb | 572 | 200 (35.0) | 144 (25.2) | <0.000 |
| Current smokers or quit <12 months ago | 595 | 88 (14.8) | 81 (13.6) | NS |
| Sufficient inhalation techniquec | 471 | 185 (39.3) | 251 (53.3) | <0.000 |
| Well controlled (ACQ<0.75) | 591 | 377 (63.8) | 383 (64.8) | NS |
| Partially controlled (ACQ⩾0.75 and <1.50) | 119 (20.1) | 123 (20.8) | ||
| Uncontrolled (ACQ⩾1.50) | 95 (16.1) | 85 (14.4) | ||
| Mean (s.d.) | Mean (s.d.) | |||
| FEV1 pre (L) | 596 | 3.1 (0.9) | 3.0 (0.9) | <0.000 |
| COPD (n=245) | n (%) | n (%) | ||
| ⩾1 exacerbationb | 244 | 85 (34.8) | 62 (25.4) | 0.010 |
| Current smokers or quit <12 months ago | 243 | 109 (44.9) | 99 (40.7) | 0.017 |
| Sufficient inhalation techniquec | 177 | 59 (33.3) | 78 (44.1) | 0.034 |
| Stable (CCQ<1) | 243 | 128 (52.7) | 136 (56.0) | NS |
| Not entirely stable (CCQ⩾1 and <2) | 79 (32.5) | 74 (30.5) | ||
| Unstable (CCQ⩾2 &<3) | 23 (9.5) | 24 (9.9) | ||
| Very unstable (CCQ⩾3) | 13 (5.3) | 9 (3.7) | ||
| Mean (s.d.) | Mean (s.d.) | |||
| FEV1 pre (L) | 244 | 2.2 (0.7) | 2.1 (0.6) | <0.000 |
| Overlap syndrome (n=88) | n (%) | n (%) | ||
| ⩾1 exacerbationb | 88 | 34 (38.6) | 25 (28.4) | NS |
| Current smokers or quit <12 months ago | 88 | 38 (43.2) | 34 (38.6) | 0.094 |
| Sufficient inhalation techniquec | 66 | 24 (36.4) | 28 (42.4) | NS |
| Well controlled (ACQ<0.75) | 87 | 37 (42.5) | 48 (55.2) | 0.032 |
| Partially controlled (ACQ⩾0.75 and <1.50) | 25 (28.7) | 21 (24.1) | ||
| Uncontrolled (ACQ⩾1.50) | 25 (28.7) | 18 (20.7) | ||
| Stable (CCQ<1) | 65 | 24 (36.9) | 30 (46.2) | 0.027 |
| Not entirely stable (CCQ ⩾1 & <2) | 27 (41.5) | 26 (40.0) | ||
| Unstable (CCQ⩾2 and <3) | 7 (10.8) | 7 (10.8) | ||
| Very unstable (CCQ⩾3) | 7 (10.8) | 2 (3.1) | ||
| Mean (s.d.) | Mean (s.d.) | |||
| FEV1 pre (L) | 88 | 2.3 (0.7) | 2.2 (0.7) | <0.000 |
Abbreviations: ACQ, Asthma Control Questionnaire; CCQ, Clinical COPD Questionnaire; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; GP, general practitioner; NS, not significant.
P values are two-sided and P values ⩾0.10 are reported as ‘ns’.
Exacerbations are defined as having used oral corticosteroids or antibiotics for lung problems last year.
Inhalation technique in patients who use medication at baseline.
Discussion
Main findings
In this study, we have evaluated over 11,000 patients referred by 360 different GPs to the Asthma/COPD (AC) service in the North of the Netherlands. With 60% of all asthma and COPD patients in the area participating, the service is well implemented in the target area. We chose to report the follow-up results from patients who were advised to change medication after 3 months separately from the patients who were advised to continue with their medication and were scheduled to have a 12-month follow-up. If a change in medication was advised, after 3 months, health status improved in COPD patients and asthma control improved in asthma patients. Asthma and COPD patients who did not need medication change and were referred to the yearly follow-up assessment stabilised in asthma control and in COPD health status. Overlap syndrome patients in this group improved in asthma control and health status. Most (65%) of the GOLD D patients moved to other categories after baseline visit; some of these patients (22%) even improved to GOLD category A. Given these results, we consider the AC service to be a feasible and effective collaboration service for primary and secondary care.
Interpretation of findings in relation to previously published work
According to the Global Initiative for Asthma guidelines, uncontrolled asthma patients would be eligible for referral to secondary care.2 The prevalence of uncontrolled asthma patients in our population (age ⩾16 years) is lower (40%) than the prevalence found in the INSPIRE study where 51% of the patients had uncontrolled asthma.20 Only 13% of our asthma patients were advised to be referred to secondary care (see Table 1), meaning that the pulmonologists did not follow the Global Initiative for Asthma guidelines. However, we do not know whether the GP followed the referral recommendation of the pulmonologist.
The accuracy of the diagnosis in Asthma/COPD support systems is of pivotal importance. Lucas et al. showed previously that a diagnosis based on paper patient data without life contact in an AC service is comparable with diagnoses acquired by a face-to-face consultation. The level of agreement on diagnoses between paper data and face-to-face diagnoses was κ=0.82,21 which exceeded the level of inter-doctor agreement from the different assessing pulmonologists (κ=0.6422). We used comparable history questions and spirometry as Lucas et al. used, and we assume that the diagnostic accuracy will be comparable as in the study of Lucas et al. Diagnoses during follow-up visits in our AC service (see Figure 1) showed that most of the diagnoses were consistent. Although diagnoses are to some extent subjective, the advantage of the system is that the GP can compare his own choices with the diagnoses and advices from the pulmonologist. We believe that this internet-based consultation in the long run might improve skills in the management of OADs.
Van den Bemt et al. 23 presented a service for COPD patients and concluded that it was not clinically effective. However, their population consisted of already-diagnosed COPD patients, and all patients had performed spirometry previously to inclusion, whereas we followed up both previously diagnosed and newly diagnosed patients. We speculate that the room for improvement in our population of a wide range of patients was higher, which might have contributed to the positive effect. Furthermore, internet is used for data exchange, and GPs receive the report from the pulmonologist mostly within 5 working days electronically in their patient information system, which makes the service user friendly. Next to that, the use of Internet and the trained lung function technicians make the service cost-effective. Direct costs were covered by reimbursement for the spirometry in primary care, including the involvement of the pulmonologist.
Generalisability
Our asthma patients differed from COPD patients, whereas the characteristics of overlap syndrome patients fell between asthma and COPD patients. This was also described by Postma et al. 24 who showed that asthma patients are younger, more frequently female and have less frequently a history of smoking. Miravitlles et al. 25 showed the same pattern of patient characteristics in their primary care population, although the proportion of male patients in their overlap syndrome population was much lower than in the AC population (Miravitlles: 26%, AC service: 49%). Characteristics of our asthma patients (⩾16 years) were comparable with asthmatics from the INSPIRE study (AC service: male, 38%; mean age, 46±17 years; current smokers, 21%. INSPIRE: male 35%; mean age, 45±17 years; current smokers, 21%).
When considering overlap syndrome as a subtype of COPD, the prevalence of overlap syndrome in our COPD population was 28%, which is comparable to the prevalence found by Ställberg et al.26 (25%) but higher than the prevalence reported by Postma et al. (13–20%).24 Our overlap patients have more frequent exacerbations compared with the asthma and COPD patients, which indicate that these patients are more at risk for future exacerbations. This high risk was confirmed in other studies.24,27,28 Although these patients are more at risk, at baseline only the CCQ scores reflect this poor health status (CCQ⩾3: COPD, 8%; overlap syndrome, 10%). Overlap patients were assessed by using the ACQ and the CCQ, because these questionnaires were part of the regular assessment in the AC collaboration service. However, no validated measurements are available to assess the health status and disease control in overlap patients. COPD patients in the AC service were distributed according to the GOLD guidelines using CCQ cutoff value of >1 (A: 28%, B: 40%, C: 8% and D: 24%). Lange et al. 29 distributed 6,628 Danish COPD patients using the modified Medical Research Council and found another distribution (A: 77%, B: 14%, C: 4% and D: 4%). Apart from the difference in symptom assessment by using the CCQ instead of the modified Medical Research Council, our COPD sample of primary care–treated patients is obviously more at risk and has more symptoms than the Danish general population.
Reversibility
Only 17% of our asthma patients had a positive bronchodilator test (BDT) response.2 In asthma patients with good lung function (FEV1/forced vital capacity (FVC) after bronchodilator ⩾90%), the proportion of patients with a positive BDT was 5%, whereas in asthma patients with very poor lung function (FEV1/FVC post bronchodilator <70%), this proportion was 40%. Others have also shown that reversibility in asthmatic patients depends on the severity of asthma as measured by the impairment in lung function.30 Although COPD is considered to be a nonreversible obstructive lung disease, 11% of our COPD patients had a BDT response, and the average reversibility was 6%. In the UPLIFT study, 50% of the COPD patients showed significant reversibility.31 Obviously, real-life COPD populations differ from selected populations, as confirmed by Kruis et al. 32 Bronchodilator response was more prevalent in COPD patients with severe disease.24The highest proportion of patients with positive BDT response was seen in the overlap syndrome patients (35%), which is consistent with the GOLD and Global Initiative for Asthma recommendations.33
Risk factors
The proportion of smoking COPD and overlap syndrome patients (COPD: 50%, overlap syndrome: 45%) was much higher than in the Dutch population, which is 27%, and also higher than the COPD population presented by Warnier et al.34 (37%). Our definition of smokers might have contributed to the higher proportion, because we considered quitters <12 months ago as current smokers. The AC service does not provide any cessation intervention but informs the GP if their patients are motivated to quit. The follow-up time of 12 months could not reveal the number of quitters (>12 months) as a result of the possible intervention of the GP.
Like in other studies,35 many patients (64%) showed an insufficient inhalation technique. Although the inhalation technique improved after 3 (50%) and 12 months (47%) of follow-up after our standard instruction as recommended in the Dutch guidelines, it is debatable whether this instruction is sufficiently effective. Further research on effective instruction seems to be needed.
Strengths and limitations of this study
The strength of this study is the large population of primary care OAD patients in real life and the strict protocol used to assess these patients. A limitation is that the AC service was not established for scientific reasons, resulting in limited follow-up results, and data could not be compared with a control group.36 Therefore, we cannot rule out that results might have been affected by regression to the mean, although regression to the mean (measured by 1−ρ) is small;37 see Supplementary 2. We also do not have data on mortality; however, on the basis of national mortality data, we assume that 1.8% of the COPD patients have died in 1 year. Leivseth et al. 38 showed that there are no large differences in mortality rates between GOLD A, B, C and D. Therefore, we assume that missed mortality rates hardly affected our COPD follow-up results.
Implications for future research, policy and practice
Relatively simple support for GPs in diagnosing and managing patients with a chronic disease by specialists might result in improved outcomes for these patients in the community. Principles of the AC service might also be suitable in other chronic diseases. The large electronic database from the AC service provides unique opportunities for further research in primary care patients with OADs.
Conclusion
The AC service is feasible, effective and efficient in supporting GPs to diagnose and manage asthma, COPD and overlap syndrome patients. The service stimulates cooperation between primary and secondary care, and delivers support to patients locally which is important in rural areas.
Acknowledgments
We thank the Northern General Practitioners Laboratory Groningen (LabNoord currently Certe Laboratories) for giving us the opportunity to realise the AC service.
RAR is a member of the advisory board of Certe Laboratories. MGP-W is the director of Certe Laboratories. TvdM is a member of the board of trustees of Certe Laboratories. JWHK is an Associate editor of npj Primary Care Respiratory Medicine, but was not involved in the editorial review of, nor the decision to publish, this article. The other authors declare no conflict of interest.
References
- World Health Organization . Burden of COPD, 2012; Available at http://www.who.int/respiratory/copd/burden/en/index.html . Accessed 29 June 2012.
- Global Initiative for Asthma . Pocket guide for asthma management and prevention, 2012; Available at http://www.ginasthma.org/ .
- Izquierdo JL, Martin A, de Lucas P, Rodriguez-Gonzalez-Moro JM, Almonacid C, Paravisini A. Misdiagnosis of patients receiving inhaled therapies in primary care. Int J Chron Obstruct Pulmon Dis. 2010;5:241–249. doi: 10.2147/copd.s11123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Goldstein RS, Guyatt GH, Blouin M, Tan WC, Davis LL. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ. 2010;182:673–678. doi: 10.1503/cmaj.091784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakhale S, Sumner A, Coyle D, Vandemheen K, Aaron S. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27–2466-11-27. doi: 10.1186/1471-2466-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DB, Yawn BP, Jones RCM. Improving the differential diagnosis of chronic obstructive pulmonary disease in primary care. Mayo Clin Proc. 2010;85:1122–1129. doi: 10.4065/mcp.2010.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirnhofer L, Lamprecht B, Firlei N, Kaiser B, Buist AS, Halbert RJ. Using targeted spirometry to reduce non-diagnosed chronic obstructive pulmonary disease. Respiration. 2011;81:476–482. doi: 10.1159/000320251. [DOI] [PubMed] [Google Scholar]
- The Dutch College of General Practitioners . Astma bij kinderen. 2014; Available at https://www.nhg.org/standaarden/samenvatting/astma-bij-kinderen . Accessed 11 February 2014.
- The Dutch College of General Practitioners . Astma bij volwassenen, 2007; Available at https://www.nhg.org/standaarden/samenvatting/astma-bij-volwassenen . Accessed 11 February 2014.
- The Dutch College of General Practitioners. COPD 2007; Available at https://www.nhg.org/standaarden/samenvatting/copd . Accessed 11 February 2014.
- Kruis AL, Smidt N, Assendelft WJ, Gussekloo J, Boland MR, Rutten-van Molken M. Cochrane corner: is integrated disease management for patients with COPD effective? Thorax. 2014;69:1053–1055. doi: 10.1136/thoraxjnl-2013-204974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavannes NH, Grijsen M, van den Akker M, Schepers H, Nijdam M, Tiep B. Integrated disease management improves one-year quality of life in primary care COPD patients: a controlled clinical trial. Prim Care Respir J. 2009;18:171–176. doi: 10.3132/pcrj.2009.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavia M, Averame G, Canonica W, Cricelli C, Fogliani V, Grassi C. Feasibility and validation of telespirometry in general practice: The Italian ‘Alliance’ study. Respir Med. 2009;103:1732–1737. doi: 10.1016/j.rmed.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Riemersma R, Meijer R, Kerstjens H, Tsiligianni I, Postma D, Molen Tvd. New developments in the treatment, diagnosing, and management of asthma in general practice. Drukkerij 1984: Appingedam, The Netherlands; 2010. [Google Scholar]
- van der Molen T, Willemse BW, Schokker S, ten Hacken NH, Postma DS, Juniper EF. Development, validity and responsiveness of the Clinical COPD Questionnaire. Health Qual Life Outcomes. 2003;1:13. doi: 10.1186/1477-7525-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thoracic Society/European Respiratory Society Task Force 2004 ; Available at http://www.thoracic.org/clinical/copd-guidelines/resources/copddoc.pdf
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global stategy for the diagnosis, management, and prevention of COPD. 2013; Available at http://www.goldcopd.org/ .
- Nationaal Kompas Volksgezondheid . Astma. 2014; Available at http://www.nationaalkompas.nl/gezondheid-en-ziekte/ziekten-en-aandoeningen/ademhalingswegen/astma/ . Accessed 23 May 2014.
- Nationaal Kompas Volksgezondheid . Hoe vaak komt COPD voor en hoeveel mensen sterven eraan? 2013; Available at http://www.nationaalkompas.nl/gezondheid-en-ziekte/ziekten-en-aandoeningen/ademhalingswegen/copd/omvang/ . Accessed 13 February 2014.
- Partridge M, van dM, Myrseth S, Busse W. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med. 2006;6:13. doi: 10.1186/1471-2466-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A, Smeenk F, Smeele I, Brouwer T, van Schayck O. The validity of diagnostic support of an asthma/COPD service in primary care. Br J Gen Pract. 2007;57:892–896. doi: 10.3399/096016407782317883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas AE, Smeenk FJ, van den Borne BE, Smeele IJ, van Schayck CP. Diagnostic assessments of spirometry and medical history data by respiratory specialists supporting primary care: are they reliable? Prim Care Respir J. 2009;18:177–184. doi: 10.3132/pcrj.2009.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bemt L, Schermer TR, Smeele IJ, Boonman-de Winter LJ, van Boxem T, Denis J. An expert-supported monitoring system for patients with chronic obstructive pulmonary disease in general practice: results of a cluster randomised controlled trial. Med J Aust. 2009;191:249–254. [PubMed] [Google Scholar]
- Postma DS, Reddel HK, ten Hacken NHT, van den Berge M. Asthma and chronic obstructive pulmonary disease: similarities and differences. Clin Chest Med. 2014;35:143–156. doi: 10.1016/j.ccm.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Miravitlles M, Andreu I, Romero Y, Sitjar S, Altes A, Anton E. Difficulties in differential diagnosis of COPD and asthma in primary care. Br J Gen Pract. 2012;62:e68–e75. doi: 10.3399/bjgp12X625111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallberg B, Janson C, Johansson G, Larsson K, Stratelis G, Telg G. Management, morbidity and mortality of COPD during an 11-year period: an observational retrospective epidemiological register study in Sweden (PATHOS) Prim Care Respir J. 2014;23:38–45. doi: 10.4104/pcrj.2013.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen H, Lampela P, Nevanlinna A, Saynajakangas O, Keistinen T. High hospital burden in overlap syndrome of asthma and COPD. Clin Respir J. 2013;7:342–346. doi: 10.1111/crj.12013. [DOI] [PubMed] [Google Scholar]
- Louie S, Zeki AA, Schivo M, Chan AL, Yoneda KY, Avdalovic M. The asthma-chronic obstructive pulmonary disease overlap syndrome: pharmacotherapeutic considerations. Expert Rev Clin Pharmacol. 2013;6:197–219. doi: 10.1586/ecp.13.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange P, Marott JL, Vestbo J, Olsen KR, Ingebrigtsen TS, Dahl M. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186:975–981. doi: 10.1164/rccm.201207-1299OC. [DOI] [PubMed] [Google Scholar]
- Yancey SW, Ortega HG. Retrospective characterization of airway reversibility in patients with asthma responsive to bronchodilators. Curr Med Res Opin. 2007;23:3205–3207. doi: 10.1185/030079907X242683. [DOI] [PubMed] [Google Scholar]
- Hanania NA, Sharafkhaneh A, Celli B, Decramer M, Lystig T, Kesten S. Acute bronchodilator responsiveness and health outcomes in COPD patients in the UPLIFT trial. Respir Res. 2011;12:6–9921-12-6. doi: 10.1186/1465-9921-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruis AL, van Schayck OC, in't Veen JC, van der Molen T, Chavannes NH. Successful patient self-management of COPD requires hands-on guidance. Lancet Respir Med. 2013;1:670–672. doi: 10.1016/S2213-2600(13)70212-1. [DOI] [PubMed] [Google Scholar]
- Global Initiative for Asthma and Global Initiative for COPD . Asthma COPD and Asthma—COPD Overlap Syndrome (ACOS), 2014; Available at http://www.ginasthma.org and http://www.goldcopd.org .
- Warnier MJ, van Riet EE, Rutten FH, De Bruin ML, Sachs AP. Smoking cessation strategies in patients with COPD. Eur Respir J. 2013;41:727–734. doi: 10.1183/09031936.00014012. [DOI] [PubMed] [Google Scholar]
- Dekhuijzen PN, Bjermer L, Lavorini F, Ninane V, Molimard M, Haughney J. Guidance on handheld inhalers in asthma and COPD guidelines. Respir Med. 2014;108:694–700. doi: 10.1016/j.rmed.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2005;34:215–220. doi: 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- Armitage P, Berry G, Matthews JNS.Regression to the mean. In: Brown A, Pattison F, Ord K (eds). Statistical Methods in Medical Research Blackwell Publishing Company: Oxford, UK; 2002204–205. [Google Scholar]
- Leivseth L, Brumpton BM, Nilsen TI, Mai XM, Johnsen R, Langhammer A. GOLD classifications and mortality in chronic obstructive pulmonary disease: the HUNT Study, Norway. Thorax. 2013;68:914–921. doi: 10.1136/thoraxjnl-2013-203270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.