Abstract
Background:
Patterns of inhaled β2-agonist therapy use during severe asthma exacerbations before hospital attendance are poorly understood.
Aims:
To assess β2-agonist use prior to hospital attendance.
Methods:
We undertook an exploratory post hoc analysis of data from a 6-month clinical trial of 303 patients randomised to combination budesonide/formoterol inhaler according to a Single combination inhaler as Maintenance And Reliever Therapy regimen (‘SMART’) or fixed-dose budesonide/formoterol with salbutamol as reliever (‘Standard’). Patterns of β2-agonist use for 14 days before hospital attendance with a severe asthma exacerbation were determined by electronic monitoring of inhaler use.
Results:
There were 22 hospital attendances in 16 patients during the study. Seven and nine hospital attendances were eligible for analysis in the SMART and Standard groups, respectively. In both regimens, β2-agonist use increased before hospital attendance, with a median (range) maximum daily number of actuations of 14 (9 to 63) budesonide/formoterol in SMART and 46 (6 to 95) salbutamol in Standard with 4 (0 to 10) budesonide/formoterol actuations on the day of maximal salbutamol use. There was delay in obtaining medical review despite high β2-agonist use, in 9/16 patients. Different patterns of use were observed, including repeated days of no inhaled corticosteroid despite marked salbutamol use, which occurred in 3/9 patients in the Standard group.
Conclusions:
Delay in obtaining medical review in association with high β2-agonist use is common in patients before hospital presentation with severe exacerbations of asthma. The SMART regimen reduced nonadherence with inhaled corticosteroid therapy during severe exacerbations.
Introduction
Observational studies report that overuse of inhaled β2-agonists in a severe asthma attack is common and is associated with an increased risk of a fatal outcome.1,2 Many mechanisms have been proposed to account for this observation.3,4 Over-reliance on β2-agonist therapy, and self-administration of high doses of β2-agonist leading to delays in seeking medical assistance in a life-threatening attack, are common behaviours reported in asthma mortality surveys.1,2,4,5 In the recent United Kingdom National Review of Asthma Deaths, 39% of patients had been prescribed more than 12 short-acting reliever inhalers in the year before death, and 4% had been prescribed more than 50 reliever inhalers. During the final attack of asthma, 45% died without seeking medical assistance or before emergency care could be provided.5 In the setting of a severe asthma attack, high β2-agonist use increases the risk of hypokalaemia6 and QTc prolongation,6 and it may cause direct cardiac toxicity aggravated by hypoxia.7 The risk of death may be greater with high-dose, poorly β2-selective, potent agonists with high intrinsic activity such as isoprenaline and fenoterol, which have been implicated in asthma mortality epidemics.8,9 The association may also be noncausal, because high β2-agonist use may be a marker of severe asthma that is poorly responsive to bronchodilator therapy, and thereby a surrogate marker for mortality risk.10
However, there are limited data on patterns of actual use of β2-agonist inhalers by patients during severe exacerbations and in particular life-threatening attacks that lead to hospital admission or death. Studies to date rely on the information from general practitioners (GPs), patient self-report or from the relatives or friends of patients who have died from asthma.1,2,11,12 All of these may provide inaccurate estimates of actual medication use.
We recently undertook a 6-month randomised controlled trial (RCT) of combination budesonide/formoterol metered-dose inhaler (MDI) therapy when used as per the Single combination inhaler as Maintenance And Reliever Therapy (SMART) regimen or as a fixed-dose treatment with salbutamol MDI as reliever (Standard regimen).13,14 Data on the actual use of inhaled treatment were measured by electronic monitoring of MDI use. The SMART regimen reduced severe exacerbations requiring systemic corticosteroids and the number of episodes of β2-agonist overuse.13 However, in both the treatment groups, in ~90% of occasions in which patients overused their β2-agonist in excess of the level at which their management plan advised to seek medical review, no such review was obtained within the subsequent 48 h.
To further investigate the patterns of β2-agonist use in the setting of severe exacerbations, we undertook an exploratory analysis of β2-agonist use in the 14 days leading up to presentation to hospital with a severe exacerbation. Our main hypotheses were that there would be extremely high β2-agonist use recorded in most patients regardless of randomised regimen, and that this would be associated with delay in obtaining medical review.
Materials and methods
An exploratory post hoc analysis of data was undertaken from a 24-week multicentre, prospective, open-label, RCT of the SMART versus Standard regimens in asthma patients aged 16 to 65 years. Eligible patients had a physician’s diagnosis of asthma, a current prescription for inhaled corticosteroid (ICS) and at least one asthma exacerbation in the preceding year. Patients who had high baseline reliever use were not excluded, and there was no step-down in maintenance inhaled treatment on entry into the study. Full details of the trial have been published.13,14 The Study Protocol is available at http://www.mrinz.ac.nz/uploads/mrinz/SMART_Protocol.pdf, and see the Supplementary Material for further details. All patients provided written informed consent, and the New Zealand Multi-Region Ethics Committee approved the study protocol (MEC/09/11/127).
There were 151 patients randomised to receive 200/6 μg budesonide/formoterol via MDI (Vannair, AstraZeneca NZ Limited, Auckland, New Zealand; this is the MDI formulation of Symbicort Turbuhaler), two actuations twice daily as maintenance with extra doses for relief of symptoms as per the SMART regimen (SMART group), and 152 patients were randomised to receive 200/6 μg budesonide/formoterol via MDI, two actuations twice daily as maintenance with salbutamol 100 μg via MDI (Ventolin, GlaxoSmithKline NZ Limited, Auckland, New Zealand) for relief of symptoms (Standard group). Five study visits occurred over 24 weeks. At the first visit, all participants had their inhaler technique checked and were provided verbal instructions and written asthma self-management plans corresponding to their randomised regimen.15,16 Patients who previously had GP-prescribed prednisone for self-initiation during an exacerbation were able to continue with this practice during the study. Patients who self-initiated prednisone for asthma were advised to also seek medical review. This analysis is restricted to patients with a severe exacerbation of asthma that resulted in an acute presentation to a hospital Emergency Department and/or admission to hospital during the study period.
Main outcomes
The main variables of interest were as follows:
Individual patient and median daily budesonide/formoterol use in the SMART group and budesonide/formoterol and salbutamol use in the Standard group, in the 14 days preceding hospital attendance with a severe exacerbation.
Median maximum number of budesonide/formoterol actuations in a 24-h period in the SMART group and median maximum number of salbutamol actuations in a 24-h period and median number of budesonide/formoterol actuations on the day of maximum salbutamol use in the Standard group.
Secondary outcomes
Proportion of patients in each treatment group who did not seek medical review within 48 h despite exceeding the level of β2-agonist use at which this was recommended by their self-management plans, i.e., >12 actuations per 24 h of budesonide/formoterol in SMART (>8 actuations in addition to the four maintenance doses) and >16 actuations per 24 h of salbutamol in Standard.
Proportion of patients in each treatment group with ICS nonadherence, defined as no budesonide/formoterol actuations in a 24-h period.
First hospital-measured serum potassium and QTc interval in SMART versus Standard groups.
Association between the first serum potassium measurement and budesonide/formoterol use for SMART or salbutamol use for Standard patients in the 7 days and 24 h preceding the hospital attendance.
Self-reported reliever use as recorded in hospital medical records versus electronic monitoring of medication use.
Electronic medication use data
Smartinhaler Tracker electronic monitors (Nexus6 Limited, Auckland, New Zealand) were incorporated in all Vannair and Ventolin MDIs dispensed in the study.17 These validated monitors are 99.7% accurate in recording MDI actuations during bench testing,18 and they were used according to detailed trial quality control procedures.19 Each MDI actuation resulted in a date and time log, which was downloaded at the next study visit. Patients were informed that their inhalers measured the total number of actuations used and were unaware of the detailed capabilities of the monitor to record patterns of use.
Actuation data on medication use for the fourteen 24-h periods before the attendance time at hospital with a severe exacerbation were extracted for each patient. Repeated hospital attendance within 7 days of the preceding visit was counted as part of the same episode.20
Hospital attendance data
At each of the scheduled study visits, participants were asked whether they had sought medical help or whether they had taken oral prednisone since the last visit. All Emergency Department (ED) visits and hospital admissions for asthma were verified by searches of the hospital databases where the patient attended. Documentation on inhaler use, first electrocardiogram recordings and serum potassium measurements were recorded.
Statistical analysis
Data description for medication use patterns for the 14 days before hospital attendance episodes was shown. Serum potassium measurements and QTc intervals were compared between randomised groups by mixed linear models, to take account of repeated measurements of some patients. The strength of association between serum potassium and medication use before the hospital attendance was shown by scatter-plots supplemented by calculation of the product–moment correlation coefficients for the association.
SAS version 9.2 and Microsoft Excel 2010 were used.
Results
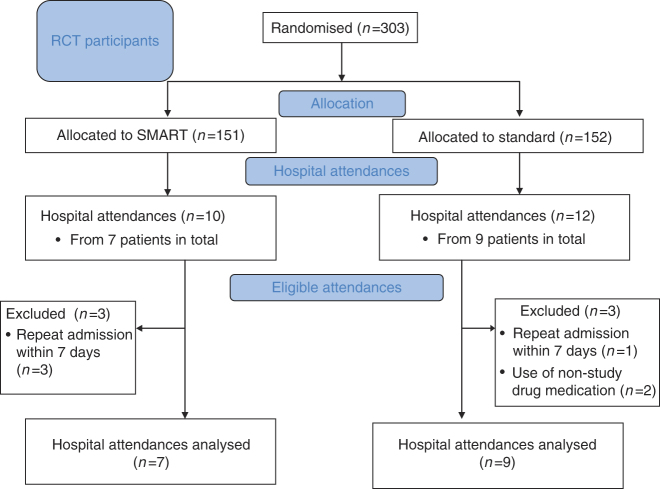
There were 22 hospital attendances (10 SMART and 12 Standard) in 16 patients (seven SMART and nine Standard; Figure 1). There were five hospital admissions (three SMART, two Standard). The baseline characteristics of the 16 patients are shown in Table 1.
Figure 1.
Flow of participants through the study, showing those presenting to hospital who were eligible for analysis.
Table 1. Baseline characteristics of patients attending hospital for severe asthma.
| Characteristic | SMART group (n=7) | Standard group (n=9) |
|---|---|---|
| Age, years | 46.9 (16.0) | 43.3 (14.8) |
| Male gender | 3 (43%) | 2 (22%) |
|
Ethnicity
| ||
| European | 6 (86%) | 7 (78%) |
| Māori | 0 (0%) | 2 (22%) |
| Pacific Islander | 1 (14%) | 0 (0%) |
| ACQ-7 score | 1.98 (1.19) | 3.10 (1.52) |
| On-treatment FEV1, litres | 2.52 (0.82) | 1.93 (1.02) |
| On-treatment FEV1, % predicted | 80.2 (22.6) | 62.7 (23.8) |
| Severe exacerbations in the prior 12 months | 2.29 (1.60) | 2.33 (1.41) |
| Zero severe exacerbations | 0 (0%) | 0 (0%) |
| One severe exacerbation | 3 (43%) | 2 (22%) |
| Two severe exacerbations | 2 (29%) | 3 (33%) |
| Three severe exacerbations | 0 (0%) | 1 (11%) |
| Four severe exacerbations | 1 (14%) | 2 (22%) |
| Five severe exacerbations | 1 (14%) | 1 (11%) |
| Number of patients with at least one prior hospital admission ever for asthma | 1 (14%) | 5 (56%) |
| Duration of asthma, years | 28.7 (20.0) | 33.0 (15.3) |
Data are mean (s.d.) or n (%). ACQ-7 is a composite score of asthma control, comprising questions on asthma symptoms, rescue bronchodilator use and forced expiratory volume in 1 s (FEV1) % predicted (overall scores range from 0 to 6, with scores ⩽0.75 suggesting ‘well-controlled’ asthma and scores ⩾1.50 suggesting ‘not well-controlled’ asthma).30,31 A severe exacerbation was defined as follows: (a) the use of systemic corticosteroids for at least 3 days, or (b) a hospitalisation or Emergency Department visit because of asthma, requiring systemic corticosteroids.20 Courses of corticosteroids separated by 7 days or more were treated as separate severe exacerbations.
Abbreviation: SMART, Single combination inhaler as Maintenance And Reliever Therapy.
In the run-up to the first hospital attendance, two patients self-initiated prednisone: one Standard (who subsequently had GP review) and one SMART. Two patients (both Standard) were prescribed prednisone by a GP (Figures 2 and 3). One patient in the SMART group went to their GP and was then referred in to the hospital that same day.
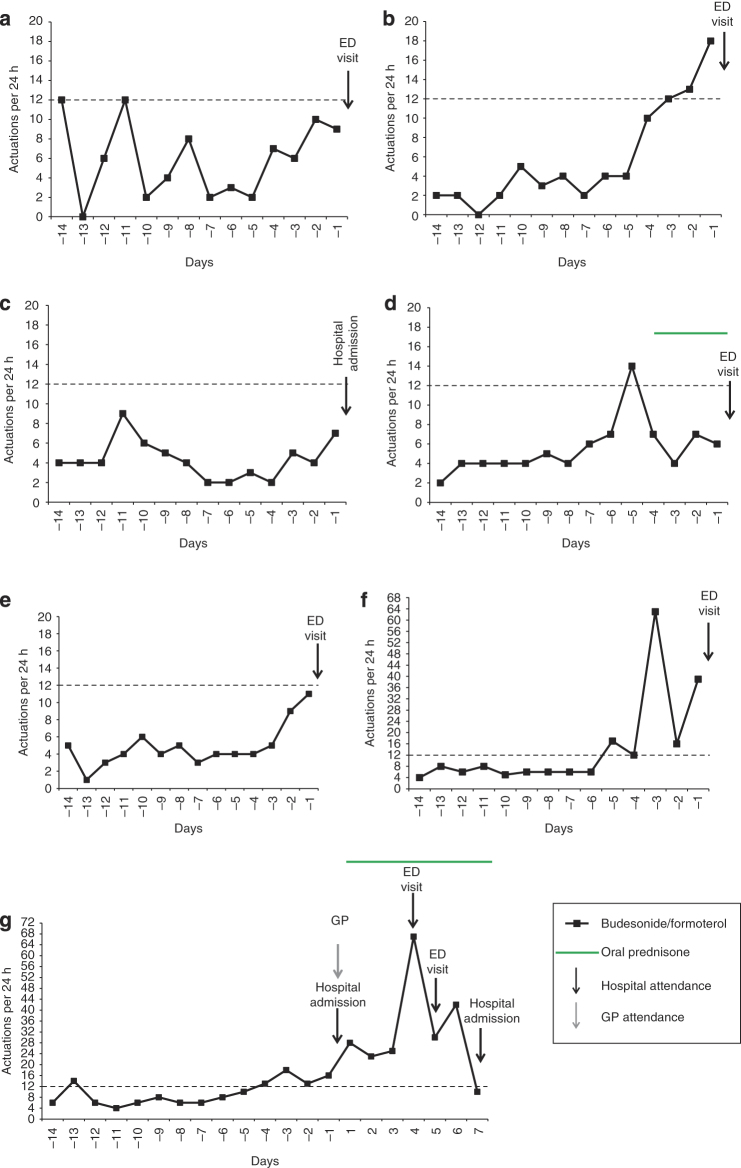
Figure 2.
Individual patterns of daily budesonide/formoterol use in the 14 days before hospital attendance in the SMART group. Hospital attendances owing to Emergency Department (ED) visit or hospital admission are specified for each participant. The x axis is days preceding or following the first hospital attendance (i.e., day −1 refers to the 24 h before the first hospital attendance, and day 1 refers to the 24 h following the first hospital attendance). Data extraction was for fourteen 24-h periods before the attendance time at hospital. The y axis is the number of actuations per 24 h. Dashed horizontal lines represent the thresholds of β2-agonist use per day above which self-management plans recommend medical review (>12 actuations of budesonide/formoterol per day for SMART patients). (d) The participant self-initiated prednisone for asthma (40 mg per day for 4 days) on day −4 (without subsequent medical review until hospital attendance). (g) The participant had four hospital attendances, identified by the solid arrows (hospital admissions occurred for the first and last attendances; ED visits occurred for the second and third attendances). Before the first ED visit, the participant who attended was seen by their general practitioner (GP). The participant was prescribed prednisone (40 mg per day for 7 days, followed by a weaning course over the next 21 days).
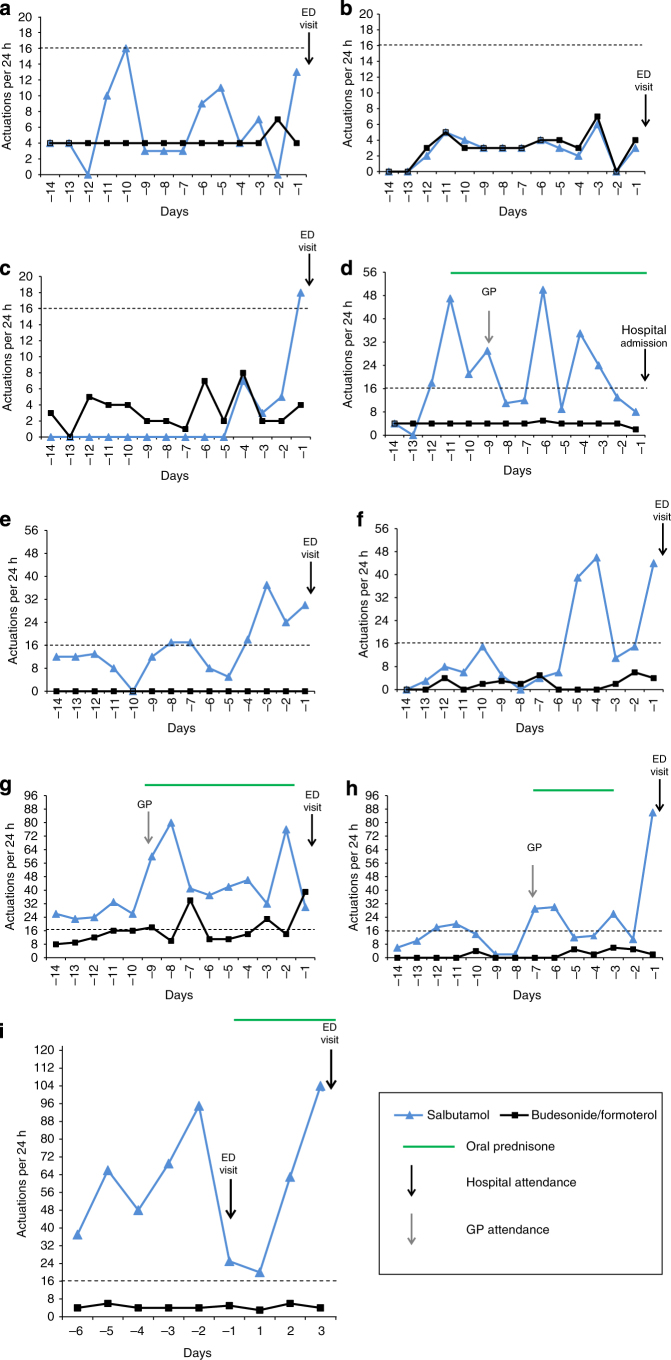
Figure 3.
Individual patterns of daily salbutamol and budesonide/formoterol use in the 14 days before hospital attendance in the Standard group. Hospital attendances owing to Emergency Department (ED) visit or hospital admission are specified for each participant. The x axis is days preceding or following the first hospital attendance (i.e., day −1 refers to the 24 h before the first hospital attendance, day 1 refers to the 24 h following the first hospital attendance). Data extraction was for fourteen 24-h periods before the attendance time at hospital. The y axis is the number of actuations per 24 h. Horizontal dashed lines represent the thresholds of β2-agonist use per day above which self-management plans recommend medical review (>16 actuations of salbutamol per day for Standard patients). (d) The participant self-initiated prednisone (40 mg per day for 14 days) for asthma on day −11 (with subsequent medical review by a general practitioner (GP) on day −9). (g) The participant was prescribed prednisone (40 mg per day for 7 days) for asthma by a general practitioner on day −9. (h) The participant was prescribed prednisone (40 mg per day for 4 days) for asthma by a general practitioner on day −7. (i) The participant had two ED visits, identified by the solid arrows, and there were no data before day −6, as this was the day of the first study visit (randomisation visit). The participant was prescribed prednisone (40 mg per day for 7 days). (c) and (f) refer to two episodes in the same participant, occurring 4 months apart.
Electronic medication data set
One patient in the SMART group had three hospital attendances in the 7 days following the first hospital admission. One patient in the Standard group had one hospital attendance within the 7 days following the first attendance. In another patient randomised to Standard treatment who had two hospital attendances, there were no recorded reliever use data before either episode, owing to the use of nonstudy inhaled medication. Thus, electronic data for seven SMART and nine Standard group episodes were included in the analysis of medication use patterns (Figure 1). There was no reported use of nonstudy inhaled asthma treatment in any of the other patients before the first hospital attendance. There was no data loss owing to inhaler loss or malfunction. There were no days on which dose dumping was observed, using the previously defined criteria.21
Medication use before hospital attendance
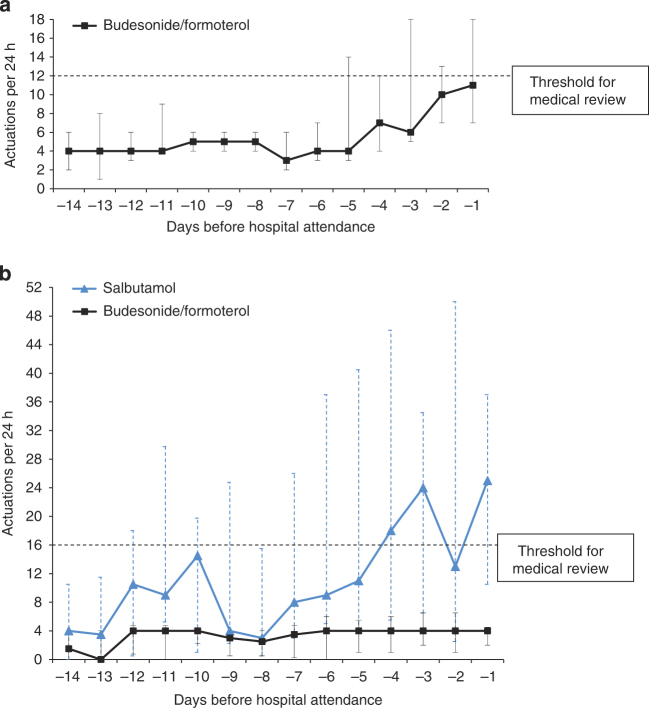
The use of budesonide/formoterol in the SMART group and salbutamol in the Standard group progressively increased 5 days before hospital attendance, and it peaked in the 24 h preceding attendance (Figure 4). The use of salbutamol increased around 10 days before hospital attendance, before reducing to a lower level and then progressively increasing in the 5 days before hospital attendance (Figure 4).
Figure 4.
Median daily medication use in the 14 days before hospital attendance in the SMART (n=7 attendances) (a) and Standard (n=9 attendances) (b) groups. There is a 1:2 dose bioequivalence (6 μg:200 μg) for formoterol to salbutamol,13 on the basis of bronchodilator studies of repeat dosing in acute asthma.24,25 Dashed lines represent the thresholds of β2-agonist use per day above which self-management plans recommend medical review (>8 actuations of budesonide/formoterol per day above the four maintenance actuations (i.e., a total of 12 actuations) for SMART patients and >16 actuations of salbutamol per day for Standard patients). The x axis is days preceding the hospital attendance (i.e., day −1 refers to the 24 h before hospital attendance). Data extraction was for the fourteen 24-h periods before the attendance time at hospital for each patient. The y axis is the median number of actuations per 24 h. Error bars are the interquartile range (IQR). For one patient in the Standard group, there were no data before day −6, as this was the day of the first study visit (randomisation visit).
For the maximum number of inhaler actuations in a 24-h period, the median(range) was 14(9 to 63) for budesonide/formoterol in the SMART group and 46(6 to 95) for salbutamol in the Standard group. On the day of maximum salbutamol use for each presentation in the Standard group, the median(range) number of budesonide/formoterol actuations was 4(0 to10).
The use of medication by individual patients showed distinct patterns (Figures 2 and 3), which are not obvious from the summary data shown in Figure 4. For the SMART regimen, the daily number of doses of budesonide/formoterol was consistent with the use of budesonide/formoterol as maintenance and reliever therapy, although it is possible that in some patients budesonide/formoterol was used according to an as-required ‘reliever’ regimen. For the Standard regimen, three predominant patterns were observed:
Maintenance budesonide/formoterol use with salbutamol ‘reliever’ use consistent with the ‘Standard regimen’ (Figure 3a,d,i).
Poorly/nonadherent budesonide/formoterol maintenance use and salbutamol ‘reliever’ use (Figure 3e,f,h).
Variable budesonide/formoterol use and variable salbutamol use consistent with ‘budesonide/formoterol and/or salbutamol reliever regimen’ (Figure 3b,c,g).
Delay in medical review in the setting of β2-agonist overuse
There were 3/7 patients in the SMART group (Figure 2d,f,g) and 6/9 patients in the Standard group (Figure 3d–i) who delayed obtaining medical review as recommended in the self-management plan in the setting of β2-agonist overuse in the lead up to hospital attendance. One SMART patient (Figure 2d) self-initiated a course of prednisone, in accordance with their self-management plan, but without medical review.
ICS nonadherence
ICS nonadherence occurred in two SMART patients, on a single day each (Figure 2a,b). Three Standard patients had repeated days of ICS nonadherence, despite ongoing extreme salbutamol overuse (Figure 3e,f,h).
Physiological and biochemical parameters
The physiological, serum potassium and QTc interval measurements at hospital presentation were similar in both treatment groups (Table 2). The lowest potassium value was 3.2 mmol/l (in the Standard patient, in whom no reliever medication use data were available owing to nonstudy medication use). All other recorded potassium values were ⩾3.6 mmol/l. There was no association between serum potassium and the total number of budesonide/formoterol or salbutamol actuations in the 24 h or 7 days preceding initial hospital attendance for SMART and Standard patients, respectively (Online Supplementary Figure OS1). One patient (in the SMART group) who was subsequently diagnosed with idiopathic hypertrophic cardiomyopathy had a QTc interval of 456 ms; all other measured values were <440 ms (Online Supplement).
Table 2. Initial physiological and biochemical parameters for all attendances to hospital for severe asthma.
| Parameter | SMART group | Standard group |
|---|---|---|
| Number of attendancesa | 9 | 12 |
| Number of ambulance transfers | 2 | 5 |
| Initial SpO2, % saturationb | 97.6 (1.3) | 96.3 (4.0) |
| Initial respiratory rate, breaths/min | 24.6 (3.6) | 27.1 (7.9) |
| Initial heart rate, beats/min | 100.8 (18.1) | 95.5 (14.7) |
| Initial systolic blood pressure, mm Hg | 142.7 (18.5) | 130.0 (17.9) |
| Initial diastolic blood pressure, mm Hg | 87.0 (14.2) | 83.2 (15.3) |
| Attendances with PEFR performed | 4 (44%) | 9 (75%) |
| PEFR, l/min | 238.8 (80.3) | 315.0 (128.0) |
| % Best value | 59.2 (14.7) | 72.3 (24.3) |
| Attendances with serum potassium performed | 9 (100%) | 8 (67%) |
| Serum potassium, mmol/lc | 4.13 (0.29) | 3.76 (0.32) |
| Attendances with an ECG performed | 7 (78%) | 3 (25%) |
| QTc interval, msd | 418.6 (21.3) | 401.1 (11.0) |
Values are mean (s.d.) or n (%). The comparison between randomised groups used a mixed linear model to take account of repeated measurements of some participants. Estimates may be numerically different from the values calculated from the tabulated mean values.
Abbreviations: ECG, electrocardiogram; PEFR, peak expiratory flow rate; SMART, Single combination inhaler as Maintenance And Reliever Therapy; SpO2, oxygen saturation by pulse oximetry.
n=Nine attendances for SMART as there were no clinical data recorded for one patient who left the Emergency Department before clinical review.
SpO2 was recorded on oxygen therapy for one SMART attendance and three Standard attendances.
SMART minus Standard mean (95% confidence interval (CI))=0.33 (−0.07 to 0.73), P=0.093.
SMART minus Standard mean (95% CI)=22.2 (−21.6 to 65.9), P=0.25.
Documented β2-agonist use in medical case notes
Self-reported β2-agonist use was documented in the medical records in 11/22 of ED attendances and, when recorded, underestimated actual use as measured by electronic monitoring for patients in both groups (Table 3).
Table 3. Self-reported β2-agonist use in medical records versus electronic monitoringa .
| Patient code b | Number of actuations measured by electronic monitor in the 24 h preceding attendance c | Self-reported β2-agonist use documented in hospital medical records |
|---|---|---|
| SMART B | 18 | Using inhaler every 20 min |
| SMART C | 7 | Using inhaler four times a day |
| SMART F | 39 | Using inhaler every 30 min |
| SMART Gd | 42 | Using multiple doses of own medicine |
| Standard A | 13 | 4 actuations of salbutamol |
| Standard B | 3 | 12 puffs of salbutamol |
| Standard C | 18 | 3 puffs of salbutamol in 2 h |
| Standard D | 8 | 6 puffs of salbutamol every 1–2 h |
| Standard E | 30 | Salbutamol 20 times per day |
| Standard H | 86 | Increasing use of salbutamol in the past 24 h |
| Standard Ie | 25 | Using salbutamol three times per day |
4/10 and 7/12 of attendances in the Single combination inhaler as Maintenance And Reliever Therapy (SMART) and Standard groups had self-reported β2-agonist use documented in the hospital medical records.
Refers to patient code on the figures displaying individual patient patterns of use.
Number of budesonide/formoterol actuations for SMART and number of salbutamol actuations for Standard.
Before the fourth attendance.
Before the first attendance.
Discussion
Main findings
This analysis shows that patients commonly take very high doses of inhaled β2-agonist therapy in the 2-week period leading up to a hospital attendance with a severe exacerbation of asthma. β2-agonist overuse was particularly observed in the Standard regimen, in which the median maximum number of actuations of salbutamol in a 24-h period was 46 with an additional median number of four actuations of budesonide/formoterol, compared with a median maximum 14 actuations of budesonide/formoterol in a 24-h period in the SMART group. Delays in seeking medical review in response to exceeding the β2-agonist level indicated in their asthma self-management plan were observed in both groups, more commonly in patients randomised to the Standard regimen. Repeated days of ICS nonadherence, in which patients took no budesonide/formoterol actuations, were observed in one-third of the Standard patients in the 2 weeks before hospital attendance, in contrast to the SMART regimen in which this pattern did not occur.
Strengths and limitations of this study
We recognise that this study was not powered to determine the statistical significance of differences between the SMART and Standard regimens, and it was limited by the relatively small sample size, because only 16 of 303 patients attended the hospital for a severe exacerbation of asthma during the trial. However, this is balanced by our use of electronic monitoring to provide data on actual patterns of medication use in the setting of severe asthma in real-world patients, which, to our knowledge, is unique.
Unlike previous similar analyses, which have relied on patient report,22 electronic monitoring provides accurate individual data on actual medication use in the setting of a severe exacerbation, and insight into patterns of behaviour in seeking medical review. We did not collect data on lung function outside of study visits, and thus we are unable to correlate the observed patterns of medication use with peak expiratory flow rates during severe exacerbations. We did not extend this analysis to include urgent GP or after-hours attendance, as this would have resulted in inclusion of some presentations of uncertain severity or clinical significance.
Entry to the study required patients to have had at least one asthma exacerbation in the preceding year and a current prescription for an inhaled corticosteroid. Although it is uncertain whether patterns of inhaler use and health-seeking behaviour before hospital attendance would differ between this group and other asthmatics with less severe asthma, recruitment of this population has the advantage of generating results relevant to those patients who are at a high risk of a future exacerbation.23
It is inherently difficult to make comparisons between formoterol and salbutamol in terms of levels of overuse, owing to their different pharmacological properties, including duration of bronchodilator action. In our study, we have used a 1:2 dose bioequivalence (6 μg:200 μg) for formoterol to salbutamol,13 on the basis of bronchodilator studies of repeat dosing in acute asthma.24,25 The high-use thresholds were based on the dose limits of β2-agonist use requiring medical review, as defined in the respective self-management plans.15,16
Interpretation of findings in relation to previously published work
For both regimens, the plots of average medication use show increasing reliever use from around 5 days preceding the hospital attendance, which is consistent with the self-reported reliever use data at the time of severe asthma exacerbations presented by Tattersfield et al. 22 In our study, the individual data on medication use from the electronic monitoring provided greater detail of how patients vary their reliever therapy during worsening asthma. Patients randomised to SMART had patterns of use broadly consistent with this regimen, although it is probable that some were taking the budesonide/formoterol as a ‘reliever’ only. In Standard regimen patients, three predominant patterns were observed, two were consistent with the Standard regimen, one of which was characterised by poor maintenance budesonide/formoterol adherence, and the third pattern was consistent with a salbutamol and/or budesonide/formoterol use only as a ‘reliever’.
We previously observed that patients randomised to the SMART regimen had fewer days of nonadherence with ICS in the form of budesonide/formoterol therapy compared with the Standard regimen.13 The present analysis indicates that differences in ICS nonadherence persist within the setting of a severe exacerbation of asthma. In the SMART group, two patients were nonadherent on one day each in the 2 weeks before presentation. One-third of the patients in the Standard group had repeated days on which they took no ICS therapy, despite concomitant extremely high salbutamol use. One patient did not take any ICS throughout the 2-week period despite up to 37 salbutamol actuations per day; another patient had a three-day period of non-ICS use despite up to 46 salbutamol actuations per day; and a third patient had eight days of no ICS use despite up to 30 salbutamol actuations on these days. We propose that these episodes of nonadherence with ICS therapy in the Standard regimen contribute to the progression of severe asthma leading to hospital presentation. It is likely that the SMART regimen prevented nonadherence to ICS therapy in patients poorly adherent to maintenance treatment, because these patients used their budesonide/formoterol inhaler ‘as needed’ in response to symptoms, rather than as per the formal SMART regimen.
We were concerned that the higher intrinsic activity of formoterol compared with salbutamol could potentially lead to greater hypokalaemic and cardiovascular effects.26,27 However, patients in the SMART group did not have lower serum potassium levels, presumably because of the markedly lower doses of budesonide/formoterol taken compared with salbutamol. The observation that only one patient had a serum potassium level below the lower limit of the normal range, despite such high β2-agonist doses, indicates that substantive tolerance to the systemic β2 effects occurs.28,29 Unfortunately, too few patients had an electrocardiogram at presentation to hospital to investigate the relative effects on QTc interval.
Documentation in hospital medical records under-reported the extent of β2-agonist overuse leading up to the hospital presentation. This observation probably reflects both under-reporting by patients and the lack of recognition of the importance of accurate determination and documentation of this feature of the history by the attending medical staff. The clinical importance of this is illustrated by the three patients, each of whom used at least 95 salbutamol actuations in the 48 h before attendance, and who were subsequently discharged from the ED on the day of their presentation. Our findings of very high doses of self-administered β2-agonist, in the form of salbutamol or formoterol, are consistent with a prior study demonstrating high blood salbutamol concentrations in patients dying of asthma.11
Implications for future research, policy and practice
There was delay in obtaining medical review or initiating oral prednisone despite high β2-agonist use above the predefined levels indicating the requirement for medical review and intervention in half of the patients. This is likely to be an underestimate of the frequency of delay in obtaining medical review and intervention in clinical practice, as the patients were closely followed up in the setting of the clinical trial with implementation of personalised asthma management plans, reinforced at regular clinic review. During periods of overuse, the opportunity exists for patients to seek medical review and receive appropriate medical intervention to reduce the risk of a life-threatening attack. The importance of this behaviour is underlined by the repeated findings from asthma mortality surveys, which showed that marked overuse of β2-agonist drugs and associated delay in seeking medical review are important factors contributing to a fatal outcome.1–4 In the recent United Kingdom National Review of Asthma Deaths, 39% of patients had been prescribed more than 12 short-acting reliever inhalers in the year before death, and 4% of the patients had been prescribed more than 50 reliever inhalers. During the final attack of asthma, 45% died without seeking medical assistance or before emergency care could be provided.5
Nonadherence to maintenance ICS therapy before hospital attendance was observed in the Standard group. The use of the SMART regimen reduced nonadherence with ICS therapy, which together with the increased self-administration of ICS therapy in the setting of worsening asthma is likely to be responsible for the reduction in severe exacerbations requiring oral corticosteroids with its use.13
Conclusions
In conclusion, many patients take very high doses of inhaled β2-agonists for prolonged periods before presentation to hospital with severe asthma. During this period, the opportunity exists to seek medical review and appropriate medical intervention to reduce the risk of a life-threatening attack. The SMART regimen led to reduced nonadherence with ICS therapy, which together with the increased self-administration of ICS therapy in the setting of worsening asthma is likely to be responsible for the reduction in severe exacerbations requiring oral corticosteroids with its use.
Acknowledgments
The authors are grateful to the study participants for their involvement in the study. The authors acknowledge the support of the hospitals involved in the collection of these data. MP is funded by a National Institute for Health Research Clinical Lectureship.
SMART Study Group. Steering Committee: Mitesh Patel (clinical coordinating investigator), Janine Pilcher, Alison Pritchard, Kyle Perrin, Justin Travers, Dominick Shaw, Shaun Holt, Matire Harwood, Peter Black, Mark Weatherall (study biostatistician), Richard Beasley (principal investigator); Auckland (Henderson Medical Centre): Clare McGuinness-Goodwin, Bill Mackey, Rodney Marks, Vikky Qi, Tyronne Tranquilino, Dirk Venter; Auckland (University of Auckland): Amy Chan; Hamilton (Waikato Hospital): Robert Hancox; Lower Hutt (Tu Kotahi Māori Asthma Trust): Cheryl Davies, Ann Smith; Tauranga (CentralMed General Practice): Andrew Corin, Colin Helm, Chris Tofield; Tauranga (Papamoa Pines Medical Centre): Davitt Sheahan; Wellington (BoydHQ Limited): Craig Boyd (database engineer); Wellington (MRINZ): Tanya Baker, Irene Braithwaite, Denise Fabian, Maureen Stretch, Mathew Williams.
The Medical Research Institute of New Zealand (MRINZ) has received research funding from AstraZeneca for the unrelated study ‘Pharmacotherapy for the different phenotypes of airways disease’. RH has received payment for lectures from GlaxoSmithKline and support to attend meetings from Boehringer Ingelheim. RB has been a member of the GlaxoSmithKline (NZ) advisory board, consulted for Cytos Biotechnology and Pharmaxis, received research grants from AstraZeneca, Cephalon, Chiesi, Genentech, GlaxoSmithKline and Novartis, payment for lectures or support to attend meetings from Boehringer Ingelheim, GlaxoSmithKline, Novartis, Nycomed and Otsuka Pharmaceuticals. MP, JP, DaS, AP, IB, DoS and MW have no conflicts of interest.
References
- Fraser PM, Speizer FE, Waters SD, Doll R, Mann NM. The circumstances preceding death from asthma in young people in 1968 to 1969. Br J Dis Chest. 1971;65:71–84. [PubMed] [Google Scholar]
- Sears MR, Rea HH. Patients at risk for dying of asthma: New Zealand experience. J Allergy Clin Immunol. 1987;80:477–481. doi: 10.1016/0091-6749(87)90079-0. [DOI] [PubMed] [Google Scholar]
- Beasley R, Pearce N, Crane J, Burgess C. Beta-agonists: what is the evidence that their use increases the risk of asthma morbidity and mortality? J Allergy Clin Immunol. 1999;104:S18–S30. doi: 10.1016/s0091-6749(99)70270-8. [DOI] [PubMed] [Google Scholar]
- Beasley R, Pearce N, Crane J, Windom H, Burgess C. Asthma mortality and inhaled beta agonist therapy. Aust N Z J Med. 1991;21:753–763. doi: 10.1111/j.1445-5994.1991.tb01385.x. [DOI] [PubMed] [Google Scholar]
- Royal College of Physicians . Why asthma kills: the National Review of Asthma Deaths (NRAD) Confidential Enquiry Report. RCP: London, UK; 2014. [Google Scholar]
- Newhouse MT, Chapman KR, McCallum AL, Abboud RT, Bowie DM, Hodder RV. Cardiovascular safety of high doses of inhaled fenoterol and albuterol in acute severe asthma. Chest. 1996;110:595–603. doi: 10.1378/chest.110.3.595. [DOI] [PubMed] [Google Scholar]
- Collins JM, McDevitt DG, Shanks RG, Swanton JG. The cardio-toxicity of isoprenaline during hypoxia. Br J Pharmacol. 1969;36:35–45. doi: 10.1111/j.1476-5381.1969.tb08301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane J, Pearce N, Flatt A, Burgess C, Jackson R, Kwong T. Prescribed fenoterol and death from asthma in New Zealand, 1981-83: case-control study. Lancet. 1989;1:917–922. doi: 10.1016/s0140-6736(89)92505-1. [DOI] [PubMed] [Google Scholar]
- Stolley PD, Schinnar R. Association between asthma mortality and isoproterenol aerosols: a review. Prev Med. 1978;7:519–538. doi: 10.1016/0091-7435(78)90265-7. [DOI] [PubMed] [Google Scholar]
- Suissa S, Blais L, Ernst P. Patterns of increasing beta-agonist use and the risk of fatal or near-fatal asthma. Eur Respir J. 1994;7:1602–1609. doi: 10.1183/09031936.94.07091602. [DOI] [PubMed] [Google Scholar]
- Abramson MJ, Bailey MJ, Couper FJ, Driver JS, Drummer OH, Forbes AB. Are asthma medications and management related to deaths from asthma? Am J Respir Crit Care Med. 2001;163:12–18. doi: 10.1164/ajrccm.163.1.9910042. [DOI] [PubMed] [Google Scholar]
- Windom HH, Burgess CD, Crane J, Pearce N, Kwong T, Beasley R. The self-administration of inhaled beta agonist drugs during severe asthma. N Z Med J. 1990;103:205–207. [PubMed] [Google Scholar]
- Patel M, Pilcher J, Pritchard A, Perrin K, Travers J, Shaw D. Efficacy and safety of maintenance and reliever combination budesonide–formoterol inhaler in patients with asthma at risk of severe exacerbations: a randomised controlled trial. Lancet Respir Med. 2013;1:32–42. doi: 10.1016/S2213-2600(13)70007-9. [DOI] [PubMed] [Google Scholar]
- Patel M, Pilcher J, Reddel HK, Pritchard A, Corin A, Helm C. Metrics of salbutamol use as predictors of future adverse outcomes in asthma. Clin Exp Allergy. 2013;43:1144–1151. doi: 10.1111/cea.12166. [DOI] [PubMed] [Google Scholar]
- Symbicort SMART Asthma Action Plan National Asthma Council Australia. Available at http://www.nationalasthma.org.au/health-professionals/tools-for-primary-care/asthma-action-plans/asthma-action-plan-library . Accessed January 2013.
- Holt S, Masoli M, Beasley R. The use of the self-management plan system of care in adult asthma. Prim Care Respir J. 2004;13:19–27. doi: 10.1016/j.pcrj.2003.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nexus 6 Limited Smartinhaler Tracker Product Details. Available at http://www.smartinhaler.com/ Accessed January 2013.
- Patel M, Pilcher J, Chan A, Perrin K, Black P, Beasley R. Six-month in vitro validation of a metered-dose inhaler electronic monitoring device: implications for asthma clinical trial use. J Allergy Clin Immunol. 2012;130:1420–1422. doi: 10.1016/j.jaci.2012.06.037. [DOI] [PubMed] [Google Scholar]
- Patel M, Pilcher J, Travers J, Perrin K, Shaw D, Black P. Use of metered-dose inhaler electronic monitoring in a real-world asthma randomized controlled trial. J Allergy Clin Immunol Pract. 2013;1:83–91. doi: 10.1016/j.jaip.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- Rand CS, Wise RA, Nides M, Simmons MS, Bleecker ER, Kusek JW. Metered-dose inhaler adherence in a clinical trial. Am Rev Respir Dis. 1992;146:1559–1564. doi: 10.1164/ajrccm/146.6.1559. [DOI] [PubMed] [Google Scholar]
- Tattersfield AE, Postma DS, Barnes PJ, Svensson K, Bauer CA, O'Byrne PM. Exacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study Group. Am J Respir Crit Care Med. 1999;160:594–599. doi: 10.1164/ajrccm.160.2.9811100. [DOI] [PubMed] [Google Scholar]
- Global Initiative for Asthma Global Statement for Asthma Management and Prevention 2014. Available at www.ginasthma.org . Accessed July 2014.
- Balanag VM, Yunus F, Yang PC, Jorup C. Efficacy and safety of budesonide/formoterol compared with salbutamol in the treatment of acute asthma. Pulm Pharmacol Ther. 2006;19:139–147. doi: 10.1016/j.pupt.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Rubinfeld AR, Scicchitano R, Hunt A, Thompson PJ, Van Nooten A, Selroos O. Formoterol Turbuhaler as reliever medication in patients with acute asthma. Eur Respir J. 2006;27:735–741. doi: 10.1183/09031936.06.00027405. [DOI] [PubMed] [Google Scholar]
- Hanania NA, Sharafkhaneh A, Barber R, Dickey BF. Beta-agonist intrinsic efficacy: measurement and clinical significance. Am J Respir Crit Care Med. 2002;165:1353–1358. doi: 10.1164/rccm.2109060. [DOI] [PubMed] [Google Scholar]
- Bremner P, Woodman K, Burgess C, Crane J, Purdie G, Pearce N. A comparison of the cardiovascular and metabolic effects of formoterol, salbutamol and fenoterol. Eur Respir J. 1993;6:204–210. [PubMed] [Google Scholar]
- Newnham DM, Grove A, McDevitt DG, Lipworth BJ. Subsensitivity of bronchodilator and systemic beta 2 adrenoceptor responses after regular twice daily treatment with eformoterol dry powder in asthmatic patients. Thorax. 1995;50:497–504. doi: 10.1136/thx.50.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg BT, Louwerse RT, Luiken GJ, Jonkers RE, van Boxtel CJ. Hypokalaemia in healthy volunteers after single and multiple doses of formoterol or salbutamol. Clin Drug Investig. 1998;15:523–529. doi: 10.2165/00044011-199815060-00009. [DOI] [PubMed] [Google Scholar]
- Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying 'well-controlled' and 'not well-controlled' asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.