Abstract
Context
Cryotherapy is used widely in sport and exercise medicine to manage acute injuries and facilitate rehabilitation. The analgesic effects of cryotherapy are well established; however, a potential caveat is that cooling tissue negatively affects neuromuscular control through delayed muscle reaction time. This topic is important to investigate because athletes often return to exercise, rehabilitation, or competitive activity immediately or shortly after cryotherapy.
Objective
To compare the effects of wet-ice application, cold-water immersion, and an untreated control condition on peroneus longus and tibialis anterior muscle reaction time during a simulated lateral ankle sprain.
Design
Randomized controlled clinical trial.
Setting
University of Hertfordshire human performance laboratory.
Patients or Other Participants
A total of 54 physically active individuals (age = 20.1 ± 1.5 years, height = 1.7 ± 0.07 m, mass = 66.7 ± 5.4 kg) who had no injury or history of ankle sprain.
Intervention(s)
Wet-ice application, cold-water immersion, or an untreated control condition applied to the ankle for 10 minutes.
Main Outcome Measure(s)
Muscle reaction time and muscle amplitude of the peroneus longus and tibialis anterior in response to a simulated lateral ankle sprain were calculated. The ankle-sprain simulation incorporated a combined inversion and plantar-flexion movement.
Results
We observed no change in muscle reaction time or muscle amplitude after cryotherapy for either the peroneus longus or tibialis anterior (P > .05).
Conclusions
Ten minutes of joint cooling did not adversely affect muscle reaction time or muscle amplitude in response to a simulated lateral ankle sprain. These findings suggested that athletes can safely return to sporting activity immediately after icing. Further evidence showed that ice can be applied before ankle rehabilitation without adversely affecting dynamic neuromuscular control. Investigation in patients with acute ankle sprains is warranted to assess the clinical applicability of these interventions.
Key Words: cryotherapy, neuromuscular control, proprioception, tilt platform
Key Points
Ten minutes of joint cooling with wet-ice application or cold-water immersion did not adversely affect muscle reaction time or muscle amplitude in response to a simulated lateral ankle sprain.
Athletes can return safely to sporting activity immediately after 10 minutes of ankle-joint cooling.
Ice can be applied before ankle rehabilitation without adversely affecting dynamic control.
Ankle sprains occur frequently during sport and exercise.1,2 In recent reviews of epidemiologic studies spanning more than 70 sports, authors3,4 have identified ligament damage as responsible for 84% of all ankle injuries, with most involving the lateral ligament complex. The most commonly reported mechanism for an ankle sprain was excessive loading with the foot in plantar flexion and inversion.5
Ankle-joint stability is achieved through the interaction of passive and dynamic systems. The lateral ligaments and joint capsule provide a passive restraint against external forces.5 These structures also contain mechanoreceptors that sense extreme or sudden movements and initiate a dynamic restraint6,7; peroneus longus activation provides a dynamic restraint against excessive inversion,5,8,9 whereas the tibialis anterior restrains excessive plantar flexion.10–12 Coordinated and correctly timed contraction of these muscles is vital to protect the ankle joint against excessive movement.13 Individuals with functional ankle instability exhibit a delay in muscle reaction time, which may explain the frequent episodes of giving way and repetitive inversion injury commonly reported within this population.11,14,15
Cryotherapy is used widely in sport and exercise medicine.16–19 It has an immediate analgesic effect20–22 achieved through a range of physiologic mechanisms, including prolonged latency and duration of sensory action potentials, decreased nerve transmission,23 suppression of nociceptive receptor sensitivity,24 and counterirritant effects.22 These effects are often used to manage acute pain after soft tissue injury; ice application on the sidelines or during half time can provide cold-induced analgesia to facilitate a return to competitive activity. A growing trend is to use cold-induced analgesia to enhance rehabilitation and therapeutic exercise, which is often called cryokinetics.25 Cryokinetic treatments are commonly 5 to 10 minutes in duration and involve cold-water immersion.18,26,27 The basic premise is that cold-induced analgesia facilitates rehabilitation and enables exercises to be performed earlier than would normally be possible.16,18
In addition to providing effective pain relief, local cooling can induce a range of concomitant physiologic effects. In a recent review, Bleakley et al28 provided consistent evidence that longer periods of ice application (>20 minutes) adversely affected strength, speed, power, and agility-based running. Another concern is that cooling tissue negatively affects neuromuscular control through changes in joint position sense29 and delayed reaction time. Authors13,25,30 of only 3 studies have assessed the effect of ice application on muscle reaction time during a simulated lateral ankle sprain. Although they all identified no change postapplication, these conclusions were limited to the effects of dry ice (crushed ice contained within a nonporous bag) on peroneus longus activity. Therefore, determining the effect of other popular modes of cooling on muscle reaction time is important. We also need to determine whether these patterns are consistent across other muscle groups contributing to dynamic ankle stability. Thus, the purpose of our study was to examine the effects of cryotherapy on muscle reaction time during a simulated lateral ankle sprain. Our specific objectives were to compare the effects of wet-ice application, cold-water immersion, and an untreated control condition on muscle reaction time of the peroneus longus and tibialis anterior muscle groups.
METHODS
The study was a randomized controlled trial. The independent variables were treatment (wet-ice application, cold-water immersion, control) and time interval (pretreatment, posttreatment). The dependent variables were muscle reaction time and muscle amplitude.
Participants
A total of 58 physically active individuals (30 men, 28 women) were recruited from the student population. We provided a participant briefing that outlined the study. To meet the inclusion criteria, volunteers had to be ages 18 to 25 years; be active in sport at least once each week; have pain-free palpation of the anterior talofibular ligament (ATFL) and calcaneofibular ligament; and have pain-free active dorsiflexion, plantar-flexion, inversion, and eversion range of motion. Exclusion criteria were a previous ankle sprain; any injury at the time of the study that might affect lower limb biomechanics, including acute trauma and muscular pain; use of foot orthoses; biomechanical abnormalities; a history of lower limb surgery; and fear or intolerance of ice, including Raynaud disease. Four volunteers (3 men, 1 woman) were excluded; 3 presented with a history of ankle sprain, and 1 had been prescribed orthoses. The remaining 54 individuals (age = 20.1 ± 1.5 years, height = 1.7 ± 0.07 m, mass = 66.7 ± 5.4 kg) participated in the study. All participants provided written informed consent, and the study was approved by the Institutional Life and Medical Sciences Ethics Committee of the University of Hertfordshire.
Instrumentation
During all testing conditions, electromyography (EMG) data were collected using a DataLINK EMG system (model DLK900; Biometrics Ltd, Gwent, United Kingdom). All EMG signals were amplified and sampled at 1000 Hz. The DataLINK incorporated both a low-pass filter at 450 Hz and a high-pass, third-order filter at 18 dB per octave. The latter was designed to remove direct current offsets due to membrane potentials and to minimize low-frequency interference caused by movement of the preamplifier on the skin surface. The preamplifier also contained an eighth-order elliptical filter at −60 dB at 550 Hz.
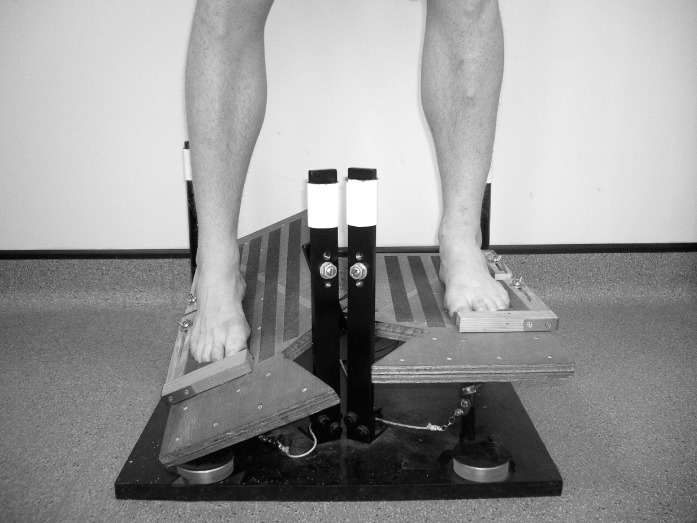
We used a tilt platform that simulated a lateral ankle sprain (Figure). It consisted of 2 independent foot plates so that either foot could experience sudden perturbation to reduce any anticipatory effect. The sprain simulation differed from previous platforms because 20° of plantar flexion was incorporated alongside 30° of inversion. The platform was constructed and first implemented by Mitchell et al.11 When the tilt began, the contact switches separated, sending a digital signal to the DataLINK system. The signal response time was 1 millisecond and was recorded on the display screen with an event marker.
Figure.
Tilt platform simulating a nonpathologic lateral ankle sprain used for muscle reaction time testing.
The wet-ice application consisted of 1 kg of crushed ice enveloped within a thin cotton bag. Before application, the bag was held under running water (30°C–34°C) for 10 seconds before being applied to the lateral aspect of the ankle. The participants lay on their sides, and the center of the bag was situated over the ATFL. For the cold-water immersion, 3 kg of crushed ice were placed into a cool box before cold water was added to achieve an immersion depth of one-third the measured distance between the lateral malleolus and the head of the fibula of the participant. The participants remained seated and, when ready, immersed their ankle and foot complex into the ice-and-water mix. The water temperature was monitored constantly to ensure that it remained between 1°C and 4°C.31 For the control condition, participants were instructed to remain seated, and no treatment was administered.
A DataLogger (model SQ800; Grant Instruments Ltd, Cambridge, United Kingdom) recorded skin temperature from the site of peroneus longus and tibialis anterior electrode placement and the ATFL using thermistors (U-type thermistor probes) that were affixed to the site with microporous tape. The modality temperature was also monitored and recorded at 60-second intervals throughout the testing session. For the wet-ice application, we placed a thermistor on the outside of the cotton bag, and for the cold-water immersion, we placed a thermistor directly into the cold water.
Participant Preparation
Electromyography data were recorded from the dominant limb, which was defined as the limb the participant would choose to kick a ball. The peroneus longus and tibialis anterior muscles were contracted and palpated. The area was shaved with disposable razors and shaving foam before being cleansed with isopropyl alcohol wipes to reduce skin impedance. In accordance with the Surface Electromyography for the Non-Invasive Assessment of Muscles (SENIAM) recommendations,32 preamplified surface electrodes (model SX230; Biometrics Ltd) with a gain of 1000, input impedance greater than 1015 Ω, common-mode rejection ratio greater than 96 dB, noise less than 5 μV, bandwidth from 20 to 60 Hz, and a fixed 20-mm intradistance were affixed parallel to the orientation of the muscle fibers. We secured a ground reference cable (R206) of the DataLINK system over the pisiform bone of the wrist. Resisted ankle dorsiflexion, plantar flexion, inversion, and eversion were performed to identify the responses of the corresponding muscles in the EMG traces on screen.
Experimental Protocol
Upon arrival at the laboratory, participants were screened for the inclusion and exclusion criteria, were educated on the experimental procedures, gave informed consent, and were assigned randomly to 1 of 3 treatment groups (wet-ice application, cold-water immersion, or control). The randomization procedure consisted of each participant selecting a numbered ball (range, 1–54), which directly corresponded with 1 of the 3 treatment groups, from an envelope. Each group, therefore, contained 18 participants.
After preparation, the participants stood barefoot on the tilt platform and were instructed to distribute their weight evenly between both feet while looking straight ahead in a relaxed stance. They stood with their feet shoulder-width apart and parallel while the 2 foot supports were adjusted to provide maximal support and safety. The investigator (P.K.T.) stood behind the participants to ensure they could not see the initiation of the tilt. Using a computer-based random-sequence generator that the investigator designed (Excel version 2010; Microsoft Corporation, Redmond, WA), we randomly exposed 1 limb to a simulated lateral ankle sprain. Participants were not informed when the perturbations would occur, and perturbations were initiated at variable intervals to reduce any anticipatory effect. Three practice perturbations were administered to ensure the participant was fully accustomed to the simulated lateral ankle sprain before the main testing commenced.
The tilt was not initiated until the EMG signal trace was at a flat baseline as viewed on screen. After the perturbation, we stopped the recording, reset the tilt platform, and carried out the remaining perturbations. This was performed 8 times (ie, each limb received 4 perturbations), with only the first 3 tilts to the dominant limb used for calculating and analyzing muscle reaction time and muscle amplitude.10 All perturbations were performed within 2 minutes, with a minimum 10-second rest between perturbations.
Participants then sat quietly for a 10-minute equilibrium period. After this equilibrium period, they received the randomly assigned treatment for 10 minutes. After application, the ankle was dried carefully with a towel, and the tilt-platform measures were repeated. Apart from analgesia, no additional adverse effects were reported.
Data Processing
We checked the raw EMG signals visually for artifacts and then processed them using a root mean square filter with a moving-window length of 10 milliseconds10 (DataLINK software version 5.0). The filtered signal was exported into an Excel 2010 spreadsheet before a specially designed computer onset detector calculated the onset of EMG muscle activity and then calculated subsequent reaction time and amplitude. For the muscle to be deemed active, the EMG readings had to be elevated by 5 SDs30 above the 1000-millisecond baseline reference point obtained before the tilt for a consecutive 20-millisecond period. Muscle reaction time was calculated as the time (in milliseconds) between the initiation of the tilt and the onset of muscle activity. Muscle amplitude (% maximum) was calculated as the average muscle amplitude (mV) over a 100-millisecond window starting from the onset of muscle activity. The average was normalized to the peak value obtained from within the same window.33
Statistical Analysis
Given that the data were normally distributed (Kolmogorov-Smirnov, P > .05), a 2-factor (mixed model: between-within) analysis of variance (ANOVA) test was used. The between-subjects factor was treatment condition (wet-ice application, cold-water immersion, control), and the within-subject factor was time (pretreatment, posttreatment). Separate ANOVAs were undertaken for each dependent variable (muscle reaction time and muscle amplitude after a sudden ankle perturbation). When we observed a main effect, we performed post hoc pairwise comparisons among the groups using the Bonferroni adjustment for multiple comparisons. The α level was set at .05. We also calculated effect sizes (ηp2 values; 0.01 = small, 0.06 = medium, and 0.14 = large).34 All statistical tests were performed with SPSS statistical software (version 20.0; IBM Corporation, Armonk, NY).
RESULTS
Peroneus longus and tibialis anterior reaction times are presented in Table 1. No main effect for time was identified for the peroneus longus (F1,51 = 0.03, P = .86, ηp2 < 0.01) or tibialis anterior (F1,51 = 0.17, P = .69, ηp2 < 0.01). In addition, we did not observe a main effect for the peroneus longus (F2,51 = 0.06, P = .94, ηp2 < 0.01) or tibialis anterior (F2,51 = 0.10, P = .91, ηp2 < 0.01) when comparing the 3 treatments. Last, we found no treatment-by-time interaction effect for the reaction time of the peroneus longus (F2,51 = 3.05, P = .06, ηp2 = 0.11) or tibialis anterior (F2,51 = 0.56, P = .57, ηp2 = 0.02), suggesting that ice application had no effect on muscle reaction time.
Table 1.
Muscle Reaction Times (ms [Mean ± SD]) of the Peroneus Longus and Tibialis Anterior During a Simulated Lateral Ankle Sprain Pretreatment and Posttreatment
| Treatment |
Peroneus Longus |
Tibialis Anterior |
||||
| Pretreatment |
Posttreatment |
Mean Difference ± 95% Confidence Interval |
Pretreatment |
Posttreatment |
Mean Difference ± 95% Confidence Interval |
|
| Control | 53.22 ± 8.89 | 51.05 ± 10.29 | 2.17 ± 6.28 | 51.53 ± 8.31 | 50.89 ± 9.40 | 0.64 ± 5.80 |
| Wet-ice application | 51.97 ± 10.95 | 51.48 ± 11.61 | 0.49 ± 7.37 | 50.34 ± 9.69 | 51.32 ± 12.82 | 0.98 ± 7.42 |
| Cold-water immersion | 49.59 ± 5.88 | 52.72 ± 6.51 | 3.13 ± 4.05 | 50.74 ± 7.74 | 49.19 ± 7.21 | 1.55 ± 4.89 |
Peroneus longus and tibialis anterior muscle amplitudes are presented in Table 2. No main effect for time was identified for the peroneus longus (F1,51 = 0.20, P = .66, ηp2 < 0.01) or tibialis anterior (F1,51 = 2.05, P = .16, ηp2 = 0.04). In addition, we did not observe a main effect for the peroneus longus (F2,51 = 2.14, P = .13, ηp2 = 0.08) or tibialis anterior (F2,51 = 1.03, P = .36, ηp2 = 0.04) when comparing the 3 treatments. Finally, we found no treatment-by-time interaction effect for muscle activity of the peroneus longus (F2,51 = 0.19, P = .83, ηp2 = 0.01) or tibialis anterior (F2,51 = 2.08, P = .14, ηp2 = 0.08), suggesting that ice application had no effect on muscle amplitude.
Table 2.
Muscle Amplitudes % of Maximum ([Mean ± SD]) of the Peroneus Longus and Tibialis Anterior During a Simulated Lateral Ankle Sprain Pretreatment and Posttreatment
| Treatment |
Peroneus Longus |
Tibialis Anterior |
||||
| Pretreatment |
Posttreatment |
Mean Difference ± 95% Confidence Interval |
Pretreatment |
Posttreatment |
Mean Difference ± 95% Confidence Interval |
|
| Control | 39.99 ± 6.31 | 40.29 ± 5.42 | 0.30 ± 3.84 | 41.07 ± 6.03 | 37.51 ± 6.96 | 3.56 ± 4.25 |
| Wet-ice application | 37.91 ± 4.41 | 37.15 ± 4.87 | 0.76 ± 3.04 | 37.17 ± 4.78 | 38.26 ± 4.48 | 1.09 ± 3.03 |
| Cold-water immersion | 40.66 ± 6.03 | 40.09 ± 5.24 | 0.57 ± 3.69 | 40.60 ± 6.15 | 39.04 ± 6.12 | 1.56 ± 4.01 |
The skin temperature at the electrode sites for the treatment groups and at the ATFL for the untreated control group declined less than 1°C throughout the 10-minute application period. The skin temperature of the ATFL and the temperatures of both cooling modalities are displayed in Table 3. Both the wet-ice application and cold-water immersion reduced the skin temperature of the ATFL to 19°C after 60 seconds of application and to 9°C at the end of treatment.
Table 3.
Skin Surface and Modality Temperatures After the 10-Minute Treatment Period
| Treatment |
Thermistor |
Temperature, °C |
||
| Mean ± SD |
Minimum |
Maximum |
||
| Wet-ice application | Anterior talofibular ligament | 9.08 ± 1.42 | 6.65 | 11.40 |
| Modality | 4.34 ± 0.50 | 3.00 | 4.90 | |
| Cold-water immersion | Anterior talofibular ligament | 8.78 ± 1.46 | 6.40 | 11.10 |
| Modality | 2.43 ± 0.76 | 1.60 | 3.95 | |
DISCUSSION
The purpose of our study was to examine the effects of wet-ice application, cold-water immersion, and an untreated control condition on muscle reaction time during a simulated lateral ankle sprain. We are the first to conduct a study that incorporated 2 popular modes of cryotherapy and recorded muscle reaction times of 2 of the major muscles involved in dynamic ankle stability.
Wet-ice application and cold-water immersion induced similar skin-temperature reductions over the ATFL. In each case, a 10-minute treatment dose resulted in skin-temperature reductions to 9°C. In addition, all participants reported numbness of the ankle directly after each icing condition ended. Optimal analgesia is elicited at skin temperatures less than 13°C.35–37 Algafly and George20 reported that skin-temperature reductions below 10°C are associated with a 33% reduction in nerve conduction velocity. Whereas these data support the use of either wet-ice application or cold-water immersion for inducing analgesia, our primary objective was to examine any concomitant adverse effect on muscle reaction time by comparison with an untreated control. We found that neither cooling condition influenced peroneus longus muscle activity during ankle-sprain simulation as compared with a control condition. This observation concurs with findings from other studies13,25,30 in which authors investigated the effects of dry-ice application.
Mok et al38 calculated that a lateral ankle sprain reaches maximal inversion at 80 milliseconds after initial contact, with a deviation from normal kinematics starting at 60 milliseconds.39 The dynamic defense mechanism needs to be initiated in this preinjury phase to prevent a lateral ankle sprain from occurring.39 We reported postapplication reaction times of 51.48 milliseconds for the wet-ice group and 52.72 milliseconds for the cold-water immersion group, which align closely with the finding reported by Hopkins et al,25 who applied 30 minutes of dry ice to the ankle. However, other researchers have observed values as low as 30 milliseconds30 and approximately 20 milliseconds (as interpreted from a graph)13 after 30 and 20 minutes of dry-ice application, respectively.
We found it interesting that despite the disparity in the reported reaction times between Cordova et al30 and our study, similar skin temperatures of 9°C were reported. Therefore, such variability in reaction time across studies may relate to differences in algorithms for detecting the onset of muscle activity. We implemented a double-threshold algorithm. First, the muscle was deemed active when a threshold of 5 SDs above the baseline activity was breached. Second, the muscle amplitude had to remain above this threshold for a consecutive 20-millisecond period. Although Cordova et al30 also set the threshold at 5 SDs, they used a single-threshold approach with no requirement for a sustained period of heightened muscle activity. Similarly, both Berg et al13 and Hopkins et al25 used single-threshold algorithms with thresholds of 2 SDs and 4 SDs, respectively, highlighting the need for a more standardized method of detecting onset of muscle activity. The testing procedures during simulated lateral ankle sprain also ranged from full weight-bearing13 to partial weight-bearing30 and walking25 protocols.
Investigators13,25,30 studying this topic have simply focused on inversion movements during simulated ankle sprains. However, the ATFL, which is the most frequently injured ankle ligament,2,40 is the primary restraint against pathologic plantar flexion.41–44 Therefore, we used a tilt platform designed and built by Mitchell et al11 that incorporated a combination of inversion and plantar-flexion movements. Thus, we are among the few researchers to assess tibialis anterior activity during a simulated lateral ankle sprain. We found no differences either between or within participants for reaction time and amplitude of the tibialis anterior muscle and found no interaction effects.
The overall mean tibialis anterior reaction time recorded before application for all 3 treatment groups was 51 milliseconds. Other researchers have reported reaction times of 56 milliseconds11 and 49 milliseconds,10 with Löfvenberg et al15 reporting the fastest time (46 milliseconds). Again, this disparity could relate to differences in the methods of detecting the onset of muscle activity. Denyer et al10 used a double-threshold detector similar to the one we used, but they set the initial threshold at 3 SDs and the consecutive period at 25 milliseconds. Mitchell et al11 outlined the use of a threshold detector but did not provide details, whereas Löfvenberg et al15 did not use a specific threshold detector but decided to use the first registered EMG response.
Our observations seem to relate to those of previous studies in which authors45,46 reported that cooling did not affect other specific components of neuromuscular control such as joint position sense. Notwithstanding this, in the most recent systematic review examining the effect of cryotherapy on joint position sense, Costello and Donnelly29 concluded that limited and equivocal evidence is available; therefore, clinicians should be cautious about returning individuals to activity immediately after ice application. In addition, the effect of cooling on more generic functional performance measures remains unclear. Such contrasting findings can be attributed to the varying cooling doses and the volume of body part that is exposed. Indeed, clear trends indicate that immersing a large portion of the lower limb in cold water causes decrements in jump height, sprinting, and agility.47–49 However, these effects seem to be diminished when topical cooling is isolated to a single joint.50–52
Perhaps surprisingly, we found no differences between cold-water immersion and isolated joint cooling. An important consideration was that our cold-water–immersion protocol involved immersion of the foot and the lower third of the leg only. This meant that the immersion level was just below the musculotendinous junction of the triceps surae muscle group and direct cooling of the peroneus longus and tibialis anterior muscle tissue was minimal. Yet peroneus longus reaction time displayed a medium effect size (ηp2 = 0.11) for the treatment-by-time comparison. The reaction time after cold-water immersion was marginally slower than the preapplication time; this same trend was not apparent in the wet-ice application or untreated control group (Table 1). This observation does not appear to be clinically important. The muscle still reacted before the reported time taken to deviate from normal kinematics,39 and the mean difference was 3.13 milliseconds with a 95% confidence interval of 4.05 milliseconds; therefore, the magnitude of this effect is negligible.
Athletes commonly undertake rehabilitative exercise during or immediately after the application of a cold treatment (cryokinetics). The basic premise is that cold-induced analgesia facilitates rehabilitation and enables exercises to be performed earlier than would normally be possible.16,18 Determining an optimal dose of cryotherapy, particularly for cryokinetics, is often difficult. Our results suggested that 10 minutes of cold-water immersion to the foot and ankle complex did not diminish peroneus longus or tibialis anterior reaction time or muscle amplitude response during a simulated lateral ankle sprain. Practitioners should consider that these findings were based on 10-minute applications and cannot be extrapolated to other joints. We also note that longer application times of more than 20 minutes can adversely affect strength, speed, power, and agility-based running.28
Our study was conducted on healthy participants. Future research should be undertaken with participants who have acute joint injury or chronic ankle instability. Evidence has suggested that postural control is already impaired during the first 2 weeks after a lateral ankle sprain.53 It is not clear whether cooling induces more impairments to postural control and muscle reaction time. In addition, our outcomes were limited to the immediate stages after icing. Intramuscular temperatures can continue to decline for approximately 15 minutes after ice application,54 and in future research, investigators should also monitor reaction time over a longer period postapplication. Moreover, with a medium effect size identified for the treatment-by-time comparison for the peroneus longus, researchers should examine the effect of repeated intermittent bouts of cryotherapy, which are used frequently for cryokinetic protocols.17 Furthermore, investigators should consider the adiposity of participants because it has been shown to affect tissue cooling.55
We examined the reaction time of the dominant limb during a simulated lateral ankle sprain, yet examining the reaction time of the musculature in the nondominant limb may also be useful. Researchers11 have shown that the dominant limb displayed a trend toward a faster reaction time during a simulated lateral ankle sprain than that of the nondominant limb. Furthermore, we incorporated a combination of inversion and plantar-flexion movements during a simulated lateral ankle sprain. However, during testing, participants were static, and we did not incorporate other important variables associated with a lateral ankle sprain. Hopkins et al25 simulated a more dynamic injury mechanism through a novel design that subjected participants to a sudden ankle-inversion perturbation during walking. Researchers should design and implement a platform that can fully examine the dynamic defense mechanism by initiating simultaneous inversion and plantar flexion of the ankle during walking.
CONCLUSIONS
Joint cooling did not adversely affect muscle reaction time or muscle amplitude during a simulated lateral ankle sprain. These observations suggest that athletes can return safely to sporting activity immediately after icing. Evidence also showed that ice can be applied before ankle rehabilitation without adversely affecting dynamic control. These effects are limited to 10-minute applications of either wet-ice application or cold-water immersion and cannot be extrapolated to other joints.
REFERENCES
- 1.Junge A, Engebretsen L, Mountjoy ML, et al. Sports injuries during the Summer Olympic Games 2008. Am J Sports Med. 2009;37(11):2165–2172. doi: 10.1177/0363546509339357. [DOI] [PubMed] [Google Scholar]
- 2.Swenson DM, Collins CL, Fields SK, Comstock RD. Epidemiology of US high school sports-related ligamentous ankle injuries, 2005/06–2010/11. Clin J Sport Med. 2013;23(3):190–196. doi: 10.1097/JSM.0b013e31827d21fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doherty C, Delahunt E, Caulfield B, Hertel J, Ryan J, Bleakley C. The incidence and prevalence of ankle sprain injury: a systematic review and meta-analysis of prospective epidemiological studies. Sports Med. 2014;44(1):123–140. doi: 10.1007/s40279-013-0102-5. [DOI] [PubMed] [Google Scholar]
- 4.Fong DT, Hong Y, Chan LK, Yung PS, Chan KM. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37(1):73–94. doi: 10.2165/00007256-200737010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Konradsen L, Voigt M, Højsgaard C. Ankle inversion injuries: the role of the dynamic defense mechanism. Am J Sports Med. 1997;25(1):54–58. doi: 10.1177/036354659702500110. [DOI] [PubMed] [Google Scholar]
- 6.Lephart SM, Pincivero DM, Giraldo JL, Fu FH. The role of proprioception in the management and rehabilitation of athletic injuries. Am J Sports Med. 1997;25(1):130–137. doi: 10.1177/036354659702500126. [DOI] [PubMed] [Google Scholar]
- 7.Michelson JD, Hutchins C. Mechanoreceptors in human ankle ligaments. J Bone Joint Surg Br. 1995;77(2):219–224. [PubMed] [Google Scholar]
- 8.Cordova ML, Ingersoll CD. Peroneus longus stretch reflex amplitude increases after ankle brace application. Br J Sports Med. 2003;37(3):258–262. doi: 10.1136/bjsm.37.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmieri-Smith RM, Hopkins JT, Brown TN. Peroneal activation deficits in persons with functional ankle instability. Am J Sports Med. 2009;37(5):982–988. doi: 10.1177/0363546508330147. [DOI] [PubMed] [Google Scholar]
- 10.Denyer JR, Hewitt NL, Mitchell AC. Foot structure and muscle reaction time to a simulated ankle sprain. J Athl Train. 2013;48(3):326–330. doi: 10.4085/1062-6050-48.2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell A, Dyson R, Hale T, Abraham C. Biomechanics of ankle instability: part 1. Reaction time to simulated ankle sprain. Med Sci Sports Exerc. 2008;40(8):1515–1521. doi: 10.1249/mss.0b013e31817356b6. [DOI] [PubMed] [Google Scholar]
- 12.Vaes P, Duquet W, Van Gheluwe B. Peroneal reaction times and eversion motor response in healthy and unstable ankles. J Athl Train. 2002;37(4):475–480. [PMC free article] [PubMed] [Google Scholar]
- 13.Berg CL, Hart JM, Palmieri-Smith R, Cross KM, Ingersoll CD. Cryotherapy does not affect peroneal reaction following sudden inversion. J Sport Rehabil. 2007;16(4):285–294. doi: 10.1123/jsr.16.4.285. [DOI] [PubMed] [Google Scholar]
- 14.Konradsen L, Ravn JB. Prolonged peroneal reaction time in ankle instability. Int J Sports Med. 1991;12(3):290–292. doi: 10.1055/s-2007-1024683. [DOI] [PubMed] [Google Scholar]
- 15.Löfvenberg R, Kärrholm J, Sundelin G, Ahlgren O. Prolonged reaction time in patients with chronic lateral instability of the ankle. Am J Sports Med. 1995;23(4):414–417. doi: 10.1177/036354659502300407. [DOI] [PubMed] [Google Scholar]
- 16.Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251–261. doi: 10.1177/0363546503260757. [DOI] [PubMed] [Google Scholar]
- 17.Knight KL. Cryotherapy in Sport Injury Management. Champaign, IL: Human Kinetics;; 1995. pp. 217–231. [Google Scholar]
- 18.Knight KL, Brucker JB, Stoneman PD, Rubley MD. Muscle injury management with cryotherapy. Athl Ther Today. 2000;5(4):26–30. [Google Scholar]
- 19.MacAuley DC. Ice therapy: how good is the evidence? Int J Sports Med. 2001;22(5):379–384. doi: 10.1055/s-2001-15656. [DOI] [PubMed] [Google Scholar]
- 20.Algafly AA, George KP. The effect of cryotherapy on nerve conduction velocity, pain threshold, and pain tolerance. Br J Sports Med. 2007;41(6):365–369. doi: 10.1136/bjsm.2006.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bleakley CM, Hopkins JT. Is it possible to achieve optimal levels of tissue cooling in cryotherapy? Phys Ther Rev. 2010;15(4):344–350. [Google Scholar]
- 22.Saeki Y. Effect of local application of cold or heat for relief of pricking pain. Nurs Health Sci. 2002;4(3):97–105. doi: 10.1046/j.1442-2018.2002.00112.x. [DOI] [PubMed] [Google Scholar]
- 23.De Jong RH, Hershey WN, Wagman IH. Nerve conduction velocity during hypothermia in man. Anesthesiology. 1966;27(6):805–810. doi: 10.1097/00000542-196611000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Kunesch E, Schmidt R, Nordin M, Wallin U, Hagbarth KE. Peripheral neural correlates of cutaneous anaesthesia induced by skin cooling in man. Acta Physiol Scand. 1987;129(2):247–257. doi: 10.1111/j.1748-1716.1987.tb08065.x. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins JT, Hunter I, McLoda T. Effects of ankle joint cooling on peroneal short latency response. J Sports Sci Med. 2006;5(2):333–339. [PMC free article] [PubMed] [Google Scholar]
- 26.Hayden CA. Cryokinetics in an early treatment program. Phys Ther. 1964;44:990–993. doi: 10.1093/ptj/44.11.990. [DOI] [PubMed] [Google Scholar]
- 27.Pincivero DM, Gieck JH, Saliba EN. Rehabilitation of a lateral ankle sprain with cryokinetics and functional progressive exercise. J Sport Rehabil. 1993;2(3):200–207. [Google Scholar]
- 28.Bleakley CM, Costello JT, Glasgow PD. Should athletes return to sport after applying ice? A systematic review of the effect of local cooling on functional performance. Sports Med. 2012;42(1):69–87. doi: 10.2165/11595970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Costello JT, Donnelly AE. Cryotherapy and joint position sense in healthy participants: a systematic review. J Athl Train. 2010;45(3):306–316. doi: 10.4085/1062-6050-45.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cordova ML, Bernard LW, Au KK, Demchak TJ, Stone MB, Sefton JM. Cryotherapy and ankle bracing effects on peroneus longus response during sudden inversion. J Electromyogr Kinesiol. 2010;20(2):348–353. doi: 10.1016/j.jelekin.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Kernozek TW, Greany JF, Anderson DR, et al. The effect of immersion cryotherapy on medial-lateral postural sway variability in individuals with a lateral ankle sprain. Physiother Res Int. 2008;13(2):107–118. doi: 10.1002/pri.393. [DOI] [PubMed] [Google Scholar]
- 32.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(5):361–374. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 33.Yang JF, Winter DA. Electromyographic amplitude normalization methods: improving their sensitivity as diagnostic tools in gait analysis. Arch Phys Med Rehabil. 1984;65(9):517–521. [PubMed] [Google Scholar]
- 34.Pallant J. SPSS Survival Manual: A Step By Step Guide to Data Analysis Using SPSS. 4th ed. Berkshire, UK: Open University Press;; 2010. p. 210. [Google Scholar]
- 35.Bleakley CM, Glasgow PD, Phillips P, et al. Management of Acute Soft Tissue Injury Using Protection Rest Ice Compression and Elevation: Recommendations From the Association of Chartered Physiotherapists in Sports and Exercise Medicine. London, UK: Association of Chartered Physiotherapists in Sports and Exercise Medicine;; 2011. [Google Scholar]
- 36.Bugaj R. The cooling, analgesic, and rewarming effects of ice massage on localized skin. Phys Ther. 1975;55(1):11–19. doi: 10.1093/ptj/55.1.11. [DOI] [PubMed] [Google Scholar]
- 37.McMeeken J, Lewis MM, Cocks S. Effects of cooling with simulated ice on skin temperature and nerve conduction velocity. Aust J Physiother. 1984;30(4):111–114. doi: 10.1016/S0004-9514(14)60682-6. [DOI] [PubMed] [Google Scholar]
- 38.Mok KM, Fong DT, Krosshaug T, et al. Kinematics analysis of ankle inversion ligamentous sprain injuries in sports: 2 cases during the 2008 Beijing Olympics. Am J Sports Med. 2011;39(7):1548–1552. doi: 10.1177/0363546511399384. [DOI] [PubMed] [Google Scholar]
- 39.Fong DT, Hong Y, Shima Y, Krosshaug T, Yung PS, Chan KM. Biomechanics of supination ankle sprain: a case report of an accidental injury event in the laboratory. Am J Sports Med. 2009;37(4):822–827. doi: 10.1177/0363546508328102. [DOI] [PubMed] [Google Scholar]
- 40.Ferran NA, Oliva F, Maffulli N. Ankle instability. Sports Med Arthrosc. 2009;17(2):139–145. doi: 10.1097/JSA.0b013e3181a3d790. [DOI] [PubMed] [Google Scholar]
- 41.Colville MR, Marder RA, Boyle JJ, Zarins B. Strain measurement in lateral ankle ligaments. Am J Sports Med. 1990;18(2):196–200. doi: 10.1177/036354659001800214. [DOI] [PubMed] [Google Scholar]
- 42.Hertel J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. 2002;37(4):364–375. [PMC free article] [PubMed] [Google Scholar]
- 43.Nigg BM, Skarvan G, Frank CB, Yeadon MR. Elongation and forces of ankle ligaments in a physiological range of motion. Foot Ankle. 1990;11(1):30–40. doi: 10.1177/107110079001100107. [DOI] [PubMed] [Google Scholar]
- 44.Renström P, Wertz M, Incavo S, et al. Strain in the lateral ligaments of the ankle. Foot Ankle. 1988;9(2):59–63. doi: 10.1177/107110078800900201. [DOI] [PubMed] [Google Scholar]
- 45.LaRiviere J, Osternig LR. The effect of ice immersion on joint position sense. J Sport Rehabil. 1994;3(1):58–67. [Google Scholar]
- 46.Ozmun JC, Thieme HA, Ingersoll CD, Knight KL. Cooling does not affect knee proprioception. J Athl Train. 1996;31(1):8–11. [PMC free article] [PubMed] [Google Scholar]
- 47.Cross KM, Wilson RW, Perrin DH. Functional performance following an ice immersion to the lower extremity. J Athl Train. 1996;31(2):113–116. [PMC free article] [PubMed] [Google Scholar]
- 48.Kinzey SJ, Cordova ML, Gallen KJ, Smith JC, Moore JB. The effects of cryotherapy on ground-reaction forces produced during a functional task. J Sport Rehabil. 2000;9(1):3–14. [Google Scholar]
- 49.Patterson SM, Udermann BE, Doberstein ST, Reineke DM. The effects of cold whirlpool on power, speed, agility, and range of motion. J Sports Sci Med. 2008;7(3):387–394. [PMC free article] [PubMed] [Google Scholar]
- 50.Atnip BL, McCrory JL. The effect of cryotherapy on three dimensional ankle kinematics during a sidestep cutting maneuver. J Sports Sci Med. 2004;3(2):83–90. [PMC free article] [PubMed] [Google Scholar]
- 51.Hart JM, Leonard JL, Ingersoll CD. Single-leg landing strategy after knee-joint cryotherapy. J Sport Rehabil. 2005;14(4):313–320. [Google Scholar]
- 52.Jameson AG, Kinzey SJ, Hallam JS. Lower extremity joint cryotherapy does not affect vertical ground-reaction forces during landing. J Sport Rehabil. 2001;10(2):132–142. [Google Scholar]
- 53.Hertel J, Buckley WE, Denegar CR. Serial testing of postural control after acute lateral ankle sprain. J Athl Train. 2001;36(4):363–368. [PMC free article] [PubMed] [Google Scholar]
- 54.Dykstra JH, Hill HM, Miller MG, Cheatham CC, Michael TJ, Baker RJ. Comparisons of cubed ice, crushed ice, and wetted ice on intramuscular and surface temperature changes. J Athl Train. 2009;44(2):136–141. doi: 10.4085/1062-6050-44.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Myrer WJ, Myrer KA, Measom GJ, Fellingham GW, Evers SL. Muscle temperature is affected by overlying adipose when cryotherapy is administered. J Athl Train. 2001;36(1):32–36. [PMC free article] [PubMed] [Google Scholar]