Abstract
Background
Iodine intake is related to thyroid disease. This study investigated the effect of the amount of iodine intake on p14ARF and p16INK4a expression of thyroid papillary carcinoma in rats.
Material/Methods
A cohort of 240 SD rats were randomly divided into control group, low iodine, normal iodine, and high iodine groups (n=60 per group). We inoculated 2×105 papillary thyroid carcinoma (PTC) cells on the left side of the thyroid gland. After 6 and 12 weeks, serum thyroid hormone level and urine iodine level were measured in addition to morphological observations of tumor tissues. Expression of p14ARF, p16INK4a was detected by immunohistochemical staining.
Results
The expression of p14ARF, p16INK4a, FT3, and FT4 levels in all iodine-treated animals were significantly lower than in the control group, while TSH level was significantly higher (P<0.05). Compared to the normal iodine group, the low and high groups had lower p14ARF and p16INK4a expression, lower FT3 and FT4 levels, higher TSH levels, and heavier tumors (P<0.05). In a further between-group comparison, p14ARF and p16INK4a expression and FT3 and FT4 levels at 12 weeks were lower than at 6 weeks. Expression of p14ARF and p16INK4a were positively correlated with FT3 and FT4, and negatively correlated with TSH and tumor weight.
Conclusions
Low and high iodine diet intake could reduce p14ARF and p16INK4a expressions and promote tumor development.
Keywords: Genes, p16; Iodine; Thyroiditis, Suppurative; Tumor Suppressor Protein p14ARF
Background
Thyroid cancer is a clinically common malignant tumor, and mainly includes anaplastic carcinoma, medullary carcinoma, follicular carcinoma, and papillary carcinoma. Papillary thyroid carcinoma (PTC) accounts for about 80% of all thyroid cancers [1]. Thyroid cancer incidence significantly increased in 2010 in the USA, with the PTC incidence increasing by about 2.3 times [2]. PTC occurrence is believed to be related to abnormal cell cycle regulation [3]. p14ARF and p16INK4a proteins, both of which are cell cycle regulation factors encoded by tumor suppressor gene INK4a-ARF, are abnormally expressed in various malignant tumors. Excess iodine intake can lead to diseases such as hyperthyroidism, while deficiency results in diseases such as endemic goiter. Epidemiological studies [4] found that PTC incidence after long-term high iodine intake was significantly higher compared to people with normal iodine intake, suggesting that long-term high iodine intake may increase PTC risk [5]. This study therefore analyzed the effect of iodine intake on in situ p14ARF and p16 expression in PTC nude rats, aiming to provide a basis for PTC prevention and iodine intake level control.
Material and Methods
Experimental animals
We obtained 240 male SD rats (aging between 10 and 12 weeks, averaged body weight=90 g) from the animal center of Kunming Medical University. All procedures were pre-approved by the Animal Ethics Committee of the home institute. The experiment started after 1 week of adaptive feeding.
All animals were randomly divided into the control group (n=60 each), the low iodine group (LI), the normal iodine group (NI), and the high iodine group (HI). Rats in each group were then randomly assigned for sacrifice at 6 weeks or 12 weeks, with 30 rats in each subgroup.
The PTC model rat was established by inoculating 2×105 PTC cells on the left side of the thyroid gland in nude rats. After inoculating, rats were given iodine-free diet and water containing KIO3. Rats in group LI, NI, HI, and control received iodine water at 30 μg/L, 300 μg/L, 3000 μg/L, and 300 μg/L, respectively. Both diet and KIO3–containing water were provided by the Fater Feed Co., LTD (Hebei, China).
Urine iodine level detection
The rats were re-housed in metabolic cages after feeding for 6 or 12 weeks. We collected and saved 24-h total urine. Acid digestion followed by arsenic-cerium catalytic spectrophotometry was used to detect the urine iodine level.
Thyroid hormone assay
We collected 5 ml of abdominal venous blood after sacrificing the rats. Automatic electrochemical luminescence instrument was used to detect the free triiodothyronine (FT3), free thyroxine (FT4), and thyroid-stimulating hormone (TSH) levels.
Immunohistochemistry
Thyroid samples were fixed in 10% formaldehyde, embedded in paraffin, and sliced at 4 μm. Immunohistochemical staining was performed to detect expression of p14ARF and p16INK4a using a staining kit and chromogenic reagents (GIBCO, USA), along with primary antibody against p14ARF and p16INK4a (Baygene Biotech, China). Five randomly selected fields from each section were counted for positive-staining cells. A field with over 5% positive staining cells was considered as positive, while less than 5% was considered negative.
Statistical analysis
All statistical analyses were performed using SPSS20.0 software (Chicago, IL). Measurement data are presented as mean ± standard deviation (x±s). Differences between groups or time course were analyzed using the chi-square test. Bonferroni method was used for pairwise comparison and Pearson analysis was used for correlation analysis, with P<0.05 considered to be statistically significant.
Results
Thyroid tissue morphology
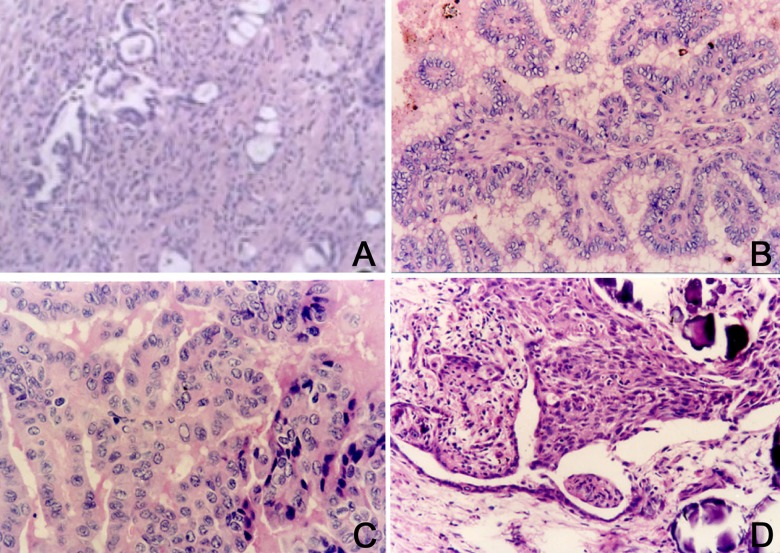
Thyroid follicles in the control animals appeared in a normal lobular pattern. As shown in Figure 1A, mature follicles with colloid distribution were uniformly surrounded by a small amount of blood vessels and fibers. Thyroid follicles in the LI group were smaller, with unevenly distributed colloid and invasive growth pattern surrounded by obvious blood vessels. Granular structure with unclear boundary and gravel formation could also be found (Figure 1B). Thyroid follicles in group NI had unevenly distributed colloid surrounded by obvious fibers and blood vessels without invasive growth but with gravel formation (Figure 1C). Thyroid follicles in group HI showed enlargement and were full of powder-like colloid and apparent squamous metaplasia with calcification. The thyroid follicles were surrounded by blood vessels and presented as diffuse development. Blood vessels axis could be found with an unclear boundary (Figure 1D).
Figure 1.
Thyroid tissue morphology observation. (A) Normal thyroid tissue, SP×200; (B) Thyroid tissue in group LI, SP×100; (C) Thyroid tissue in group NI, SP×100; (D) Thyroid tissue in group HI, SP×200.
p14ARF and p16INK4a expression
p14ARF and p16INK4a showed weak positive expression after 12 weeks. p14ARF positive expression was presented as brown granules distributed in nucleus, with less expression in cytoplasm. p16INK4a protein expression was concentrated in cancer cell cytoplasm as brown granules with less in the nucleus (Figure 2).
Figure 2.
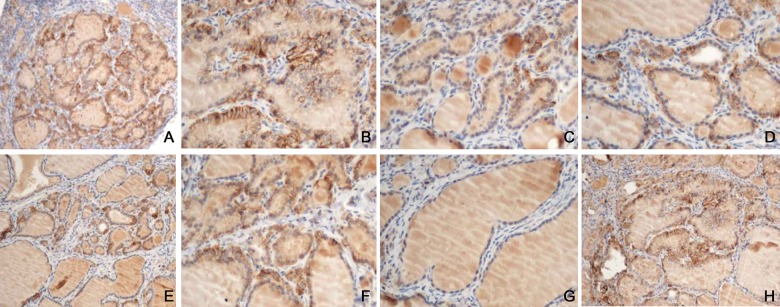
p14ARF and p16INK4a expression. (A) p14ARF expression in the control, SP×100; (B) p16INK4a expression in the control, SP×100; (C) p14ARF expression in group LI, SP×100; (D) p16INK4a expression in group LI, SP×100; (E) p14ARF expression in group NI, SP×100; (F) p16INK4a expression in group NI, SP×100; (G) p14ARF expression in group HI, SP×100; (H) p16INK4a expression in group HI, SP×100.
Among different groups, the positive rates of p14ARF and p16INK4a expression in group LI, NI, and HI were significantly lower than those in the control group (P<0.05). The expression level was lower in the LI and HI groups compared to the NI group (P<0.05). Within each group, rats in LI and HI at 6 weeks presented lower positive rate than those at 12 weeks, while no significant difference existed between rats at 6 weeks and 12 weeks in the NI group (P>0.05, Table 1).
Table 1.
p14ARF and p16INK4a expression positive rate comparison [n(%)].
| Group | Cases | p14ARF | p16INK4a | ||
|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||
| Group LI | |||||
| 6 weeks | 30 | 11 (36.67)* | 19 (63.33)* | 13 (43.33)* | 17 (56.67)* |
| 12 weeks | 30 | 3 (10.00)*,**,# | 27 (90.00)*,**,# | 5 (16.67)*,**,# | 25 (83.33)*,**,# |
| Group NI | |||||
| 6 weeks | 30 | 14 (46.67)* | 16 (53.33)* | 16 (53.33)* | 14 (46.67)* |
| 12 weeks | 30 | 10 (33.33)* | 20 (66.67)* | 13 (43.33)* | 17 (56.67)* |
| Group HI | |||||
| 6 weeks | 30 | 10 (33.33)* | 20 (66.67)* | 12 (40.00)* | 18 (60.00)* |
| 12 weeks | 30 | 2 (6.67)*,**,# | 28 (93.33)*,**,# | 3 (10.00)*,**,# | 27 (90.00)*,**,# |
| Control | |||||
| 6 weeks | 30 | 24 (80.00) | 6 (20.00) | 27 (90.00) | 3 (10.00) |
| 12 weeks | 30 | 26 (86.67) | 4 (13.33) | 28 (93.33) | 2 (6.67) |
Compared with control, P<0.05;
compared with group NI, P<0.05;
compared with 6 weeks, P<0.05.
Serum thyroid hormone levels and urine iodine level
Among different groups, FT3 and FT4 levels in the iodine-feeding group were significantly lower than in the control group, while TSH level was higher (P<0.05). Further analysis showed that TSH level was lower in the LI and HI groups compared to the NI group, while FT3 and FT4 levels exhibited the opposite pattern (P<0.05). LI group showed higher TSH level and lower FT3 and FT4 levels than the HI group (P<0.05). Within each group, rats in the LI and HI groups at 12 weeks presented lower levels of FT3 and FT4 and higher TSH levels than those at 6 weeks. No significant difference existed between rats at 6 weeks and 12 weeks in the NI group (P>0.05, Table 2).
Table 2.
Serum thyroid hormone levels and urine iodine level comparison (χ̄±s).
| Group | Cases | FT3 (ng/ml) | FT4 (ng/ml) | TSH (uIU/ml) | Urine iodine (μg/L) |
|---|---|---|---|---|---|
| Group LI | |||||
| 6 weeks | 30 | 2.41±0.54*,** | 0.83±0.29*,** | 3.59±1.01*,** | 33.14±1.26*,** |
| 12 weeks | 30 | 2.29±0.49*,**,# | 0.74±0.21*,**,# | 3.71±1.13*,**,# | 34.51±1.39*,** |
| Group NI | |||||
| 6 weeks | 30 | 2.81±0.78* | 1.01±0.36* | 3.04±0.97* | 85.17±4.22 |
| 12 weeks | 30 | 2.79±0.74* | 0.99±0.34* | 3.06±0.92* | 84.46±4.53 |
| Group HI | |||||
| 6 weeks | 30 | 2.19±0.36*,**,# | 0.69±0.21*,**,# | 3.81±0.94*,**,# | 2470.02±106.12*,** |
| 12 weeks | 30 | 2.05±0.27*,**,# | 0.62±0.16*,**,# | 3.92±1.03*,**,# | 2490.26±112.07*,** |
| Control | |||||
| 6 weeks | 30 | 3.46±0.82 | 1.33±0.17 | 2.05±0.57 | 84.21±4.63 |
| 12 weeks | 30 | 3.49±0.87 | 1.32±0.15 | 2.07±0.48 | 85.22±4.57 |
Compared with control, P<M0.05;
compared with group NI, P<0.05;
compared with 6 weeks, P<0.05.
Thyroid tumor weight
Thyroid tumor weight at 6 weeks showed no significant differences among different groups (P>0.05). The weight at 12 weeks was higher than at 6 weeks in the same group (P<0.05). Tumor weight was heavier in the LI and HI groups compared to the NI group (P<0.05). Differences in tumor weights between groups LI and HI were not significant (P>0.05, Table 3).
Table 3.
Thyroid tumor weight changes comparison (χ̄±s, mg).
| Group | Cases | Thyroid tumor weight |
|---|---|---|
| Group LI | ||
| 6 weeks | 30 | 2.01±0.24 |
| 12 weeks | 30 | 4.19±0.52*,** |
| Group NI | ||
| 6 weeks | 30 | 1.97±0.21 |
| 12 weeks | 30 | 2.32±0.34* |
| Group HI | ||
| 6 weeks | 30 | 2.03±0.35 |
| 12 weeks | 30 | 4.27±0.49*,** |
Compared with 6 weeks, P<0.05;
compared with group NI, P<0.05.
Correlation analysis
Rates of positive p14ARF and p16INK4a expression in the model rats were positively correlated with FT3 (r=0.651, P<0.05) and FT4 (r=0.584, P<0.05), and negatively correlated with TSH (r=0.592, P<0.05) and tumor weight (r=0.571, P<0.05).
Discussion
Iodine is indispensable for the bio-synthesis of thyroid hormone, which is necessary for normal physiological functions of various organs and tissues. Thyroid hormone can promote human growth, and has important function on the reproductive system, skeletal muscle, and nervous system. Epidemiological surveys [6,7] suggested that the global incidence of thyroid cancer increased in recent decades. Thyroid cancer may be related to mental, psychological, environmental, and genetic factors. Recent research [8] revealed that the occurrence of thyroid carcinoma was also closely related to the iodine level in diet or water – deficient or excessive iodine intake may lead to thyroid carcinoma. Some investigators found lower urinary iodine level in thyroid cancer patients compared to patients with benign thyroid disease or healthy people [9]. Ergin et al. [10], however, showed that thyroid cancer patients had higher urinary iodine level than patients with benign thyroid disease or healthy people. Harch et al. [11] studied residents of Iceland, who had higher daily iodine intake than other regions, and found higher incidence of thyroid cancer. This information collectively suggests that iodine intake level is closely related with the occurrence and development of thyroid carcinoma. In the present study, thyroid tumor weight at 12 weeks was higher than that at 6 weeks, and was heavier in the LI and HI groups than in the NI group, indicating that deficient or excessive iodine intake may promote PCT development in rats. However, the clinical relationship between iodine intake and PCT incidence still requires epidemiological data to confirm [12].
Located on the short arm of chromosome 9, Ink4a-arf gene loci contain 2 promoters directing 2 transcripts – p14ARF and p16INK4a. Both of those proteins can negatively regulate cell cycle through different pathways. p14ARF may combine with MDM2 to facilitate induced apoptosis and regulate the function of p53 protein in the cell cycle [13]. p16INK4a, as a cell cycle-related protein kinase inhibitor, can competitively bind with protein-dependent kinase to inhibit its activity, thereby preventing cell cycle into infinite growth, division, and amplification, and preventing cells in G1 phase from entering S phase. p14ARF and p16INK4a gene inactivation, mutation, or absence may reduce tumor cell proliferation inhibition ability, resulting in continuous malignant tumor cell growth. Multiple clinical studies [14] have shown the low expression of p14ARF and p16INK4a in pancreatic gastric, lung, esophageal, breast cancer, and melanoma. Olson et al. [15] found that p14ARF and p16INK4a genes were positively expressed in healthy people, and were positively expressed in 80% of those with benign uterine disease, while all of the 10 endometrial carcinoma patients exhibited negative expression, suggesting that p14ARF and p16INK4a genes were down-regulated in endometrial carcinoma. Our study observed weak p14ARF and p16INK4a gene expression, as well as serum thyroid hormone and urine iodine levels in PCT model rats with different amounts of iodine intake. The rates of positive p14ARF and p16INK4a expression in iodine-treated rats were significantly lower than in controls, indicating the low expression level of p14ARF and p16INK4a in PCT rats. The gene expression level was lower in groups LI and HI than in group NI, and optimal iodine intake increased the p14ARF and p16INK4a expression. These results collectively indicate that the iodine intake level is closely related to p14ARF and p16INK4a gene expression in PCT rats.
In the thyroid system, TSH has a direct effect on thyroid function. TSH stimulation can significantly enhance the activity of the thyroid follicular epithelial cells, leading to gradual activation of the hyperplasia. Clinical studies [16] showed that some of the thyroid follicular epithelial cells retained hormone receptors even with thyroid malignant tumor. Meantime, Yamada et al. [17] found that TSH levels in PCT patients were significantly higher than in healthy people and in patients with benign thyroid disease. TSH is a potential thyroid cancer cell growth factor, as high level of TSH may increase thyroid cancer recurrence. In this study, TSH level in all iodine intake rats were significantly higher than those of the controls; TSH level was lower in the LI and HI groups than in the NI group, indicating that different iodine intake levels have remarkable effect on TSH level in PCT rats, and optimal iodine intake can help to lower the TSH level. We also found that the HI group had a significantly higher TSH level than the LI group. The results suggest that excessive iodine intake may elevate TSH level in PCT rats. This is because excessive iodine intake may block the organic process of iodine, and inhibit the hydrolysis of thyroglobulin, thus increasing TSH level as a feedback [18]. Thyroxine mainly consists of 2 key hormones – triiodothyronine and thyroxine – which exist in both free and combined form. FT3 and FT4 levels detection can accurately reflect hormone levels and body thyroid functions [19]. Zerrouqi et al. [20] found that PCT patients presented significantly lower levels of serum FT3 and FT4. Our research revealed that FT3 and FT4 levels in iodine-treated rats were significantly lower than in the controls, and they were lower in the LI and HI groups compared to the NI group, indicating that iodine intake has obvious impact on FT3 and FT4 levels in PCT rats. Our study further found that LI rats exhibited lower FT3 and FT4 levels than in the HI group, while rats in the LI and HI groups at 12 weeks presented lower FT3 and FT4 levels compared to those at 6 weeks. This suggests that long-term high iodine intake inhibits the synthesis and secretion of FT3 and FT4, resulting in hypothyroidism. Simultaneously, extreme insufficient iodine intake may cause thyroid hormone hydrolysis, which decreases FT3 and FT4 levels.
Conclusions
Low or high iodine intake can reduce expression of p14ARF and p16INK4a, promote PCT development, and have obvious effects on thyroid function. The expression of p14ARF and p16INK4a was positively correlated with FT3 and FT4, and negatively correlated with TSH and tumor weight.
Footnotes
Source of support: Departmental sources
References
- 1.Ebina A, Sugitani I, Fujimoto Y, Yamada K. Risk-adapted management of papillary thyroid carcinoma according to our own risk group classification system: Is thyroid lobectomy the treatment of choice for low-risk patients? Surgery. 2014;156:1579–89. doi: 10.1016/j.surg.2014.08.060. [DOI] [PubMed] [Google Scholar]
- 2.Stanojevic B, Saenko V, Todorovic L, et al. Low VHL mRNA expression is associated with more aggressive tumor features of papillary thyroid carcinoma. Plos One. 2014;9:e114511. doi: 10.1371/journal.pone.0114511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Overhoff MG, Garbe JC, Koh J, et al. Cellular senescence mediated by p16INK4A-coupled miRNA pathways. Nucleic Acids Res. 2014;42:1606–18. doi: 10.1093/nar/gkt1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JY, Kim EK, Lee HS, Kwak JY. Conventional papillary thyroid carcinoma: effects of cystic changes visible on ultrasonography on disease prognosis. Ultrasonography. 2014;33:291–97. doi: 10.14366/usg.14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Khalaf HH, Mohideen P, Nallar SC, et al. The cyclin-dependent kinase inhibitor p16INK4a physically interacts with transcription factor Sp1 and cyclin-dependent kinase 4 to transactivate microRNA-141 and microRNA-146b-5p spontaneously and in response to ultraviolet light-induced DNA damage. J Biol Chem. 2013;288:35511–25. doi: 10.1074/jbc.M113.512640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moritani S. Impact of invasive extranodal extension on the prognosis of patients with papillary thyroid carcinoma. Thyroid. 2014;24:1779–83. doi: 10.1089/thy.2014.0167. [DOI] [PubMed] [Google Scholar]
- 7.McFadden DG, Dias-Santagata D, Sadow PM, et al. Identification of oncogenic mutations and gene fusions in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2014;99:E2457–62. doi: 10.1210/jc.2014-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaPak KM, Burd CE. The molecular balancing act of p16(INK4a) in cancer and aging. Mol Cancer Res. 2014;12:167–83. doi: 10.1158/1541-7786.MCR-13-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J, Dong J, Sun Q, et al. Should radioactive iodine therapy be administrated in patient of papillary thyroid carcinoma? Chin Med J (Engl) 2014;127:3039–40. [PubMed] [Google Scholar]
- 10.Ergin AB, Saralaya S, Olansky L. Incidental papillary thyroid carcinoma: Clinical characteristics and prognostic factors among patients with Graves’ disease and euthyroid goiter, Cleveland Clinic experience. Am J Otolaryngol. 2014;35:784–90. doi: 10.1016/j.amjoto.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Ye WC, Gao L, Huang J, et al. Suppressed Kruppellike factor 17 expression induces tumor proliferation, metastasis and a poor prognosis in papillary thyroid carcinoma. Mol Med Rep. 2014;10:2087–92. doi: 10.3892/mmr.2014.2429. [DOI] [PubMed] [Google Scholar]
- 12.Wakasa T, Li Y, Bai Y, et al. Up-regulation of urinary-type plasminogen activator correlates with high-risk papillary thyroid carcinoma with BRAF(V600E) mutation and its possible molecular mechanism. Pathol Res Pract. 2014;210:733–38. doi: 10.1016/j.prp.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Peng Y, Li C, Luo DC, et al. Expression profile and clinical significance of microRNAs in papillary thyroid carcinoma. Molecules. 2014;19:11586–99. doi: 10.3390/molecules190811586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bian XH, Sun H, Xue H, et al. Expression and clinical significance of Shh/Gli-1 in papillary thyroid carcinoma. Tumour Biol. 2014;35:10523–28. doi: 10.1007/s13277-014-2365-3. [DOI] [PubMed] [Google Scholar]
- 15.Olson AC, Haugen BR, Walter J, et al. SPECT/CT and I131 therapy of brain metastases from follicular variant papillary thyroid carcinoma (FVPTC) J Clin Endocrinol Metab. 2014;99:3511–12. doi: 10.1210/jc.2014-1875. [DOI] [PubMed] [Google Scholar]
- 16.Bai Y, Xue W. [Correlation between histological characteristics, molecular expression and clinical outcomeof papillary thyroid carcinoma]. Zhonghua Bing Li Xue Za Zhi. 2014;43:341–43. [in Chinese] [PubMed] [Google Scholar]
- 17.Yamada O, Miyauchi A, Ito Y, et al. Changes in serum thyroglobulin antibody levels as a dynamic prognostic factor for early-phase recurrence of thyroglobulin antibody-positive papillary thyroid carcinoma after total thyroidectomy. Endocr J. 2014;61:961–65. doi: 10.1507/endocrj.ej14-0275. [DOI] [PubMed] [Google Scholar]
- 18.Renaud F, Gnemmi V, Devos P, et al. MUC1 expression in papillary thyroid carcinoma is associated with BRAF mutation and lymph node metastasis; the latter is the most important risk factor of relapse. Thyroid. 2014;24:1375–84. doi: 10.1089/thy.2013.0594. [DOI] [PubMed] [Google Scholar]
- 19.Veena MS, Wilken R, Zheng JY, et al. p16 Protein and gigaxonin are associated with the ubiquitination of NFkappaB in cisplatin-induced senescence of cancer cells. J Biol Chem. 2014;289:34921–37. doi: 10.1074/jbc.M114.568543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zerrouqi A, Pyrzynska B, Brat DJ, Van Meir EG. P14ARF suppresses tumor-induced thrombosis by regulating the tissue factor pathway. Cancer Res. 2014;74:1371–78. doi: 10.1158/0008-5472.CAN-13-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]