Commentary
Excitatory Effects of Parvalbumin-Expressing Interneurons Maintain Hippocampal Epileptiform Activity via Synchronous Afterdischarges.
Ellender TJ, Raimondo JV, Irkle A, Lamsa KP, Akerman CJ. J Neurosci 2014;34(46):15208–15222.
Epileptic seizures are characterized by periods of hypersynchronous, hyperexcitability within brain networks. Most seizures involve two stages: an initial tonic phase, followed by a longer clonic phase that is characterized by rhythmic bouts of synchronized network activity called afterdischarges (ADs). Here we investigate the cellular and network mechanisms underlying hippocampal ADs in an effort to understand how they maintain seizure activity. Using in vitro hippocampal slice models from rats and mice, we performed electrophysiological recordings from CA3 pyramidal neurons to monitor network activity and changes in GABAergic signaling during epileptiform activity. First, we show that the highest synchrony occurs during clonic ADs, consistent with the idea that specific circuit dynamics underlie this phase of the epileptiform activity. We then show that ADs require intact GABAergic synaptic transmission, which becomes excitatory as a result of a transient collapse in the chloride (Cl−) reversal potential. The depolarizing effects of GABA are strongest at the soma of pyramidal neurons, which implicates somatic-targeting interneurons in AD activity. To test this, we used optogenetic techniques to selectively control the activity of somatic-targeting parvalbumin-expressing (PV+) interneurons. Channelrhodopsin-2-mediated activation of PV+ interneurons during the clonic phase generated excitatory GABAergic responses in pyramidal neurons, which were sufficient to elicit and entrain synchronous AD activity across the network. Finally, archaerhodopsin-mediated selective silencing of PV+ interneurons reduced the occurrence of ADs during the clonic phase. Therefore, we propose that activity-dependent Cl− accumulation subverts the actions of PV+ interneurons to perpetuate rather than terminate pathological network hyperexcitability during the clonic phase of seizures.
Epilepsy is a network disorder that requires specificity in the manipulation of these networks for optimal treatment. Current pharmacologic approaches lack specificity, affecting virtually all cells expressing their target of action, often resulting in unwanted and even offsetting effects, which can impact unaffected networks. The development of optogenetics, which offers spatial, temporal, and cell-type specific control, has generated enthusiasm for the use of this technology in the treatment of the epilepsies (1).
Optogenetics refers to the approach of combining genetics with optical technology for the ability to specifically control target cells. Different opsins have been developed to specifically activate (e.g., channelrhodopsin-2 [ChR2]) or inhibit (e.g., halorhodopsin [NpHR] and archaerhodopsin [Arch]) cells. These opsins can be genetically targeted to specific subsets of neurons, such as interneuron subtypes, allowing control over their activity with light. Thus, optogenetics represents a useful tool to investigate the role of specific populations of neurons in the generation and progression of seizure activity. However, basic science investigations into the antiseizure potential of optogenetics have raised some issues to consider regarding the translational potential of optogenetics.
Employing optogenetics to silence excitatory neurons is effective at suppressing seizures (1). However, attempts to suppress seizures via activation of interneurons have led to interesting observations regarding the role of interneurons in seizure progression, which is the focus of this commentary. Here, we highlight a recent study by Ellender et al. demonstrating a role for parvalbumin (PV)-positive interneurons in contributing to the generation of a specific type of epileptiform activity, specifically rhythmic bouts of synchronized network activity termed afterdischarges. These findings are supported by more recent reports demonstrating that optogenetic activation of interneurons exacerbates epileptiform activity in vitro (Table 1) (2, 3).
TABLE 1.
Optogenetic Manipulation of Interneurons on Epileptiform Activity*
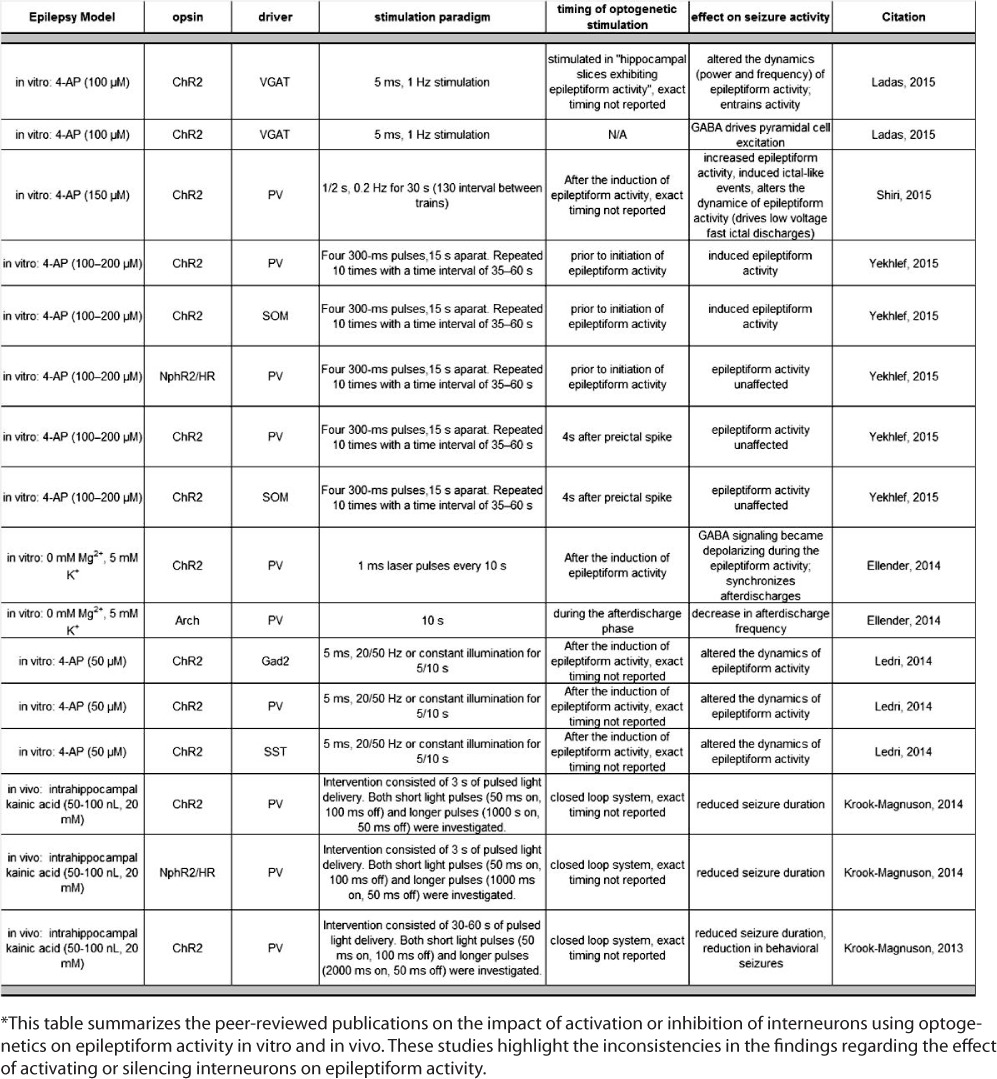
Ellender et al. demonstrated that excitatory actions of GABA during seizure progression, due to a collapse in the chloride gradient, drive afterdischarges. Optogenetic activation of PV-interneurons entrained and increased the duration of afterdischarges; whereas, silencing PV-interneurons reduced afterdischarges in an in vitro epilepsy model. The involvement of excitatory actions of GABA is supported by recent findings demonstrating that activation of GABAergic interneurons induces pyramidal cell excitation in the 4-aminopyridine (4-AP) model (4, 5). A single in vitro study claimed that optogenetic activation of interneurons suppressed epileptiform activity (5). Indeed, activation of interneurons suppressed ictal-like activity, but closer examination demonstrates that activation of interneurons may alter the nature of epileptiform activity, similar to other reports (Table 1) (2, 4), driving what appears to be lower frequency epileptiform activity (5). The majority of these studies utilized the 4-AP model of epilepsy. The highlighted study was critical in demonstrating that interneuron activation contributed to the generation of epileptiform activity in a different in vitro model (O-Mg2+), suggesting that these findings are not model specific. In patients with epilepsy, large-scale recordings from the neocortex demonstrate that fast-spiking interneurons fire throughout the tonic phase of a seizure then cease firing about halfway through a seizure event (6). The cessation of fast-spiking interneuron firing is associated with an increase in spike-and-wave activity, suggesting that the activity of interneurons may modulate the type of epileptiform activity, mediating the transition from tonic and clonic phases of seizure activity (6). Of interest, these data suggest that GABAergic inhibition may become ineffective or even detrimental throughout seizure progression, contributing to the generation of epileptiform activity and raising questions as to the therapeutic potential of optogenetically activating these neurons for seizure control.
In contrast to the highly reproducible findings reviewed above demonstrating a role of interneurons in driving epileptiform activity in vitro, the same does not appear to be the case in vivo. The two studies that have investigated the ability of optogenetic activation of interneurons to control seizure activity in vivo have demonstrated antiseizure effects (7, 8). Using a closed-loop system, optogenetic activation of PV-interneurons at the onset of seizure activity reduced the duration of both electrographic and behavioral seizures (7, 8). These data support an inhibitory role for interneurons throughout seizure progression and suggest that activation of interneurons may be beneficial for seizure control. Of interest, this group observed similar antiseizure effects by optogenetically inhibiting PV-interneurons (7), suggesting that disruption of the epileptic network, either by activating or inhibiting PV-interneurons, is sufficient to reduce seizure activity.
It is clear that the neuronal networks in vivo are more complex than those studied in vitro. However, there may be additional differences in the experimental design that contribute to conflicting observations regarding the impact of interneurons on epileptiform activity. It is possible that the timing of interneuron activation during seizure progression is essential to their role in seizure activity. For example, in the in vivo studies, interneurons were activated early in the onset of electrographic seizure activity, prior to the manifestation of behavioral seizures (8). In contrast, the in vitro studies largely focused on the activation of interneurons once epileptiform activity had already developed (Table 1). It remains to be determined whether the role of interneurons in modulating epileptiform activity differs at the onset of epileptiform activity versus during prolonged epileptiform activity. Prolonged epileptiform activity may be necessary to cause a collapse in the chloride gradient, resulting in excitatory actions of GABA, contributing to the progression of epileptiform activity. It is also possible that the pattern of stimulation may be important for promoting synchrony and entraining principal neurons to drive epileptiform activity. However, Krook-Magnuson et al. (7, 8) used different stimulation patterns that were both capable of reducing electrographic and behavioral seizures. Further, the location of interneuron activation in the epileptic network may be important. Temporally controlled focal seizure induction enables investigation into the role of timing and localization of interneuron activation on epileptiform activity (9, 10). Optogenetic activation of PV-interneurons within the epileptogenic focus prior to seizure induction exacerbated epileptiform activity (9, 10). Of interest, activation of interneurons within the epileptogenic focus rapidly induced and synchronized afterdischarges (11), similar to the observations in the currently highlighted study. However, activation of interneurons distal from the epileptogenic focus restricted seizure propagation (9). Krook-Magnuson et al. (7) demonstrated that activation of interneurons in the cerebellum is sufficient to reduce epileptiform activity in a temporal lobe model of epilepsy. These findings highlight that epilepsy is a network disorder and that interactions between brain regions must be considered.
Another important issue to consider is that the interneuron networks may be altered in some epilepsies. As mentioned above, GABAergic signaling may become dysfunctional during seizure progression. Alterations in the connectivity of interneurons have been demonstrated in experimental models of epilepsy (11), and stimulation of these aberrant inhibitory networks may have adverse consequences. However, there is evidence that optogenetic activation of grafted GABAergic interneurons enhances inhibition (12) and may be an effective strategy for seizure control.
These incongruent findings regarding the role of interneurons in seizure generation and progression demonstrate that it is imperative that we develop a better understanding of the role of interneurons in different brain regions in modulating seizure activity before attempting to manipulate them for seizure control.
Footnotes
Editor's Note: Authors have a Conflict of Interest disclosure which is posted under the Supplemental Materials (209.1KB, docx) link.
References
- 1.Paz JT, Huguenard JR. Optogenetics and epilepsy: Past, present and future. Epilepsy Curr. 2015;15:34–38. doi: 10.5698/1535-7597-15.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiri Z, Manseau F, Lévesque M, Williams S, Avoli M. Interneuron activity leads to initiation of low-voltage fast-onset seizures. Ann Neurol. 2015;77:541–546. doi: 10.1002/ana.24342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yekhlef L, Breschi GL, Lagostena L, Russo G, Taverna S. Selective activation of parvalbumin- or somatostatin-expressing interneurons triggers epileptic seizurelike activity in mouse medial entorhinal cortex. J Neurophysiol. 2015;113:1616–1630. doi: 10.1152/jn.00841.2014. [DOI] [PubMed] [Google Scholar]
- 4.Ladas TP, Chiang CC, Gonzalez-Reyes LE, Nowak T, Durand DM. Seizure reduction through interneuron-mediated entrainment using low frequency optical stimulation. Exp Neurol. 2015;269:120–132. doi: 10.1016/j.expneurol.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ledri M, Madsen MG, Nikitidou L, Kirik D, Kokaia M. Global optogenetic activation of inhibitory interneurons during epileptiform activity. J Neurosci. 2014;34:3364–3377. doi: 10.1523/JNEUROSCI.2734-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed O, Kramer M, Truccolo W, Naftulin JS, Potter NS, Eskandar EN, Cosgrove GR, Blum AS, Hochberg LR, Cash SS. Inhibitory single neuron control of seizures and epileptic traveling waves in humans. BMC Neurosci. 2014;15:1. doi:10.1186/1471-2202-15-S1-F3. [Google Scholar]
- 7.Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. Eneuro. 2014;1:e.2014. doi: 10.1523/ENEURO.0005-14.2014. doi:10.1523/ENEURO.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun. 2013;4:1376. doi: 10.1038/ncomms2376. doi:10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcon I, Sessolo M, Bovetti S, Losi G, Mariotti L, Fellin T, Carmignoto G. Activation of parvalbumin interneurons at the epileptogenic focus facilitates focal seizures generation in a mouse model of temporal lobe epilepsy. Title of Publication. 2014. In: Name XX, Name XX, eds. Proceedings of the Society for Neuroscience Meeting, Washington, DC, November 2014. Publisher City: Publisher Name. XX.
- 10.Sessolo M, Marcon I, Losi G, Mariotti L, Bovetti S, Berardi N, Fellin T, Carmignoto G. Optogenetic activation of parvalbumin interneurons synchronizes afterdischarges of seizures in a mouse model of temporal lobe epilepsy. Title of Publication. 2014. In: Name XX, Name XX, eds. Proceedings of the Society for Neuroscience Meeting, Washington, DC, November 2014. Publisher City: Publisher Name. Poster 220.10/D54.
- 11.Peng Z, Zhang N, Wei W, Huang CS, Cetina Y, Otis TS, Houser CR. A reorganized GABAergic circuit in a model of epilepsy: Evidence from optogenetic labeling and stimulation of somatostatin interneurons. J Neurosci. 2013;33:14392–14405. doi: 10.1523/JNEUROSCI.2045-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson KW, Gupta J, Tagliatela S, Litvina E, Zheng X, Van Zandt MA, Woods N, Grund E, Lin D, Royston S, Yanagawa Y, Aaron GB, Naegele JR. Long-term seizure suppression and optogenetic analyses of synaptic connectivity in epileptic mice with hippocampal grafts of GABAergic interneurons. J Neurosci. 2014;34:13492–13504. doi: 10.1523/JNEUROSCI.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


