1. The Demographic Imperative and Age-Associated Rick for Cardiovascular Disease
Cardiovascular aging is a promising frontier in preventive cardiology that is ripe for and in dire need of attention! Aging hearts and arteries operate “on the edge of cardiovascular disease”. Both the incidence and prevalence of hypertension, atherosclerosis, coronary and cerebral artery disease. increase sharply at about age 45 in men and a decade later in women [1]. The long-term sequellae of these diseases lead to an age-associated increase in the prevalence of congestive heart failure and stroke. The remaining lifetime risk for CVD and other diseases among men and women free of disease at 40 years of age is staggering (Fig. 1): The odds of having a chronic CV disease are 50%, for hypertension 85%, and for chronic heart failure 20% [2; cf. also 3 in this volume]. Moreover, at age 70, the lifetime risk of CVD in individuals free of disease is virtually the same as that at age 40, and is indicative of the extremely high likelihood for incurring CVD during one’s lifetime (Fig. 1).
Figure 1. The remaining lifetime risk for CVD and other diseases among men and women is staggering.
The odds of having a chronic CV disease are 50%, for hypertension 85%, and for chronic heart failure 20%. At age 70, the lifetime risk of CVD in individuals free of disease is virtually the same as that at age 40, and is indicative of the extremely high likelihood for incurring CVD during one’s lifetime. (Adapted from Ref. 2)
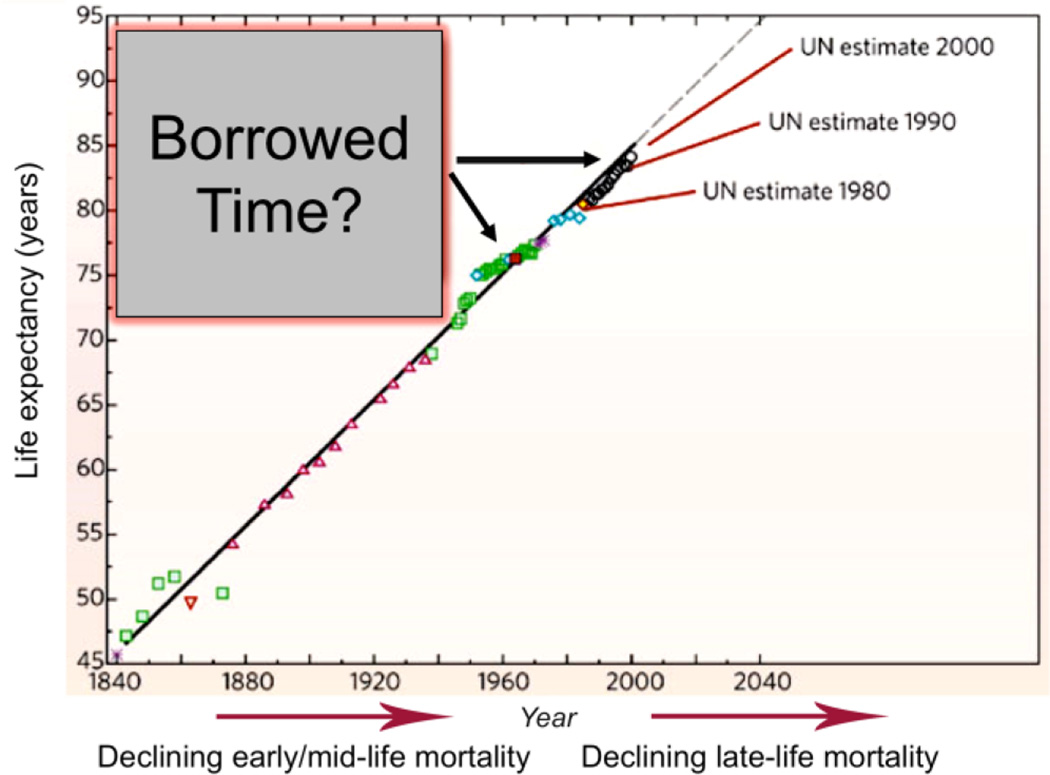
The epidemic of cardiovascular diseases has taken on a global dimension and is no longer restricted to Western societies. Cardiovascular diseases now represent more than 30% of all deaths worldwide, and by the year 2020, they are expected to surpass infectious diseases as the leading cause of mortality and disability. According to the World Health Report, 1 cardiovascular diseases were responsible for 15 million annual deaths worldwide, of which 9 million were in developing countries and 2 million in economies in transition [4]. This situation is expected to worsen as the world population is aging. In the United States, 35 million people are older than 65 years, and the number of older Americans is expected to double by the year 2030. The older population is also progressively becoming more predominant as life expectancy increases in many developing countries [5]. The clinical and economic implications of this demographic shift are staggering [5]. Adding years to the end of the lifespan (Fig. 2), when chronic age-associated diseases become rampant (Fig. 1), raises the issue that surviving to these older ages is living on “borrowed time” (Fig. 2). The data in Fig. 2 clearly raise the question of whether aging itself is a disease.
Figure 2. Life expectancy around the world has increased steadily for 200 years.
The older population is progressively becoming more predominant as life expectancy increases in many developing countries. Adding years to the end of the lifespan, when chronic age-associated diseases become rampant (Fig. 1), raises the issue that surviving to these older ages is living on “borrowed time” (adapted from Ref. 5).
2. The reality of cardiovascular aging
A view of the reality of CV aging begins with the realization of the extremely high lifetime risks of arterial diseases, e.g. atherosclerosis and hypertension, depicted in Fig. 1; cf. also 17 in this volume. Progressive changes in the structure and function of the heart and arteries occur throughout life and include diffuse intimal and medial thickening, and increased stiffening and reduced distensibility of central arteries [1,6–7]. Because the lifetime risk after the age of 40 years for predominantly systolic hypertension and atherosclerosis increases in epidemic proportion (Fig. 1), it behooves us to examine whether specific mechanisms that underlie phenotypic alterations in the heart and arterial substrates that accompany “aging” [8–10] are intimately linked to this risk (Fig. 1; cf. also 9 and 11 in this volume). But no discussion about the realty of cardiovascular aging or CVD, however, can beg the question of: what is the reality of aging, per se?
2.1 So, what is aging?
This is a tough question, about which there have been, and continue to be numerous different perspectives. Even most scientists who participate in aging research have never stopped to think about asking, “What is aging?” because there is no definitive answer. To begin to understand aging, we need to address numerous facets of life that change over time and thus to appreciate how organisms, not just cells, tissues or organs, change over time. My view is that “Aging is a shift in an organism’s reality” [10].
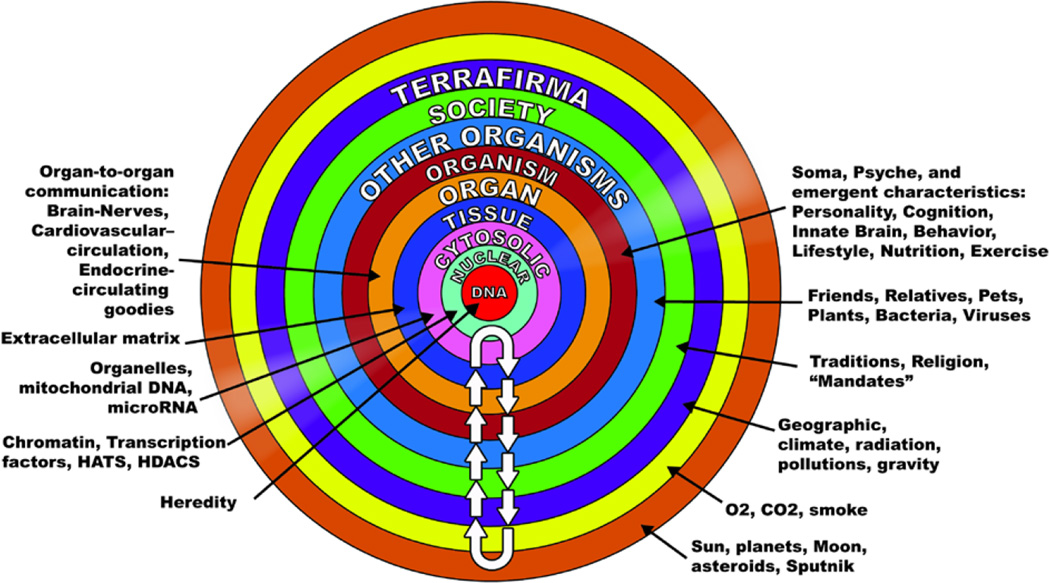
So what’s reality? This is another tough question. My view is that reality can be comprehensively defined as a “mutually enslaved” system of “DNA and its environment [10]. If this appears to be a naïve assessment of reality, check out what constitutes the DNA environment (Fig. 3).
Figure 3. Reality is a mutually enslaved “system” of DNA and its environment.
To begin to understand aging, we need to address numerous facets of life that change over time and thus to appreciate how organisms, not just cells, tissues or organs, change over time. Reality can be comprehensively defined as a “mutually enslaved” system of “DNA and its environment.” The arrows in the diagram indicate continual bidirectional signaling that must occur to sustain our existence. This continual signaling back and forth across each of these environments, confers “mutual enslavement” or ordered function among the components within the coupled DNA-environment system. Different signals across these environments are transmitted with different kinetics and vary in acute or chronic impact on the coupled system. (See text for details.) (from Ref. 10)
The intracellular DNA environment has nuclear, organelle and cytosolic components. Tissues constitute environments for cells, and compose organs that define the organisms, with somas, psyche’s innate brain function and cognition and personality, which emerge from these interactions (Fig. 3). The ontogeny of an organism is extremely complicated and requires an unfathomable degree of ordered molecular functions that evolves with time. Communication between the cardiovascular system and other organ systems is crucial to preservation of healthspan of the organism. Organisms differ in the development of their cognitive and stress coping mechanisms, in part, due to differences in personality characteristics, which, in part, give rise to the development of distinct behavior lifestyles, e.g. what and how much food we consume, how much we exercise, etc. And there are other organisms in our reality: friends, relations, plants, bugs, etc. And, as organisms, we are all immersed societies, which issue mandates, traditions, and religion, etc.. And then beyond that, we are surrounded by geographical realities of climate, radiation, pollution, etc., and of course, gravity (Fig. 3). Some perspectives about reality even include a cosmic connection (Fig. 3). So, the integrated constellation of different environments that surround our DNA and its function, in my opinion, constitutes our reality. “Epigenetics,” therefore, embraces the entire concentric series of environments depicted in Fig. 3, not just chromatins, histone acetylases (HATs), and deacetylases (HDACs), noncoding RNAs, and microRNAs, as narrowly preached by most scientific cognoscenti [cf. 12–14 in this volume]. The arrows in the diagram indicate continual bidirectional signaling that must occur to sustain our existence. This continual signaling back and forth across each of these environments, confers “mutual enslavement” or ordered function among the components within the coupled DNA-environment system. Different signals across these environments are transmitted with different kinetics and vary in acute or chronic impact on the coupled system.
2.2 Phenotypic aging is a manifestation of time-dependent failure of signaling within the DNA-environment system
“Inside every old person is a young person wondering what happened.” ---Anonymous
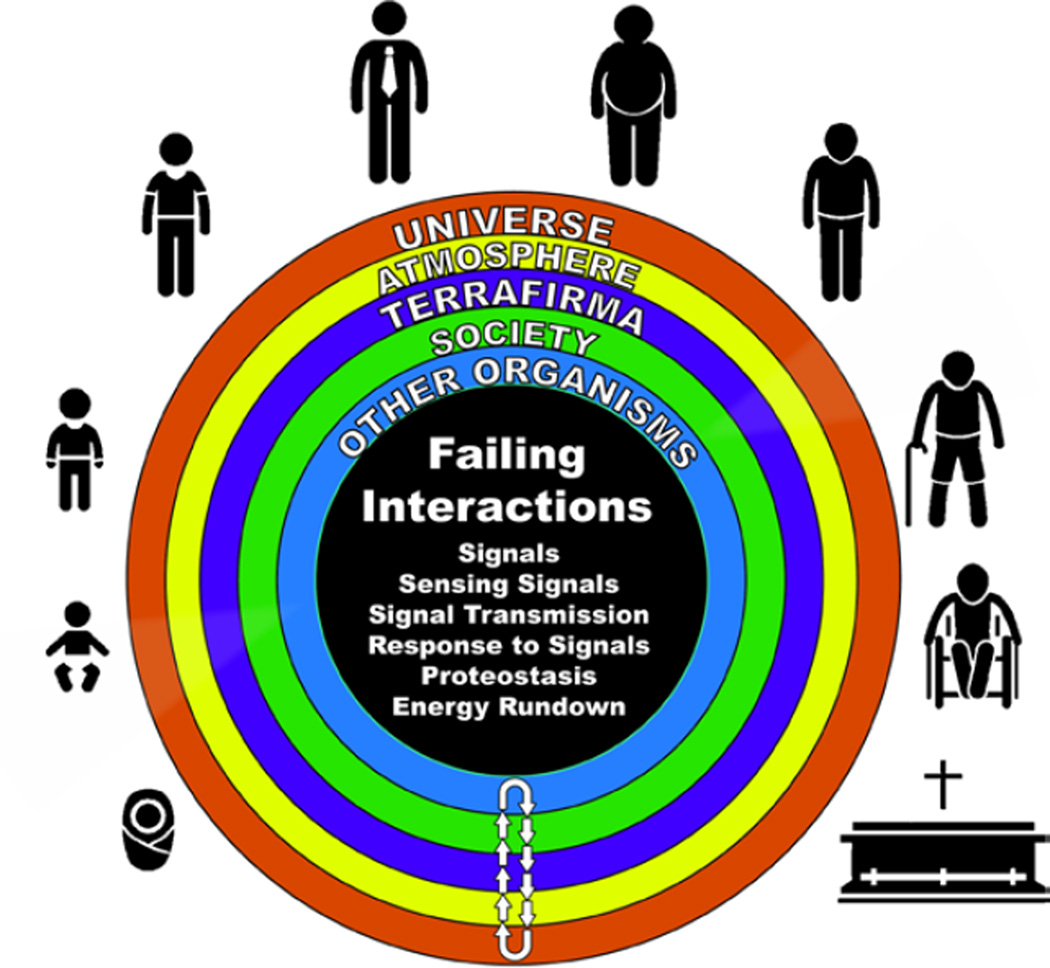
Aging can be construed as a series of failures in the signaling that occur over time within the DNA environmental system (Fig. 4). Because signaling within this system must operate in a rigorously ordered manner for an organism to function properly, failure of the system can be perceived to indicate a generalized disorder among molecular mechanisms and their interactions. Thus, the aging phenotype is a manifestation of time-dependent failing molecular mechanisms that emerges wthin system of DNA and its environment that creates disorder within the system, which masquerade as stochastic “epigentic drift” [15–16]. Age-associated cardiovascular failure and CVD play major roles in this time-dependent disorder that accrues within the system, contributing to stochastic epigenetic drift within the system.
Figure 4. Some age-associated signaling failures within the DNA-Environment System.
As we age, signals within the DNA-environment system change, as does sensing of the signals, transmission of signals and responses to signals. Aging is characterized by impaired cell renewal changes in the proteome due to alterations in genomic transcription, mRNA translation, and the local protein environments and proteostasis. The density of some molecules becomes reduced and post-translational modifications, e.g. oxidation, nitration phosphorylation, etc., lead to altered misfolding and disordered molecular interactions that alter the stoichiometry and kinetics of reactions that remove damaged organelles and proteins and underlie crucial cell functions and robust reserve mechanisms.
Nearly all aspects of signaling within the DNA-Environment system become disordered with advancing age and characterize the “reality of aging.” As we age, signals within the DNA-environment system change, as does sensing of the signals, transmission of signals and responses to signals (Fig. 4). Aging is characterized by impaired cell renewal [cf. 17, this volume] changes in the proteome due to alterations in genomic transcription, mRNA translation, and the local protein environments and proteostasis. The density of some molecules becomes reduced and post-translational modifications, e.g. oxidation, nitration phosphorylation, etc., lead to altered misfolding and disordered molecular interactions that alter the stoichiometry and kinetics of reactions that remove damaged organelles and proteins [cf. 18 in this volume] and underlie crucial cell functions and robust reserve mechanisms. The system loses its robustness and flexibility. Physical and psychic energies dwindle as reality shifts with aging in the context of age-associated molecular disorder. Not only our CV system, but our entire bodies and minds become different. We’re not exactly the same organism that we were 10 years ago, or 10 years before that. As a result of changes in the environments and in their interfaces (Fig. 3), aged organisms appear different to younger members of society and vice versa (Fig. 5).
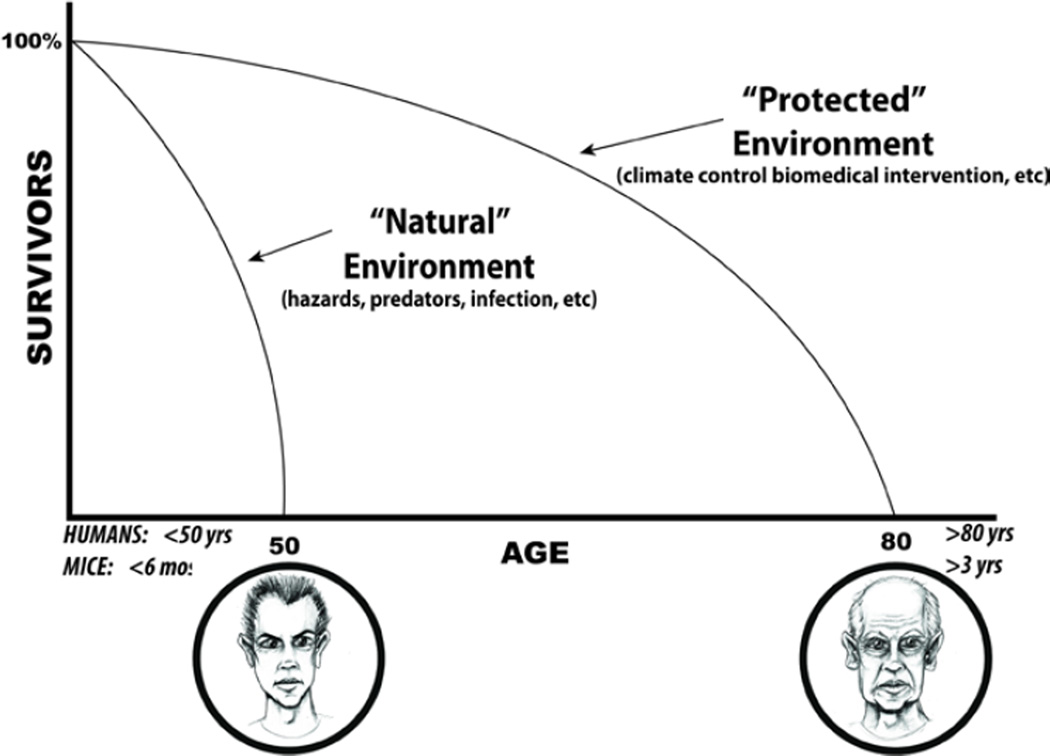
Figure 5. Natural and protected environments.
According to theory promulgated by evolutionary biology, the main reason for our reality is to perpetually ensure the existence of the next generation of our species. Thus, most of us are “wired” to be very healthy in order to procreate. After accomplishing this, in the evolutionary biology perspective, there is not a valid reason for us to remain intact, or even alive, e.g. beyond about 50 years. But although our “Natural Selection Insurance Policy” expires with advancing adult age, we remain alive because our environment has been protected by better hygiene, better nutrition, better healthcare that keep us alive well beyond our evolutionary life expectancy. (Adapted from Judy Campisi, personal communication)
2.3 The concept of aging as “borrowed time”
Another tough question is, when does aging begin? Is it a progressive run-down of the system in Fig. 3 that begins at an early age? Or does it begin to accelerate after a certain age? Some evidence points to the latter. Evolutionary biologists tell us that the main reason for our reality is to perpetually ensure the existence of the next generation of our species. Thus, most of us are “wired” to be very healthy in order to procreate. After accomplishing this, in the evolutionary perspective, there is not a valid reason for us to remain intact, or even alive. As our Natural Selection Insurance Policy “expires” with advancing adult age, we remain alive because our environment has been protected by better hygiene, better nutrition, better healthcare that keep us alive well beyond our evolutionary life expectancy. Aging, may thus, be conceptuazlied as progressive, time-dependent molecular disorder within the DNA-environment system of an organism depicted in Figs. 3 and 4, accompanied by reduced complexity, increased entropy within the DNA-environment system, leading to reduced efficiency and efficacy of molecular interactions that regulate cell, tissue, organ structure coordinated function among organ systems . Reference is often made to the “aging process.” But what is the evidence that aging is a “process”? MY OPINION: Aging appears not to be a “Process,” but rather a manifestation of time-dependent, stochastic, molecular disorder that ensues when our Natural Selection Insurance Policy expires.
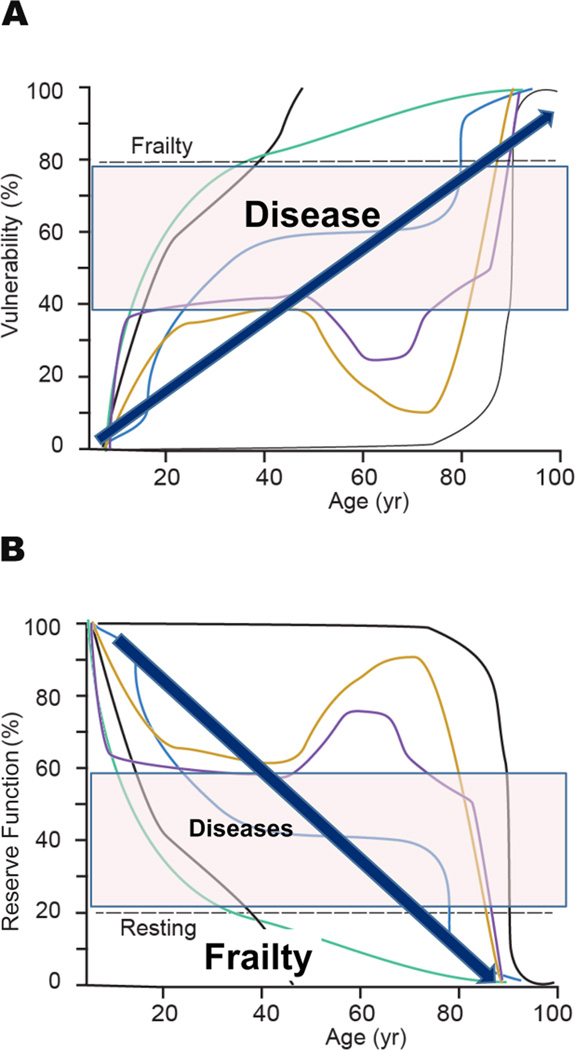
Increasing molecular and cellular disorder as we age, leads to loss of tissue organ and system reserve functions (Fig. 6A). Because numerous redundant mechanisms work together to maintain order within the DNA-environment system, the evolution of a functional decline in one mechanism enlists redundant mechanisms that compensate for that decline in order to preserve overall function (but with reduced reserve). Thus, functional declines are initially subtle and go unnoticed, as a substantial degree of order in the highly ordered DNA-environment system still exists. Eventually, as molecular disorder increases these adaptive compensations fail and disequilibrium of decline among specific functional decline ensues. Reflections upon these decline at this stage raise the issue of antagonistic pleiotropy [19]. During this advanced stage of disorder, the concept of functional homeostasis [20] is no longer applicable. The organism is “well on the road” to frailty (Fig. 6A) which, in general, can be described as an inability to perform activities of daily living. The “allostatic load,” i.e. the cumulative physiologic toll exacted on the body to adapt [21] exponentially increases and allostasis [22] fails. Accumulation of cellular defects leads to the cumulative loss of our reserve functions over time leads to (Fig. 6A) age-associated frailty, disability and vulnerability to diseases [5] (Fig. 6B) from which we were protected at earlier ages. Cardiovascular disease loom large on this vulnerability profile. The rate of increased vulnerability of reserve in our various functions (thin lines) is variable, and not always monotonic but sometimes biphasic or oscillatory due to compensatory mechanisms that occur among functions. The eigen vector for vulnerability, however (thick arrows), progressively increases with advancing adult age and underlies phenomena presently referred to as diseases and “frailty”, or the inability to perform normal activities of daily living.
Figure 6. The cumulative loss of reserve over time functions leads to increasing organismal vulnerability.
Increasing molecular and cellular disorder as we age leads to loss of tissue organ and system reserve functions. A. The rate of increased vulnerability of reserve in our various functions (thin lines) is variable, and not always monotonic but sometimes biphasic or oscillatory due to compensatory mechanisms that occur among functions. The eigen vector for loss of reserve function, however (thick arrows), progressively increases with advancing adult age and underlies phenomena presently referred to as diseases and “frailty”, or the inability to perform normal activity of daily living. The lines are arbitrary functions that decline at arbitrary rates. B. The cumulative loss of our reserve functions over time leads to age-associated increasing vulnerability to diseases and frailty from which we were protected at earlier ages.
2.4 So, is aging, per se, a disease?
This question has been debated from the era of famous Greek philosophers . One opinion, and an opinion that has underpinned modern gerontology, the study of aging, is that “aging and disease are not synonymous. There are processes of aging and etiologies of disease. The relationship between the two are important, but not inevitable.”—Nathan Shock [23]. This is not a unique opinion that has survived. Others would argue that “… to draw a distinction between disease and normal aging is to attempt to separate the undefined from the undefinable.”—J.G. Evans [24].
3. Is Cardiovascular Aging a Disease?
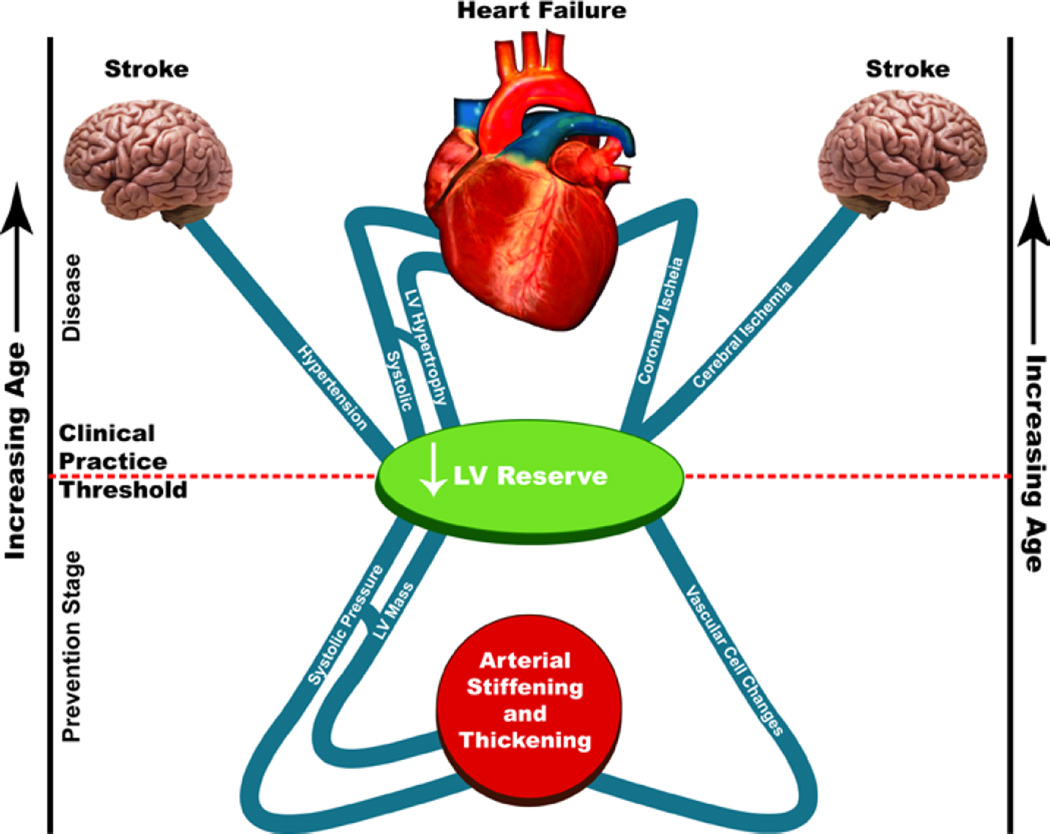
Structural and functional disorder within the hearts and arteries that clearly become manifest in the context of CVD have a preeminent role in the widespread disorder within the organism that accompanies advanced age because the cardiovascular system is crucial to the survival of other organ systems. One way to conceptualize why the clinical manifestations and the prognosis of CV diseases worsen with age is that in older individuals, the specific pathophysiologic mechanisms that cause clinical disorders depicted in Figure 1 become superimposed on heart and vascular substrates that are modified by aging [1,7,25] (Fig. 7).
Figure 7. Cardiovascular aging is the major rick factor for morbidity and mortality.
Imagine that age increases as one moves from the lower to the upper part of the figure and that the line bisecting the top and bottom parts represents the clinical practice “threshold” for disease recognition. Age-associated changes in cardiac and vascular properties (depicted below the clinical practice threshold line) alter the substrate on which cardiovascular disease (entities above the line). Thus, entities above the line are presently classified as “diseases” that lead to heart and brain failure. Vascular and cardiac changes presently thought to occur as a result of a “normal aging process” are depicted below the line. These age-associated changes in cardiac and vascular properties alter the substrates on which cardiovascular disease (entities above the line) is superimposed. Those age-associated changes in CV structure or function below the line 7 ought not to be considered to reflect “normal” CV aging. Rather, in the context of molecular and cellular disorder that accompanies “borrowed time,” they might be construed as specific risk factors for the CV diseases that they relate to, and thus might be construed as targets of interventions designed to decrease the occurrence or manifestations of cardiovascular disease at later ages (From Ref. 26).
Imagine that age increases as one moves from the lower to the upper part of Figure 7 and that the line bisecting the top and bottom parts represents the clinical practice “threshold” for disease recognition. Thus, entities above the line are presently classified as “diseases” that lead to heart and brain failure. Vascular and cardiac changes presently thought to occur as a result of a “normal aging process” are depicted below the line. These age-associated changes in cardiac and vascular properties alter the substrates on which cardiovascular disease (entities above the line) is superimposed. First, they lower the extent of disease severity required to cross the threshold that results in clinically significant signs and symptoms. Age-associated changes may also alter the manifestations and presentation of common cardiac diseases and can also influence the response to and therefore the selection of different therapeutic interventions in older individuals with cardiovascular disease. Those age-associated changes in CV structure or function below the line in Figure 7 ought not to be considered to reflect “normal” CV aging. Rather, in the context of molecular and cellular disorder that accompanies “borrowed time,” (Fig. 2) they might be construed as specific risk factors for the CV diseases that they relate to and thus might be targets of interventions designed to decrease or delay the occurrence or severely reduce cardiovascular disease at later ages.
4. Chronic inflammation degrades the landscape of cardiovascular structure/function, promoting a markedly increased risk for cardiovascular diseases in older persons
4.1 The reality of aging viewed from the arterial wall
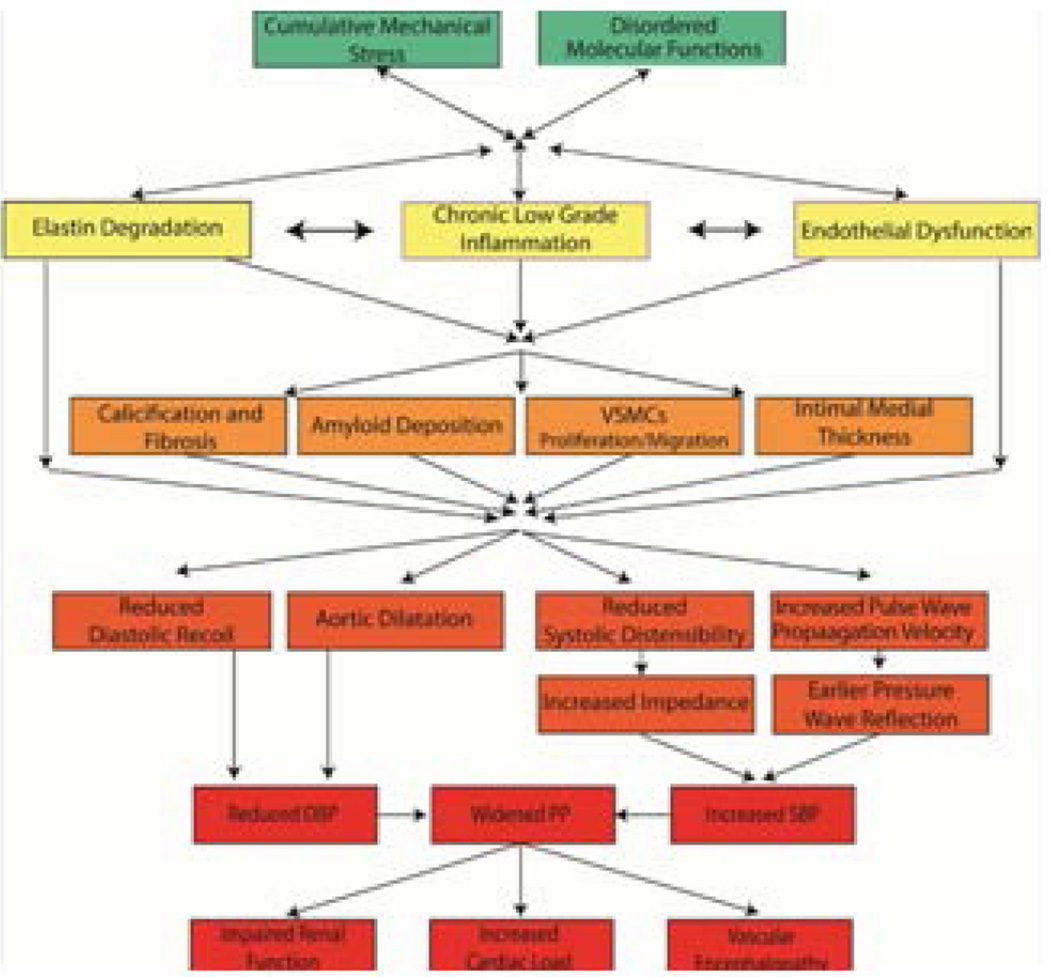
In order to determine whether or not to consider arterial aging a disease, we must understand the mechanisms that lead to age-associated changes in the arterial wall beneath the clinical disease threshold in Fig. 7 [7]. Age-associated phenotypic changes in central arteries result from a myriad of progressive structural and functional disorder, ranging from changes in molecules to cells to arterial tissue, the blood it transports, and the hormonal and neural factors that modulate molecules, cells, tissues, etc. that comprise our cardiovascular system. These events act in concert to reduce central arterial distensibility and render the arterial wall stiffer, which results in a more rapid pulse wave velocity and early return of the reflection wave to occur during systolic ejection (Fig. 8; refs 1, 6).
Figure 8. Conceptual model of arterial aging and arterial decline.
Age-associated molecular disorders and cumulative mechanical stress lead to a state of chronic inflammation, elastin degradation and endothelial and VSMC dysfunction. These products interact and lead to arterial wall calcification, fibrosis, amyloid deposition, VSMCs proliferation, and increased intimal medial thickness. These structural changes lead to functional alterations resulting in widened pulse pressure. The increase in pulsality leads to increase left ventricular load, chronic kidney disease, and vascular dementia. (From Ref. 6)
As a result, the systolic blood pressure increases, diastolic pressure decreases and the pulse pressure increases with aging. [1,6] The chronic increase in pulse pressure transmitted to the brain and kidney damage the arterial supply of those organs, leading to vascular encephalopathy and chronic renal failure (Fig. 8; cf. also 10 in this volume). Thus, while our textbooks usually describe the characteristics of central arterial changes that accompany advancing age as "physiologic" arterial aging, these changes are far from being “physiologic” and are more aptly construed as pathophysiologic.
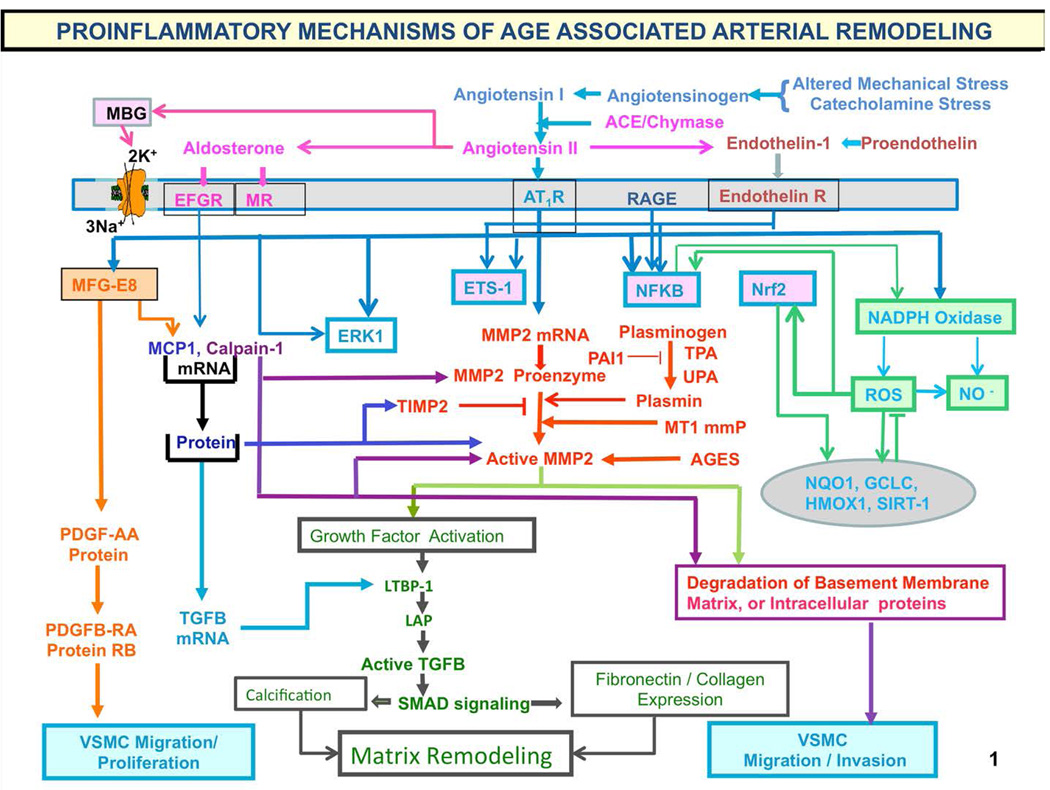
There is a substantial gap between our knowledge about what's going on in the arterial wall under the microscope with respect to structure or function of the large arteries and what can be measured in vivo [1,7]. Characteristically, there is fragmentation and calcification of elastic fibers, increased deposition of collagen and collagen cross linking, amyloid deposition in the medial layer, and migration/proliferation of vascular smooth muscle cells (VSMC) (Fig. 8, 9) [8, 27]. Processes that lead to cellular and matrix structural and functional age-associated changes of the arterial wall are driven by a proinflammatory microenvironment, mediated by mechanical and humoral factors (Fig. 9). These processes are driven by oxidative stress and low-grade inflammation [cf. 28 in this volume]. Our body's initial response to stress is moderated by increased adrenergic signaling; the downstream receptor signaling cascade results in increased activation of renin-angiotensin-aldosterone, and endothelin signaling (Fig. 9), mechanisms utilized to respond to chronic stress. Importantly, this stress defense is not executed by “professional” inflammatory cells (i.e. white blood cells) but by endothelial and VSM cells that shift their phenotypes to produce inflammatory cytokines.
Figure 9. Proinflammatory mechanisms of age-associated arterial remodeling.
Processes that lead to cellular and matrix structural and functional age-associated changes of the arterial wall are driven by a proinflammatory microenvironment and low-grade inflammation, mediated by mechanical and humoral factors. These processes are driven by oxidative stress. Abbreviations: AAASP, age-associated arterial secretory phenotype; ACE, angiotensin converting enzyme; Ang II, angiotensin II; AT1R, angiotensin II type 1 receptor; AGE, advanced glycation endproducts; ECM, extracellular matrix; ET-1, endothelin-1; ETA, endothelin-1 receptor A; Ets-1, v-ets erythroblastosis virus E26 oncogene homolog 1; LAP, latency-associated peptide; LTBP-1; latent transforming growth factor (TGF)-binding protein-1 (LTBP-1) and MCP-1, monocyte chemoattractant protein-1; MFG-E8, milk fat globule epidermal growth factor-8; MMP, matrix metalloprotease; MR, aldosterone/mineralocorticoid receptor; NF-kB, nuclear factor k light-chain-enhancer of activated B cells; Nrf-2, NF-E2-related factor 2; NO, nitric oxide; PAI, plasminogen activator inhibitor; PDGF, platelet-derived growth factor; RAGE, receptor for AGE; ROS, reactive oxygen species; TGF-b1, transforming growth factor b1; t-PA/u-PA, tissue-type/plasminogen-type plasminogen activators; VMSC, vascular smooth muscle cell.
The chronic proinflammatory profile within aged central arteries is driven by alterations in signaling systems that include Ang II signaling via its receptor AT1, as well as MR, and ET-1/ETA and RAGE signaling. NF-kB and Ets-1 are activated within, whereas other factors such as Nrf-2 are reduced. Downstream signaling molecules include MFG-E8, MMPs, calpain-1, MCP-1, and TGF-b1. Calpain-1, MMPs, TGF-b1, NADPH oxidase activation increases and NO bioavailability decreases. VSMCs from old arteries secrete increased amounts of MFG-E8, MCP-1, MMP-2, and TGF-b1 inducing VSMC proliferation, migration, secretion, senescence, extracellular arterial matrix deposition becomes markedly altered with aging. Disruption of the endothelium, intima-media thickening, arterial amyloidosis, fibrosis, calcification, elastin fracture, and matrix glycoxidative modifications are consequences of the enhanced signaling via the depicted receptor signaling cascades and can lead to changes in arterial clinical phenotype that can be measured non-invasively in humans. Our body's initial response to stress is moderated by increased adrenergic signaling; the downstream receptor signaling cascade results in increased activation of renin-angiotensin-aldosterone, and endothelial dysfunction, mechanisms that our body utilizes to respond to chronic stress. Importantly, this stress defense is not executed by “professional” inflammatory cells (i.e. white blood cells) but by endothelial and VSM cells that shift their phenotypes to produce inflammatory cytokines. (adapted from Ref. 8)
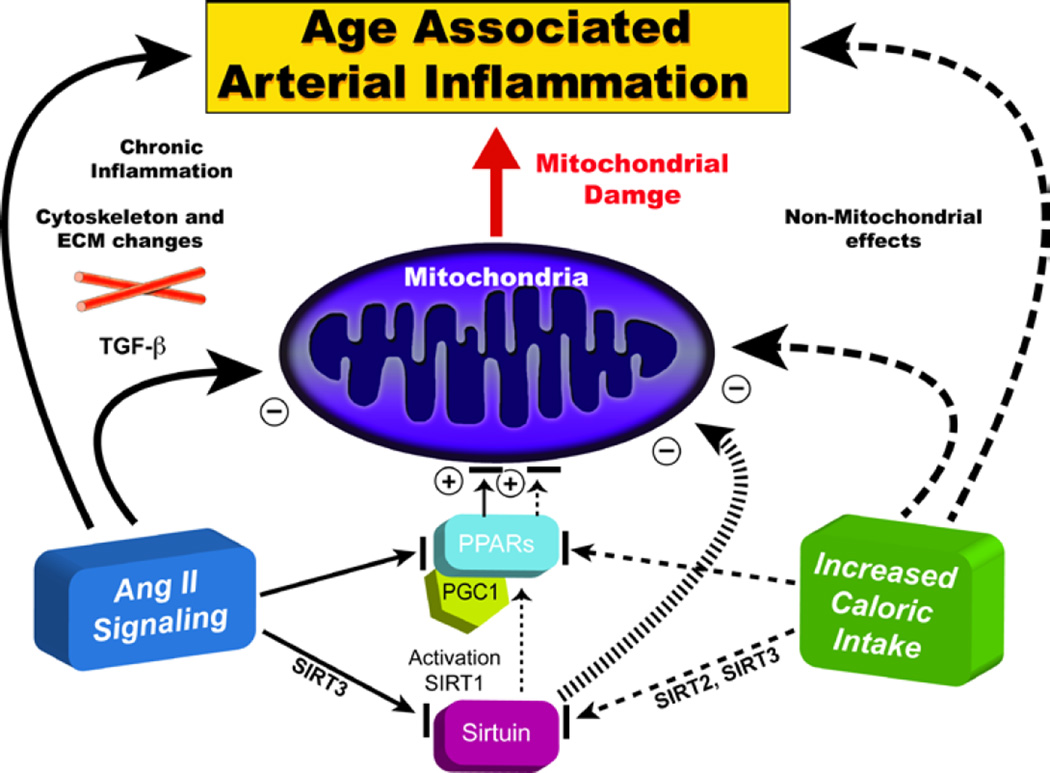
Other factors also play a key role in arterial inflammation. AGTRAP and SIRT1 (not shown in Fig. 9) are negative regulators of angiotensin receptor signaling, that leads to proinflammation and thus to age-associated arterial remodeling. Expression of both SIRT1 and AGTRAP are reduced by an increase in miR34a which accompanies advancing age [29,30]. It is noteworthy that caloric intake mimics Ang II signaling (Fig. 10) [31]. Ang II signaling and caloric intake converge to affect sirtuin and PPARs signaling, impacting upon metabolic function and resulting in mitochondrial damage (Fig. 10; cf. 32 and 33 in this volume].
Figure 10. Decreased age-associated arterial inflammation effects on the heart and arteries.
Caloric intake mimics Ang II signaling. Ang II signaling and caloric intake converge to affect sirtuin and PPAR signaling, impacting upon metabolic function and resulting in mitochondrial damage (Adapted from Ref. 31)
Although increased activation renin-angiotensin-aldosterone system, endothelin and RAGE signaling cascades (Figs 9,10) are ways that our bodies are wired to respond to chronic stress, it remains debatable as to the extent to which activation of these signaling cascades results an “overshoot” in the chronic inflammatory response creating additional oxidative stress that contributes to the progression of age-associated structural and functional arterial remodeling. The overshoot might be expected to occur because the molecular disorder that elicits the response cannot be resolved, resulting in a chronic increase in inflammatory signaling. A similar and well-known overshoot scenario occurs in the context of catecholamine signaling in chronic heart failure. In other terms, these overshoots that accompany advancing age and are generated by the body’s cells or modifies the behavior of other cells, leading to altered cell behavior, injury or cell death.
Arterial wall aging is quite similar in humans, non-human primates, rabbits and rats (Fig. 10) and involves inflammatory processes associated with oxidative stress. “Aging”-associated arterial changes and those associated with hypertension (and early atherosclerosis and diabetes) are fundamentally intertwined at the cellular and molecular levels (Fig. 11). Arteries of younger animals, in response to experimental induction of hypertension or early atherosclerosis or diabetes, parts of this proinflammatory profile within the arterial wall that have been studied to date are strikingly similar to the Ang II-mediated profile that occurs with advancing age (Fig. 11). Remarkably, continuous administration of angiotensin II to young animals for 30 days induces a rapid deterioration of their arteries, which look older [34]. Moreover, inhibition of Ang II signaling [31, 35–39] or caloric restriction [40] exerts marked amelioration of chronic inflammation (Figs. 9, 11) and, in some instances, also increases longevity [31, 38–40].
Figure 11. Ang II-regulated molecular and cellular remodeling that occurs with aging in different species, and in expressed hypertension, atherosclerosis and diabetes in younger animals.
Arterial wall aging is quite similar in humans, non-human primates, rabbits and rats and involves inflammatory processes associated with oxidative stress. Arteries of younger animals, in response to experimental induction of hypertension or early atherosclerosis or diabetes, parts of this proinflammatory profile within the arterial wall that have been studied to date are strikingly similar to the Ang II-mediated profile that occurs with advancing age (From Ref. 27)
4.2 Arterial diseases as manifestations of exaggerated age-associated arterial wall inflammation: Is atherosclerosis a manifestation of advanced age-associated arterial inflammation?
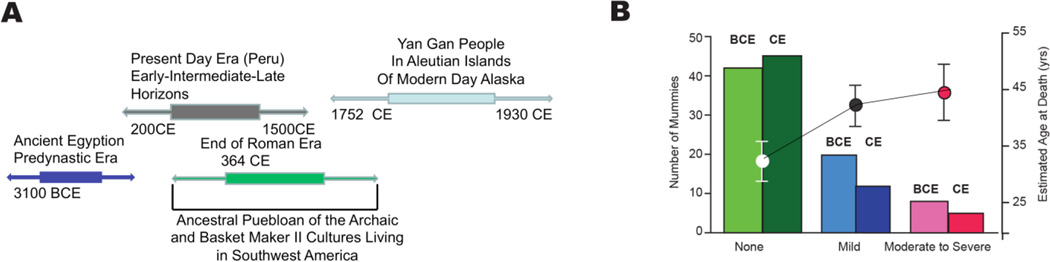
In humans, other well-known risk factors (e.g., excessive food intake (Fig. 10), altered dietary lipid and metabolism, smoking, and lack of exercise) likely interact with the arterial substrate that becomes altered during aging, and that renders the aging artery a “fertile soil” that facilitates the initiation and progression of these arterial diseases. New evidence (Fig. 12) that aging and atherosclerosis are intimately intertwined is gleaned from a recent study that imaged arteries of mummies [41]. This study imaged the arteries of mummies from over 4000 years of human history ranging from before the Common Era (BCE) to the Common Era (CE) (Fig. 12A) to detect the presence and severity of atherosclerosis defined as calcified plaques within the arterial wall. The major finding was that although diet and lifestyle differed widely among the populations studied (Fig. 12A) the prevalence and severity of atherosclerosis in BCE was nearly identical to that of CE (Fig. 12B). However, the prevalence of atherosclerosis varied by age, regardless of the era (Fig. 12B). The conclusion of this study, “Atherosclerosis across 4000 Years of Human History: The Horus Study of Four Ancient Populations,” was that “the presence of atherosclerosis in modern human beings suggests that it is an inherent component of human aging and NOT characteristic of any specific diet or lifestyle.”
Figure 12. Aging and atherosclerosis are intimately intertwined.
This study imaged the arteries of mummies from over 4000 years of human history ranging from before the Common Era (BCE) to the Common Era (CE) to detect the presence and severity of atherosclerosis defined as calcified plaques within the arterial wall. The major finding was that although diet and lifestyle differed widely among the populations studied (Panel A) the prevalence and severity of atherosclerosis in BCE was nearly identical to that of CE. In both epochs, the severity of atherosclerosis was associated with increasing age (Panel B) (Adapted from Ref. 41)
5. The Reality of Aging Viewed from the Heart
5.1 Cardiac structure and function at rest
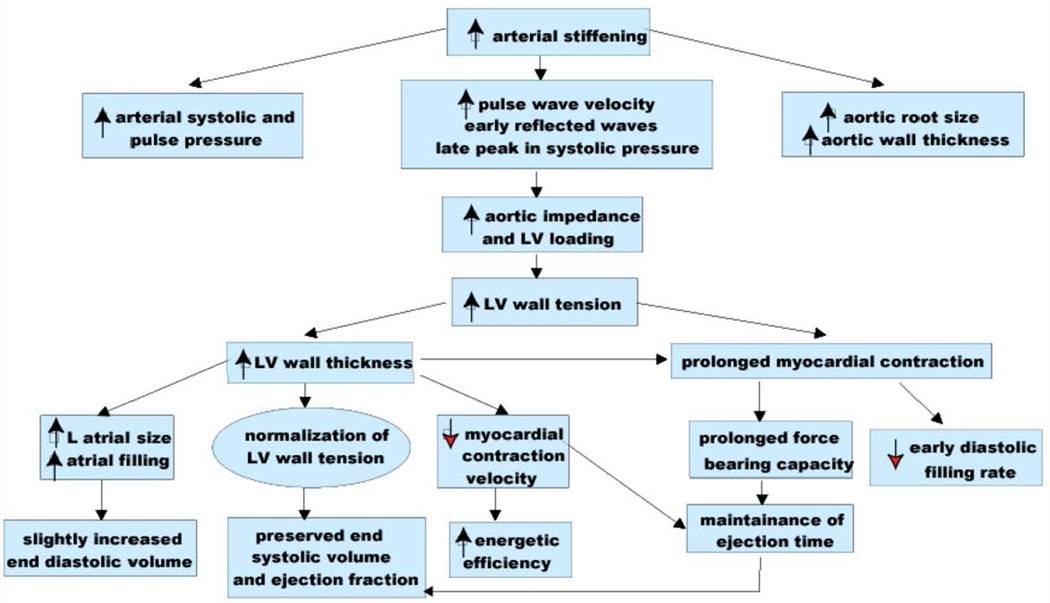
A unified interpretation of identified cardiac changes that accompany advancing age and their interaction with age-associated arterial changes in otherwise apparently clinically healthy persons is depicted in the schematic in Fig. 13 [25]. Some cardiac changes are, at least in part, adaptive, occurring to some extent in response to arterial changes (Figs. 8–9) that occur with aging. With advancing age, the walls of the left ventricle increase in thickness, largely because of an increase in ventricular myocyte size and an increase in vascular impedance, and this helps moderate the increase in LV wall tension [cf. 3 and Fig. 8]. Cardiac muscarinic receptor density and function are also diminished with increasing age [42] and might contribute to reductions in heart rate variability and baroreflex activity observed in older persons.
Figure 13. Age-associated changes in resting cardiovascular structure/function in the absence of a textbook clinical diagnosis.
Prolonged contraction of the thickened LV wall which maintains a normal ejection time in the presence of the late augmentation of aortic impedance is preserved by systolic cardiac pumping function at rest. A downside of prolonged contraction is that myocardial relaxation at the onset of diastole is relatively more incomplete in older than in younger individuals and reduces the early LV filling rate to in older vs. younger individuals. Structural changes and functional heterogeneity occurring within left ventricular tissue with aging may also contribute to this reduction in peak LV filling rate. Additional concomitant adaptations—left atrial enlargement and an enhanced atrial contribution to ventricular filling, however, compensate for the reduced early filling and maintain a normal end diastolic volume. (modified from Ref. 43)
5.2 Cardiovascular Reserve
Cardiovascular function at rest accounts for only about a third of the overall peak cardiovascular capacity, which declines substantially with advancing age. An age-associated reduction in peak cardiac reserve function accounts for about half of the age-associated decline in peak oxygen consumption. The peak cardiac output decline is solely due to chronotropic insufficiency as peak stroke volume is preserved (Table 1). Impaired heart rate acceleration and impaired augmentation of blood ejection from the left ventricle are the most dramatic changes in cardiac reserve capacity that occur with aging in healthy, community-dwelling persons (Table 1).
Table 1.
Changes in cardiorespiratory reserve in healthy, community-dwelling persons during peak cycle exercise between the ages of 20 and 80 years (from Ref. 44)
| Peak (A–V) O2 | (25%) | ↓ |
| Cardiac index | (25%) | ↓ |
| Heart rate | (25%) | ↓ |
| Stroke volume | No change | |
| End diastolic volume | (30%) | ↑ |
| Peripheral vascular reserve | (30%) | ↑ |
| End diastolic volume | (275%) | ↑ |
| LV contractility | (60%) | ↓ |
| LV ejection fraction | (15%) | ↓ |
| Plasma catecholamines | ↑ | |
| Cardiac and vascular response to | ↓ | |
| beta-adrenergic stimulation |
Mechanisms that underlie the age-associated reduction in peak heart rate and ejection fraction are multifactorial and include a reduction in intrinsic sinoatrial pacemaker cell function [45; cf. 46 in this volume] and ventricular myocyte intrinsic contractility [43; also cf. 47 in this volume], and increased arterial-ventricular load mismatching [48], which interferes with myocardial contraction and the ejection of blood.
A sizeable component of the age-associated deficit in cardiovascular reserve is due to a withering of brain-heart signaling, which includes diminished effectiveness of the autonomic modulation of heart rate, LV contractility, and arterial afterload [43] (Table 1). Sympathetic stimulation of the cardiovascular system during stress ensures that the heart beats faster; to ensure that it retains a small size, by reducing the diastolic filling period, LV afterload is reduced; myocardial contractility and relaxation are enhanced; blood is redistributed to working muscles and to skin to dissipate heat. Each of the components of autonomic cardiovascular regulation becomes compromised with advancing age. Substantial evidence points to the reduced efficiency of postsynaptic beta-adrenergic signaling with aging [43 for review]. Thus, cardiovascular responses including those of pacemaker tissue [45] to beta-adrenergic agonist infusions at rest decrease with age, and acute beta-adrenergic receptor blockade changes the exercise hemodynamic profile of younger persons to make it resemble that of older individuals. During exercise the heart rate reduction by acute beta-adrenergic blockade is greater in younger versus older subjects, as are the age-associated deficits in LV early diastolic filling rate, both at rest and during exercise. It has also been observed that the increase in aortic impedance during exercise in older dogs results from deficient β-adrenergic signaling [49]. Thus, age-associated impaired cardiovascular reserve manifest during exercise is at least in part, attributable to withering of brain-heart communication.
These deficits in sympathetic modulation of cardiac and arterial functions with aging occur in the presence of exaggerated neurotransmitter levels [cf. 43 for review]. Plasma levels of norepinephrine and epinephrine response to stress increase to a greater extent in older compared with younger healthy humans (Table 2), due to an increased spillover into the circulation and, to a lesser extent, reduced plasma clearance. Due to reduced norepinephrine reuptake at nerve endings, that differs among body organs, but increases within the heart.
Table 2.
Manifestations of molecular disorder characterize the aging heart “under the microscope”
|
5.3 Ventricular Cells Within the Older Heart Operate “on the Edge” of Disease
Tables 3 and 4 summarize changes in specific aspects of cardiac structure, function, and expression of some genes that occur in the pressure- overloaded myocardium and in the normotensive senescent heart. Because similar reductions in the cellular RNA concentration and the rate of protein synthesis are observed with aging and chronic myocardial overload in the rat model, it has been suggested that the latter (which is usually accompanied by myocardial hypertrophy) represents “accelerated aging” [50]. Thus, reflection on mechanisms that underlie structural, functional, and molecular changes in experimental hypertrophy may provide clues as to what factors underlie the age-associated changes described in the previous sections. The multiple changes in cardiac excitation, myofilament activation, contraction mechanisms, and gene expression that occur with aging are interrelated. Many of these age-associated structural and functional changes can be interpreted as adaptive in nature as they also occur in the hypertrophied myocardium of younger animals adapted to experimentally-induced chronic hypertension. Note that a similar pattern occurs in hypertension in young animals, in aging in normotensive animals, and in neonatal heart cells exposed to growth factors [51 for review].
Table 3.
Chronic Alterations in Scruture Function are Similar in Aging and Experimental Left Ventricualr Pressure Loading (From Ref. 52)
| Structural or Functional Measure Measure |
Experimental LV Pressure Loading (Rodent) |
Normotensive Aging (Rodent) |
|---|---|---|
| Myocardial cell size | ↑ | ↑ |
| Collagen content | ↑ | ↑ |
| Twitch duration | ↑ | ↑ |
| Myosin isozyme composition | ↓β1 ↑β3 | ↓β1 ↑β3 |
| SR Ca2+ pumping rate | ↓ | ↓ |
| Cai transient duration | ↑ | ↑ |
| Myofilament Ca2+ sensitivity | ↔ | ↔ |
| Actional potential repolarization time | ↑ | ↑ |
| B-adrenergic intropic response | ↓ | ↓ |
| Cardiac glycoside response | ↓ | ↓ |
| Threshold for Ca2+ overload | ↓ | ↓ |
Table 4.
Altered Myocardial Gene Expression Steady mRNA Levels in Advanced Age, Hypertension, Heart Failure or after Growth Factors (from Ref. 52)
|
GROWTH FACTORS* |
|||||
|---|---|---|---|---|---|
| RODENT Aging |
RODENT Hypertension |
TGFβ | FGF |
||
| acidic | basic | ||||
| SR Ca2+-ATPase | ↓ | ↓ | ↓ | ↓ | ↓ |
| Calsequestrin | ↔ | ↔ | |||
| Phospholamban | ↑ (rabbit) | ||||
| α Myosin Heavy Chain | ↓ | ↓ | ↓ | ↓ | ↑ |
| β Myosin Heavy Chain | ↑ | ↑ | ↑ | ↑ | ↑ |
| β Tropomyosin | ↓ | ↑** | |||
| α Skeletal Actin | ↓ | ↑** | ↑ | ↓ | ↑ |
| ANF | ↑ | ↑ | ↑ | ↑ | ↑ |
| Proenkephalin | ↑ | ↓** | |||
| Gsα | ↓ | ||||
| β1 receptor | ↓ | ||||
| Fibronectin | ↑ | ↑ | |||
| Collagen type I | ↑ | ↑ | |||
| Collagen type III | ↑ | ↑ | |||
| Angiotensinogen | ↑ | ↑ | |||
| ACE | ↑ | ↑ | |||
| Retinoid X Receptor | |||||
| α | ↔ | ||||
| β | ↔ | ||||
| δ | ↓ | ||||
| Thyroid Receptors | |||||
| α1 | ↔ | ||||
| α2 | ↔ | ||||
| β1 | ↓ | ||||
Notes:
In neonatal cultured cardiocytes
Only transient changes occur in situ following cardiac pressure loading
Thus, it is tempting to speculate that this nearly identical pattern of change in gene expression may indicate that a common set of transcription factors regulates cellular adaptation during pressure-overload hypertrophy and aging. This particular constellation of shifts in gene expression (Table 4) appears to be adaptive in that it allows for an energy-efficient and prolonged contraction. In the hypertensive rodent heart, it can be inferred that these changes in gene expression permit functional adaptations in response to an increased vascular “afterload.”
All the components of the renin-angiotensin system (RAS) are present in cardiac myocytes [53–54] . The Ang II-mediated proinflammatory profile manifested in aged arteries (Fig. 9–11) is also observed within the older heart (cf. ref. 11 in this volume). The mRNAs for both angiotensinogen and ACE are markedly increased in hearts of older vs. younger rats [55]. Ang II inhibition has been shown to regress hypertrophy induced by constriction of the abdominal aorta without altering the apparent pressure load on the heart [53]. The relationship between Ang II and myocyte hypertrophy is well-established [56–58]. In addition, there is a growing body of evidence suggesting that Ang II may play a role in functional abnormalities associated with cardiac hypertrophy [57]; thus, changes in the RAS with aging may contribute to age-associated cardiac functional deficits as well.
Reduced glucose tolerance also occurs in aged rats [59] as in humans and could possibly relate to some of the changes noted above. Alterations of thyroid status can produce alterations in some of the variables depicted in Tables 3 and 4. Finally, a reduction of physical activity occurs with aging even in rats "in captivity" [40,60]. As studies in older animals have shown that exercise training can modify some of these changes noted in Tables 3 and 4 [61–62], the physical deconditioning that accompanies aging of experimental animals may contribute to some of the changes attributed to aging per se.
Finally, some characteristics of the senescent heart may be influenced primarily by the host environment When cardiac myocytes from neo- natal rats were surgically implanted into the peritoneal cavity of two-month-old, seven-month-old, and 18-month-old host rats, those in the youngest rats displayed greater growth as assessed by a number of indices [63]. Similarly, when aortic segments were cross-transplanted among young and old rats and animals were subsequently subjected to restraint stress, the induction of heat shock protein gene expression was markedly attenuated in older host rats, whereas the response was largely preserved in younger rats irrespective of donor age [64].
6. Clinical Implications of Cardiovascular Aging
An emerging school of thought, however, proposes that the effects of cardiovascular aging, at the molecular, enzymatic, biochemical, cellular, histologic, and organismal levels, constitute the risky components of aging for the increased likelihood of clinical signs and symptoms of cardiovascular disease to emerge as age increases [1, 7,25]. Many of the age-associated alterations in cardiovascular structure and function, at both the cellular and molecular levels, are the same as those that underlie the pathophysiology of cardiovascular diseases. Because these factors can be modified by changes in lifestyle, e.g. diet [65] and exercise [65–69], or by presently available drugs, e.g. those that reduce the impact of Ang II signaling [31, 36–37,39], there is an urgency to incorporate cardiovascular aging into clinical medicine. But in spite of the interest in the physiology of the age-associated changes in cardiovascular structure and function, the risk of cardiovascular aging, per se, has remained, for the most part, outside of mainstream clinical medicine! The pathophysiologic implications of age-associated cardiovascular changes are largely underappreciated and are not well disseminated in the medical community, mainly because many of these manifestations of CV aging cannot be detected in the blood, as is the case for “traditional” arterial disease risk factors. Thus, although age is the dominant risk factor for cardiovascular diseases [1] (Fig. 1) and some effects of aging can be modified [31, 35, 36–37, 65–69], most of the research efforts on prevention of these diseases have ignored focusing on the effects of aging of the arterial wall, and, instead, have focused on development of interventions that target other “traditional” cardiovascular risk factors (such as hypertension and hyperlipidemia).
7. Conclusion
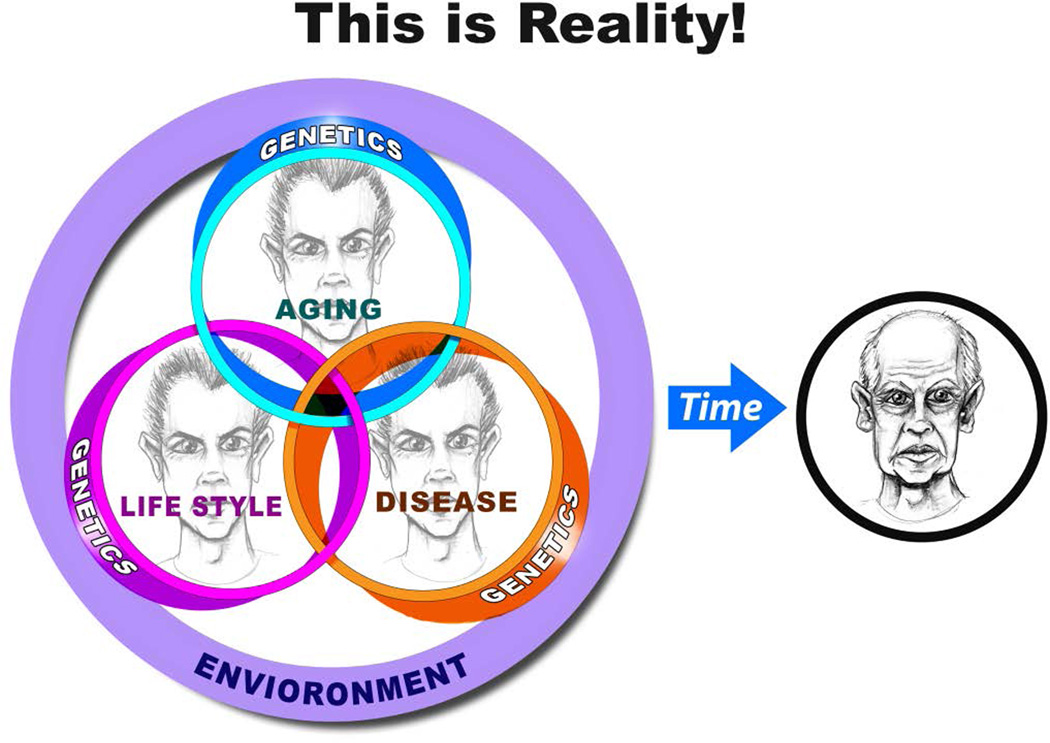
So, again, is aging a disease? My perspective, based upon the evidence presented herein, is that aging is a disease that is eventually fatal! Accelerated age-associated molecular disorder within the heart and arteries ought to be referred to as cardiovascular disease. Usually, molecular disorder that accompanies increasing age does not cause clinical symptoms; rather, aging-associated molecular disorder is a disease that becomes the dominant risk factor for other clinical syndromes that have been and continue to be referred to as cardiovascular diseases [1,7,25]. As life expectancy increases, a systems-biology approach is needed to ensure that we have a healthy lifespan [5]. We must not lose sight of the reality that age, disease, lifestyle, genetics and environmental factors are intertwined and that there are interactions that vary over time (Fig. 14) [cf. 68–69 in this volume]. To understand aging, we must discover failures that occur in cardiovascular tissue and define their underlying mechanisms, and integrate and translate these discoveries [5,65]. But, instead of integrating discoveries, we (mortals) usually fragment knowledge: organisms into systems (e.g. CNS CVS, etc.), losing the site of the organism; systems into cells – losing site of organs, systems, and organisms; cells into “departments” (e.g. biochemistry, physiology, etc.) – losing site of cells, organs, systems, organisms.
Figure 14. The reality of the evolution of the “senescent” phenotype.
Aging, lifestyle (e.g. dietary and physical exercise habits), and disease, and their genetic components interact with each other and with the environmental components shown in Fig. 3. Changes over time result in what is referred to as a senescent phenotype.
Age-associated alterations in arterial structure and function could represent the link that explains, at least in part, the risky component of aging. Indeed, a steady stream of incremental knowledge, derived from both animal and human studies, has established that several of the aging-associated changes within the walls of the central arteries are themselves potent and independent risk factors for cardiovascular diseases and many of these aging effects can be modified, e.g. increasing physical activity, by dietary modifications [40,65], or pharmacologic inhibition of Ang II signaling [31, 35–39]. Our present understanding of the age-associated alterations in cardiac and arterial structure and function at both the system, cellular and molecular levels provides valuable clues that may assist in the development of additional novel therapies to prevent, to delay, or to attenuate the cardiovascular changes that accompany advancing age. Changes in the perspectives of the reality of aging are long overdue in the clinical practice of medicine. Policymakers, researchers, and clinicians should intensify their efforts toward retarding age-associated interactions depicted in Fig. 14, particularly in individuals in whom these alterations are accelerated but not discovered in otherwise apparently “healthy” individuals. Future studies would then examine whether these strategies (i.e., those targeting cardiovascular aging) can have a salutary impact on the adverse cardiovascular effects of accelerated, cardiovascular aging. and attenuate the impact of age as the dominant risk factor for cardiovascular diseases. Importantly, this should help alter our view of the effects of aging from immutable risk factors to those amenable to modification and retardation. Cardiovascular aging is a promising frontier in preventive cardiology that is ripe for and in dire need of attention!
Acknowledgment
The author would like to thank Ruth Sadler for editorial assistance and Harold Spurgeon and Jimmy Burril for work on figures. This research was supported entirely by the Intramural Research Program of the NIH, National Institute on Aging.
References
- 1.Lakatta EG, Levy D. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises. Part I: Aging Arteries: A “Set Up” for Vascular Disease. Circulation: New Frontiers. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update A report from the American Heart Association. Circulation. 2010;121:e1–e170. [Google Scholar]
- 3.Upadhya B, Taffet GE, Cheng CP, Kitzman DW. Heart failure with preserved ejection fraction in the Elderly: Scope of the Problem. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The World Health Report 1997—conquering, suffering, enriching humanity. Geneva: World Health Organization; [PubMed] [Google Scholar]
- 5.Kirkwood TB. A systematic look at an old problem. Nature. 2008 Feb 7;451(7179):644–647. doi: 10.1038/451644a. [DOI] [PubMed] [Google Scholar]
- 6.AlGhatrif M, Lakatta EG. The Reality of Aging Viewed from the Arterial Wall. In: Safar ME, O’ Rourke MF, Frohlich ED, editors. Blood Pressure and Arterial Wall Mechanics in Cardiovascular Diseases. London: Springer; 2014. pp. 137–154. [Google Scholar]
- 7.Lakatta EG. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises Part III: Cellular and Molecular Clues to Heart and Arterial Aging. Circulation: New Frontiers. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Lakatta EG. Central Arterial Aging: Humans to Molecules. In: Safar M, editor. Handbook of Hypertension: Arterial Stiffness in Hypertension. Vol. 9. Elsevier; 2006. pp. 137–160. [Google Scholar]
- 9.Harvey A, Montezano AC, Touyz RM. Vascular biology of aging - implications in hypertension. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakatta EG. The Reality of Aging Viewed from the Arterial Wall. Artery Res. 2013;7:73–80. doi: 10.1016/j.artres.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Shah A. Age-associated pro-inflammatory remodeling in the heart and large arteries. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Illi B, Ciarapica R, Capogrossi M. Chromatin methylation and cardiovascular aging. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson BS, McKinsey T. Non-sirtuin histone deacetylases in the control of cardiac aging. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greco S, Gorospe M, Martelli F. Non-coding RNA in age-related cardiovascular diseases. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin GM. Epigenetic gambling and epigenetic drift as an antagonistic pleiotropic mechanism of aging. Aging Cell. 2009;8:761–764. doi: 10.1111/j.1474-9726.2009.00515.x. [DOI] [PubMed] [Google Scholar]
- 16.Martin GM. Stochastic modulations of the pace and patterns of ageing: impacts on quasi-stochastic distributions of multiple geriatric pathologies. Mech Ageing Dev. 2012;133:107–111. doi: 10.1016/j.mad.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hariharan N, Sussman MA. Cardiac Aging - Getting to the Stem of the Problem. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linton PJ, Gurney M, Sengstock D, Mentzer RM, Gottlieb RA. This old heart: Cardiac aging and autophagy. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2014.12.017. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin GM. Modalities of gene action predicted by the classical evolutionary biological theory of aging. Ann N Y Acad Sci. 2007;1100:14–20. doi: 10.1196/annals.1395.002. [DOI] [PubMed] [Google Scholar]
- 20.Cannon WB. Organization for Physiological Homeostasis. Physiol Rev. 1929;9:399–431. [Google Scholar]
- 21.Seeman TB, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of Adaptation--Allostatic Load and Its Health Consequences: MacArthur Studies of Successful Aging. Arch Intern Med. 1997;157:2259–2268. [PubMed] [Google Scholar]
- 22.Sterling P. Principles of allostasis: optimal design, predictive regulation, pathophysiology and rational therapeutics. In: Schulkin J, editor. Allostasis, Homeostasis, and the Costs of Adaptation. Cambridge: Cambridge University Press; 2004. [Google Scholar]
- 23.Shock NW. Physiological aspects of aging in man. Annu Rev Physiol. 1961;23:97–122. [Google Scholar]
- 24.CIBA Foundation Symposium. Research and the Ageing Population. Chichester UK: John Wiley & Sons Ltd; 1988. [Google Scholar]
- 25.Lakatta EG, Levy D. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises Part II: The Aging Heart in Health: Links to Heart Disease. Circulation: New Frontiers. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 26.Lakatta EG, Schulman SP, Gerstenblith G. Cardiovascular Aging in Health and Therapeutic Considerations with Respect to Cardiovascular Disease in Older Patients. In: Fuster V, Alexander RW, O’ Rourke RA, editors. Hurst’s The Heart. Vol. 86. McGraw-Hill; 2001. pp. 2329–2355. Chapter. [Google Scholar]
- 27.Wang M, Jiang L, Monticone RE, Lakatta EG. Proinflammation: the key to arterial aging. Trends Endocrinol Metabol. 2014;25:72–79. doi: 10.1016/j.tem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez-Ilasaca M, Liu X, Tamura K, Dzau VJ. The angiotensin II type I receptor-associated protein, ATRAP, is a transmembrane protein and a modulator of angiotensin II signaling. Mol Biol Cell. 2003;14(12):5038–5050. doi: 10.1091/mbc.E03-06-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min LJ1, Mogi M, Tamura K, Iwanami J, Sakata A, Fujita T, et al. Angiotensin II type 1 receptor-associated protein prevents vascular smooth muscle cell senescence via inactivation of calcineurin/nuclear factor of activated T cells pathway. J Mol Cell Cardiol. 2009;47(6):798–809. doi: 10.1016/j.yjmcc.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 31.de Cavanagh EMV, Inserra F, Ferder L. Angiotensin II blockade: a strategy to slow ageing by protecting mitochondria? Cardiovasc Res. 2011;89(1):31–40. doi: 10.1093/cvr/cvq285. [DOI] [PubMed] [Google Scholar]
- 32.Cencioni C, Spallotta F, Mai A, Martelli F, Farsetti A, Zeiher AM, et al. Sirtuin function in aging hearts and vessels. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2014.12.023. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Biala AK, Kirshenbaum LA. Mitochondrial dynamics: orchestrating the journey to advanced age. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Wang M, Zhang J, Spinetti G, Monticone R, Zhao D, Cheng L, et al. Angiotensin II Activates Metalloproteinase Type II and Mimics Age-Associated Carotid Arterial Remodeling in Young Rats. Am J Pathol. 2005;167:1429–1442. doi: 10.1016/S0002-9440(10)61229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saavedra JM. Angiotensin II AT(1) receptor blockers ameliorate inflammatory stress: a beneficial effect for the treatment of brain disorders. Cell Mol Neurobiol. 2012;32:667–681. doi: 10.1007/s10571-011-9754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michel JB, Heudes D, Michel O, Poitevin P, Philippe M, Scalbert E, et al. Effect of chronic ANG I-converting enzyme inhibition on aging processes. II. Large arteries. Am J Physiol. 1994 Jul;267(1 Pt 2):R124–R135. doi: 10.1152/ajpregu.1994.267.1.R124. [DOI] [PubMed] [Google Scholar]
- 37.Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007;293(3):H1351–H1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- 38.Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linz W, Heitsch H, Scholkens BA, Wiemer G. Long-Term Angiotensin II Type 1 Receptor Blockade With Fonsartan Doubles Lifespan of Hypertensive Rats. Hypertension. 2000;35:908–913. doi: 10.1161/01.hyp.35.4.908. [DOI] [PubMed] [Google Scholar]
- 40.Yu BP, Masoro EG, McMahan CA. Nutritional influences on aging of Fischer344 rats: I. Physical, metabolic, and longevity characteristics. J. Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 41.Thompson RC, Allam AH, Lombardi GP, Wann LS, Sutherland ML, Sutherland JD, et al. Atherosclerosis across 4000 years of human history: the Horus study of four ancient populations. Lancet. 2013 Apr 6;381(9873):1211–1222. doi: 10.1016/S0140-6736(13)60598-X. [DOI] [PubMed] [Google Scholar]
- 42.Brodde OE, Konschak U, Becker K, et al. Cardiac muscarinic receptors decrease with age. In vitro and in vivo studies. J Clin Invest. 1998;101:471. doi: 10.1172/JCI1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993;73:413. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- 44.Najjar SS, Lakatta EG, Gerstenblith G. Cardiovascular Aging: The Next Frontier in Cardiovascular Prevention. In: Blumenthal R, Foody J, Wong NA, editors. Prevention of Cardiovascular Disease: Companion to Braunwald’s Heart Disease. Philadelphia: Saunders; 2011. pp. 415–432. [Google Scholar]
- 45.Liu J, Sirenko S, Juhaszova M, Sollott SJ, Shukla S, Yaniv Y, et al. Age-associated abnormalities of intrinsic automaticity of sinoatrial nodal cells are linked to deficient cAMP-PKA-Ca2+ signaling. Am J Physiol Heart Circ Physiol. 2014;306:H1385–H1397. doi: 10.1152/ajpheart.00088.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monfredi O, Boyett MR. Sick sinus syndrome and atrial fibrillation in older persons—a view from the sinoatrial nodal myocyte. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Fendooni HA, Dibb KM, Howlett SE. How cardiomyocyte excitation, calcium release and contraction become altered with age. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2014.12.004. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Najjar SS, Schulman SP, Gerstenblith G, Fleg JL, Kass DA, O’Connor F, et al. Age and Gender Affect Ventricular-Vascular Coupling During Aerobic Exercise. J Am Coll Cardiol. 2004;144:611–617. doi: 10.1016/j.jacc.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 49.Yin FC, Weisfeldt ML, Milnor WR. Role of aortic input impedance in the decreased cardiovascular response to exercise with aging in dogs. J Clin Invest. 1981;68(1):28–38. doi: 10.1172/JCI110245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meerson FZ, Javich MP, Lerman MI. Dercrease in the rate of RNA and protein synthesis and degradation in the myocardium under long-term compensatory hyperfunction and on aging. J Mol Cell Cardiol. 1978;10:145–159. doi: 10.1016/0022-2828(78)90039-1. [DOI] [PubMed] [Google Scholar]
- 51.Lakatta EG. Regulation of cardiac muscle function in the hypertensive heart. In: Cox RH, editor. Cellular and Molecular Mechanisms of Hypertension. New York: EdPlenum; 1991. pp. 149–173. [DOI] [PubMed] [Google Scholar]
- 52.Boluyt MO, Lakatta EG. Cardiovascular Aging in Health. In: Altschuld RA, Haworth RA, editors. Heart Metabolism in Failure. 4B. JAI Press Inc; 1998. pp. 257–303. [Google Scholar]
- 53.Baker KM, Chernin MI, Wixson SK, Aceto JF. Renin angiotensin system involvement in pressure overload cardiac hypertrophy in rats. Am. J. Physiol. 1990;259:H324–H332. doi: 10.1152/ajpheart.1990.259.2.H324. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Dostal DE, Reiss K, Cheng W, Kajstura J, Li P, Huang H, Sonnenblick EH, Meggs LG, Baker KM, Anversa P. Identification and activation of autocrine renin-angiotensin system in adult ventricular myocytes. Am J Physiol. 1995;269:H1791–H1802. doi: 10.1152/ajpheart.1995.269.5.H1791. [DOI] [PubMed] [Google Scholar]
- 55.Heymes C, Swynghedauw B, Chevalier B. Activation of angiotensinogen and angiotensin-converting enzyme gene expression in the left ventricle of senescent rats. Circulation. 1994;90:1328–1332. doi: 10.1161/01.cir.90.3.1328. [DOI] [PubMed] [Google Scholar]
- 56.Sadoshima J, Xu Y, Slayter HS, lzumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 57.Schunkert H, Dzau V, Tang S, Hirsch A, Apstein C, Lorell B. Increased rat cardiac angiotensin-converting enzyme activity and mRNA expression in pressure overload left ventricular hypertrophy. J.Clin. Invest. 1990;86:1913–1920. doi: 10.1172/JCI114924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Makino N, Hata T, Sugano M, Dixon IMC, Yanaga T. Regression of hypertrophy after myocardial infarction is produced by the chronic blockade of angiotensin type-1 receptor in rats. J.MoL Cell. Cardiol. 1996;28:507–517. doi: 10.1006/jmcc.1996.0047. [DOI] [PubMed] [Google Scholar]
- 59.Reaven EP, Reaven GM. Structure and function changes in the endocrine pancreas of aging rats with reference to the modulating effects of exercise and caloric restrictions. J. Clin. Invest. 1981;68:75–84. doi: 10.1172/JCI110256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng MT, Kang M. Circadian rhythms and patterns of running-wheel activity, feeding and drinking behaviors of old rats. Physiol. Behav. 1984;33:615–620. doi: 10.1016/0031-9384(84)90380-9. [DOI] [PubMed] [Google Scholar]
- 61.Spurgeon HA, Steinbach MF, Lakatta EG. Chronic exercise prevents characteristic age-related changes in rat cardiac contraction. AJP. 1983;244:H513–H518. doi: 10.1152/ajpheart.1983.244.4.H513. [DOI] [PubMed] [Google Scholar]
- 62.Lakatta EG, Spurgeon HA. Effect of exercise on cardiac muscle performance in aged rats. FASEB. 1987;46:1844–1849. [PubMed] [Google Scholar]
- 63.Komatsu M, lsoyama S, Takishima T. Effects of aging on induction of cardiocyte by extracardiac factors. Am. J. Physiol. 1994;266:H2279–H2286. doi: 10.1152/ajpheart.1994.266.6.H2279. [DOI] [PubMed] [Google Scholar]
- 64.Udelsman R, Li D, Stagg C, Holbrook N. Aortic crosstransplantation between young and old rats: Effect upon the heat shock protein 70 stress response. J Geront Biol Sci. 1995;50A:B187–B192. doi: 10.1093/gerona/50a.4.b187. [DOI] [PubMed] [Google Scholar]
- 65.Seals DR, Justice JN, LaRocca TJ. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol. 2015 Jan 29; doi: 10.1113/jphysiol.2014.282665. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwak HB. Aging, exercise, and extracellular matrix in the heart. J Exerc Rehabil. 2013;9:338–347. doi: 10.12965/jer.130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vigorito C, Giallauria F. Effects of exercise on cardiovascular performance in the elderly. Front Physiol. 2014;5:51. doi: 10.3389/fphys.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corbi G, Conti V, Russomanno G, Rengo G, Vitulli P, Ciccarelli AL, Filippelli A, Ferrara N. Is physical activity able to modify oxidative damage in cardiovascular aging? Oxid Med Cell Longev. 2012;2012:728547. doi: 10.1155/2012/728547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santos-Parker JR, LaRocca TJ, Seals DR. Aerobic exercise and other healthy lifestyle factors that influence vascular aging. Adv Physiol Educ. 2014;38:296–307. doi: 10.1152/advan.00088.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith JG, Newton-Cheh CH. Genome-wide association studies of cardiovascular traits of aging. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blackmore HL, Ozanne SE. Programming of cardiovascular disease across the life-course. J Mol Cell Cardiol. 2015 doi: 10.1016/j.yjmcc.2014.12.006. epub ahead of print. [DOI] [PubMed] [Google Scholar]