Abstract
Recent neuroimaging work has observed activity in cortical midline structures (CMS) such as medial prefrontal and posterior cingulate cortices during self-referential processing. Moreover, items rated as self-relevant produce increased activity in these regions relative to items that are deemed not self-relevant. A common thread among previous reports has been reliance upon experimental tasks that encourage or require online self-referential processing. In this paper, we report findings from two experiments that manipulated requirements for self-reflection. In experiment one, subjects rated trait adjectives for social desirability and for self-relevance. Results revealed increasing activity in CMS with increasing self-relevance, but only during explicit ratings of self-relevance. In experiment two, we examined CMS activity during passive viewing of personal semantic facts (such as subjects’ own first names). Taken together, these results suggest that highly self-relevant information captures attention through neural mechanisms that are comparable to those engaged during explicit self-reflection, namely— via recruitment of CMS structures.
Keywords: medial prefrontal cortex, posterior cingulate cortex, fMRI, self-relevance, social cognitive neuroscience
Within this last decade, social neuroscience has made excellent strides in identifying brain regions that are involved in processing information about the self (Heatherton, Macrae & Kelley, 2004). Research using functional neuroimaging has demonstrated activity in cortical midline structures (CMS, Northoff and Bermpohl, 2004) such as medial prefrontal (MPFC) and medial parietal/posterior cingulate (PCC) cortices during self-referential processing (Craik et al., 1999, d’Argembeau et al., 2007; Fossati et al., 2003, 2004, Heatherton et al., 2006; Johnson et al., 2002, M. K. Johnson et al., 2006; S. C. Johnson et al., 2007, Kelley et al., 2002, Khircher et al., 2000, Kjaer et al., 2002, Lou et al., 2004, Macrae et al., 2004, Moran et al., 2006, Saxe et al., 2006, Pfeifer, Lieberman, & Dapretto, 2007, Phan et al., 2004, Schmitz et al., 2004, Schmitz and Johnson, 2006, Zhang et al., 2006, Zysset et al., 2002, 2003). In a large meta-analysis investigating self-referential processing, Northoff et al. (2006) concluded that activations cluster around three regions of the cortical midline: vMPFC, dMPFC and PCC. These results were obtained regardless of sensory presentation domain (auditory, visual, mental), and regardless of the conceptual/task domain (verbal, spatial, memory, emotional, facial, social, agency) used in any specific study. The findings of this meta-analysis strongly suggest a domain general role for these CMS in supporting the kinds of processing operations that give rise to a structured and relatively stable sense of self.
Work from our laboratory and others (Macrae et al., 2004; Phan et al., 2004; Moran et al., 2006) has revealed that CMS are more responsive to items that are endorsed as self-relevant relative to those endorsed as not self-relevant. This result is somewhat surprising, given that neuroimaging studies of self-referential processing thus far have relied on tasks that promote explicit judgments of self-relatedness of a given stimulus. Two explanations emerge for these findings. First, it is possible that highly self-relevant information, by its very nature, encourages further self-referential processing than does material that is not self-relevant. Alternatively, these regions may indeed be truly sensitive to item-to-item differences in self-relevance, in which case we would expect activity in the CMS to track with item-based self-relevance more generally, regardless of whether subjects are focusing on performing the self-referencing task. Interestingly, this hypothesis has yet to be tested, since the literature to date has not investigated self-relevance in an implicit manner, that is, in the absence of explicit instructions to relate individual items to the self.
Here we report two experiments that varied instructions to self-reflect as well as the extent to which the material was self-relevant for subjects. In Experiment one, we asked subjects to perform self-referencing and general semantic processing tasks on trait adjectives, obtaining self-relevance ratings on all trait adjectives. The goal of this experiment was to determine whether item self-relevance would modulate CMS activity during experimental conditions where subjects were engaged in a different cognitive task (implicit self-reflection). In Experiment two, we used canonically self-relevant material (such as subjects’ own names) to determine whether this class of information could modulate CMS activity in the absence of any goal-directed task (i.e., during passive viewing).
Materials and Methods
Participants
In Experiment 1, Tewnty-three participants between the ages of 18 and 31 (16 male, mean age = 20.2 years) were recruited from the local Dartmouth community. Twenty-seven subjects between the ages of 18 and 26 (15 male, mean age = 19.6) participated in Experiment 2. Subjects reported no significant abnormal neurological history, had normal or corrected-to-normal visual acuity, and were strongly right-handed as measured by the Edinburgh Handedness Inventory (Raczkowski et al., 1974). Subjects received course credit or were paid for their participation and gave informed consent in accordance with the guidelines set by the Committee for the Protection of Human Subjects at Dartmouth College. In both Experiment 1 and Experiment 2 respectively, four subjects were excluded from further analysis due to excessive head motion (>1mm between successive EPI acquisitions).
Functional imaging
Anatomical and functional whole-brain imaging was performed on a 3.0T Phillips Intera Achieva Scanner (Phillips Medical Systems, Bothell, WA). An Apple Powerbook G3 computer running PSYSCOPE V.1.2.5 (Cohen et al., 1993) was used for stimulus display. Anatomical images were acquired using a high-resolution 3-D magnetization prepared rapid gradient echo sequence (MP-RAGE; 160 sagittal slices, TE = 4.6 ms, TR = 9.9 ms, flip angle = 8°, 1 × 1 × 0.89 mm voxels). In Experiment 1, functional images were collected in six functional runs of 135 time points each. In Experiment 2, functional images were collected in five functional runs of 150 time points each. Both experiments used a fast field echo (FFE), echo-planar sequence sensitive to blood-oxygen level-dependent contrast (T2*) (Both experiments: 30 axial slices per whole-brain volume, 3mm in-plane resolution, 4mm thickness, 1mm skip, TR = 2000ms, TE = 35 ms, flip angle = 90°).
Procedure
Experiment One
In an event-related design, subjects viewed 540 trait adjectives taken from Anderson’s (1968) normed list. During the first three runs of functional imaging (270 word trials) subjects were required to indicate via a button press “How socially desirable is this trait?” on a scale from 1 (least) to 4 (most socially desirable). At the completion of run three, subjects were instructed to change their responses, by answering the question “How much does this trait describe me (the subject)?” for the remainder of the study. This presentation order was chosen in order to ensure that subjects did not self-reference when performing the social desirability judgment. Subjects used the same 4-point scale (1=least, 4=most) during the second half (runs four-six) of the study. Presentation of words was counterbalanced across subjects. Words were presented for 1250ms followed by a fixation crosshair for 750ms. Two hundred and seventy null events consisting of a fixation crosshair presented for 2000ms were pseudorandomly interspersed to introduce jitter into the fMRI time series.
Following scanning, subjects viewed all 540 trait adjectives a second time. Words that were judged for self-relevance during scanning warranted social desirability ratings on the post-scan task and vice versa. In this way, we were able to obtain a rating of social desirability and self-descriptiveness for each adjective from each subject. Further, each judgment category contained judgments made implicitly (post-scanning) and explicitly (during scanning). Figure one details the experimental procedure schematically.
Figure 1.
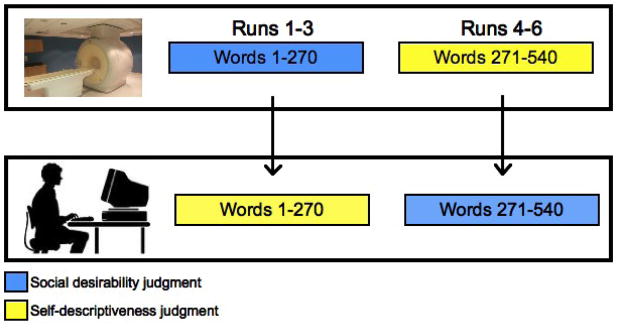
During scanning, subjects viewed all 540 trait adjectives in six functional runs. During runs one through three, they judged each adjective for its social desirability. During runs four through six, they judged adjectives for their self-descriptiveness. After the scan session, subjects viewed all 540 trait adjectives once more. This time, they judged adjectives 1–270 for their self-descriptiveness, and adjectives 271–540 for their social desirability. In this way, we are able to obtain both self-descriptiveness and social desirability ratings for each adjective for each subject.
This paradigm permitted two analyses. First, neural activity during explicit ratings of self-reference could be contrasted to explicit ratings of social desirability (SELF vs. SOCIAL). Second, we were further interested in investigating whether regions whose activity indexed self-descriptiveness during explicit judgments of self-reference would also do so in the absence of explicit task demands. To accomplish this, post-scan ratings of self-relevance were entered as a parametric regressor for trait adjectives that were endorsed for social desirability during scanning.
Experiment Two
During experiment two subjects viewed 500 words from 30 categories of personal semantic information. Items were categorized as either SELF (n=50, white font), NEUTRAL (n=400, white font) or ODDBALL (n=50, green font). Prior to scanning, participants completed a questionnaire probing categories of autobiographical information (e.g., ‘Father’s first name’, ‘hometown’, ‘phone number’, ‘initials’). Autobiographical categories were identical to those used in a similar event-related potential study by Gray and colleagues (2004). Participants were instructed to provide as many exemplars for each category as were appropriate (i.e., if the subject had two pets, to provide both pets’ names). On average, subjects provided 30 (±3 s.d.) responses. In order to increase the number of SELF items to 50 to facilitate analysis using the GLM, some responses were chosen at random to be presented twice. While repeated presentation is known to introduce repetition suppression effects in neuroimaging data (Buckner et al., 1995), it was felt that effects of this nature would bias against finding effects in support of the experimental hypothesis. More simply put, any significant effects would then be achieved under more conservative experimental conditions. Approximately 500 control stimuli were acquired by sampling lists of relevant exemplars from each category of information available on the worldwide web. Of those 500 stimuli, 50 were selected at random to appear as perceptual oddballs (displayed in green rather than white font). In order to ensure that no subject saw a self-relevant control item as part of the NEUTRAL or ODDBALL conditions, lists for these conditions were inspected and self-relevant items replaced with non self-relevant items. Participants were given simple task instructions to make a button press to each green word, thus ensuring their vigilance throughout the experiment. In addition, participants were told that their responses to the personal information questionnaire were to be included in the stimulus set in order to make the study more interesting. Words were presented for 1000ms followed by a fixation crosshair for 1000ms. 250 null events consisting of a fixation crosshair presented for 2000ms were pseudorandomly interspersed to introduce jitter into the fMRI time series. Of interest was neural activity in response to physically unique targets (external attention) and the self-relevant targets (internal attention).
Data Analysis
fMRI data were analyzed using the general linear model for event-related designs in SPM2 (Wellcome Department of Cognitive Neurology, London, UK). For each functional run, data were preprocessed to remove sources of noise and artifact. Functional data were corrected for differences in acquisition time between slices for each whole-brain volume, realigned within and across runs to correct for head movement, and transformed into a standard anatomical space (3-mm isotropic voxels) based on the ICBM 152 brain template (Montreal Neurological Institute) which approximates Talairach and Tournoux’s (1988) atlas space. Normalized data were then spatially smoothed (8mm full-width-at-half-maximum [FWHM]) using a Gaussian kernel. Analyses took place at two levels: formation of statistical images and regional analysis of hemodynamic responses.
In Experiment one, a general linear model incorporating task effects for SELF and SOCIAL task conditions, and covariates of no interest (a session mean, a linear trend, and six movement parameters derived from realignment corrections) was used to compute parameter estimates (β) and t-contrast images (containing weighted parameter estimates) for each comparison at each voxel and for each subject. Four parametric regressors were included to identify brain regions whose activity tracked linearly with EXPLICIT and IMPLICIT ratings of SELF-RELEVANCE and EXPLICIT and IMPLICIT ratings of SOCIAL DESIRABILITY. Explicit ratings of self-relevance and social desirability were those ratings that were obtained during the two scanning tasks, respectively. Implicit ratings were those ratings obtained following scanning and were used as an intrinsic self-relevance value for words that were judged for social desirability during scanning (IMPLICIT SELF) and as an intrinsic social desirability value for words that were judged for self-relevance during scanning (IMPLICIT SOCIAL).
Contrasts of interest were the comparison of SELF vs. SOCIAL trials, parametrically increasing self-relevance during SELF trials (EXPLICIT SELF), parametrically increasing self-relevance during SOCIAL trials (IMPLICIT SELF), parametrically increasing social desirability during SOCIAL trials (EXPLICIT SOCIAL), and parametrically increasing social desirability during SELF trials (IMPLICIT SOCIAL). These contrast images were then submitted to a second-level random effects analysis using one-sample t-tests, which compared weighted parameter estimates against zero for each comparison of interest, across all subjects.
In Experiment two, a general linear model incorporating task effects for SELF, ODDBALL and NEUTRAL conditions and covariates of no interest (a session mean, a linear trend, and six movement parameters derived from realignment corrections) was used to compute parameter estimates (β) and t-contrast images (containing weighted parameter estimates) for each comparison at each voxel and for each subject. Contrasts of interest were the comparison of SELF v. NEUTRAL trials, SELF v. ODDBALL trials, ODDBALL vs. NEUTRAL trials and ODDBALL vs. SELF trials. These contrast images were then submitted to a second-level random effects analysis using one-sample t-tests, which compared weighted parameter estimates against zero for each comparison of interest, across all subjects. All contrasts in this experiment were thresholded at a corrected False Discovery Rate (FDR; Genovese et al., 2002) of p < .05 and an extent threshold of 10 voxels.
For both experiments, regions of interest in medial prefrontal (BA10, [−6 54 9], MNI) and posterior cingulate (BA23/30, [−6 51 15], MNI) cortices were derived from coordinates described in Moran et al., (2006) which were obtained from a contrast of parametrically increasing self-relevance in that paper. Average parameter estimates for each condition relative to baseline fixation across these two regions were extracted for each subject and averaged together to give a measure of mean signal change from baseline for each task condition.
Experiment One Results
Behavioral Results
Two subjects were excluded from behavioral analysis as their reaction times during both tasks were more than two standard deviations from the mean. One further subject gave only ‘1’ and ‘4’ responses and was also excluded. Reaction times from the remaining 28 subjects were considered in a 2 (Task: SELF and SOCIAL) X 4 (Response: 1–4) repeated measures ANOVA (Figure 2). A main effect of Task (F(1,26) = 12.39, p < 0.001) revealed that subjects responded significantly slower when self-referencing than when rating adjectives for social desirability (F(1,26) = 12.29, p < 0.005, SELF: 1224±27ms, SOCIAL: 1172±27ms, mean±s.e.m.). A main effect of Response (F(3,78) = 34.17, p < 0.0001) on subjects’ reaction times revealed that subjects responded more slowly when giving a ‘2’ or ‘3’ response than when giving a ‘1’ or ‘4’ response (F(1,26) = 92.43, p < 0.0001) (‘1’ 1163±19ms; ‘2’ 1255±23ms; ‘3’ 1224±22ms; ‘4’ 1133±19ms, mean±s.e.m.). A significant interaction between TASK and RESPONSE (F(3,75) = 3.39, p < 0.05) was evidenced in shorter reaction times when making 1, 2, or 4 responses during SOCIAL vs. SELF trials (all Fs(1,26) > 7, all ps<.01) but not when making 3 responses (F<1).
Figure 2.
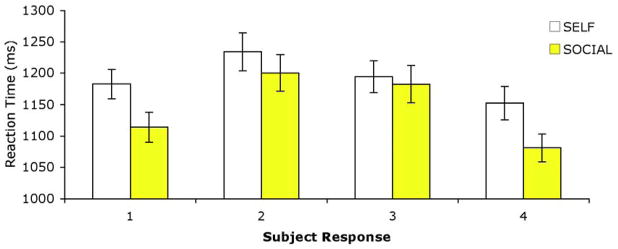
Response latencies were shorter for SOCIAL than SELF trials (p<0.0016). Response latencies were also shorter at the tail ends of the scale (1s and 4s quicker than 2s and 3s, p<0.0001) regardless of task. A significant interaction (p<.0025) between task and rating type revealed that subjects responded more quickly when making 1, 2 and 4 responses during SOCIAL vs. SELF judgments (all ps<0.01), but not when making 3 responses (p>0.05). Error bars indicate standard error of the mean (SEM).
Two separate ANOVAs considered the proportion of items afforded a given response during SELF and SOCIAL trials. A main effect of Response during SELF trials (F(3,81) = 32.09, p < 0.0001) revealed that subjects were more likely to endorse an item as either ‘2’ or ‘3’ than ‘1’ or ‘4’ (F(1,81) = 92.48, p < 0.0001), replicating findings from our previous study (Moran et al., 2006). A main effect of response during SOCIAL trials (F(3,81) = 7.48, p < 0.002) revealed again that subjects were more likely to endorse items as either ‘2’ or ‘3’ than ‘1’ or ‘4’ (F(1,81) = 18.91, p < 0.0001).
fMRI Results
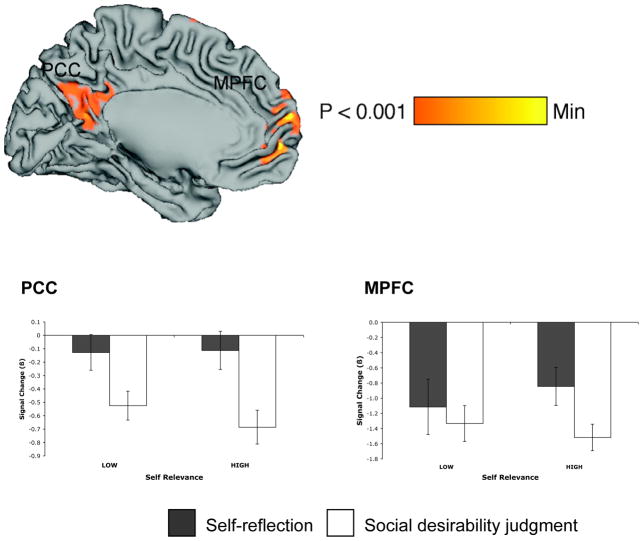
Contrasting self-referencing versus social desirability judgments (regardless of rating) revealed activity in posterior cingulate cortex (Brodmann’s area [BA] 30 [−6 −57 19]), medial prefrontal cortex (BA10 [−3 56 11] and [0 55 −5]), and in right superior frontal cortex (BA8 [21 38 50], see Figure 3A). Extracting signal change during these conditions from regions of MPFC and PCC defined in our previous study (Moran et al., 2006) revealed relatively greater activity during SELF relative to SOCIAL trials in both regions (Figure 3B). To investigate these activations further, A 2 (self-relevance) X 2 (explicit task) ANOVA was performed for both MPFC and PCC. This ANOVA revealed in MPFC a significant main effect of task (F(1,28) = 5.218, p < 0.05), such that activity was higher in this region for SELF relative to SOCIAL trials. Further, there was a significant interaction, such that activity was higher for high versus low self-relevant items (F(1,28) = 6.972, p < 0.05), but only during SELF trials. In PCC a main effect of task emerged (F(1,28) = 13.790, p < 0.001, but there was no significant interaction between task and self-relevance (F(1,28) = 2.576 p < 0.13).
Figure 3.
(A) Regions showing increased activity during self-referencing relative to social desirability rating. (B) Extracting parameter estimates from regions of MPFC and PCC defined in a contrast of increasing self-relevance in Moran et al. (2006) revealed increased activity in both regions during self-referencing relative to social desirability rating, and increased activity in MPFC with greater self-relevance, but only during self-referencing.
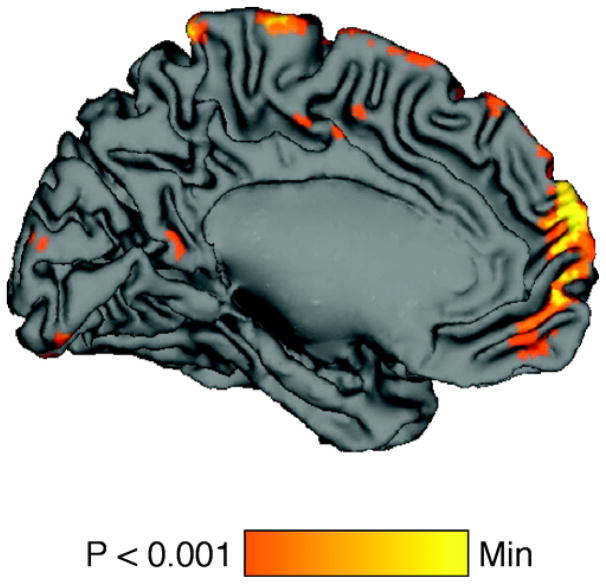
Next, we investigated regions whose activity increased in a linear fashion with increasing self-relevance ratings, either explicitly during the SELF judgment task or implicitly during the SOCIAL judgment task. Regions that showed a positive relationship with explicit self relevance ratings (p<.005, k=10) included medial prefrontal cortex (BA10 [−3 63 −3]) and left caudate nucleus ([−9 17 −6]) (Figure 4). Regions that increased their activity with implicit self-relevance included right globus pallidus ([18 −3 −2]) and medial frontal gyrus (BA9 [18 31 26]). Regions that showed this relationship with both explicit and implicit self-relevance were limited to the left caudate nucleus ([−12 17 −6]).
Figure 4.
Regions increasing their activity linearly with increasing self-relevance, while subjects rated items for their self-relevance (Explicit self-relevance ratings) included MPFC. Only the caudate nucleus showed this relationship with self-relevance during ratings of social desirability (Implicit self-relevance ratings, not shown).
Regions that increased their activity linearly with increasing social desirability during the SOCIAL task were limited exclusively to left insula ([−36 0 0]). No voxels increased their activity with implicit social desirability during the SELF task.
Experiment One Discussion
These findings both replicate and extend the extant literature on self-referencing and CMS activity. First, these results demonstrate that explicit self-referencing produces greater neural activity in both vMPFC and PCC relative to a control semantic task. Importantly, these results are not a function of task efficiency, as subjects were significantly slower to respond during SELF than SOCIAL trials. This finding argues against the notion that task-independent decreases in CMS are necessarily related to the degree of difficulty of the goal-directed task, regardless of that task’s nature, and is line with other reports of self-referential processing tasks (Kelley et al., 2002).
Secondly, our findings also replicate those of Moran et al., (2006) and Phan et al., (2004) in that MPFC activity during self-referencing increases linearly as a function of differences in individual item self-relevance. Further, a significant interaction between self-relevance and task condition in MPFC demonstrated that activity increased as a function of self-relevance in this region, but only during trials on which subjects explicitly self-referenced. Thirdly, CMS activity increases with self-relevance regardless of subjects’ attentional focus did not emerge. That is, when subjects were engaged in a task that did not encourage self-reflection, trait adjective self-relevance did not modulate CMS activity. This finding suggests that activity in CMS is not purely a function of item-to-item differences in self-relevance (in the case of trait adjectives), but may require explicit self-referencing.
Experiment 2 seeks to clarify these findings by using information that is overtly and potently self-relevant as stimuli. That is, it seems likely that subjects simply do not identify with trait adjectives at a general level. However, it seems possible that highly relevant personal information could produce self-referencing even during a task that does not explicitly demand self-referencing. To this end, we modified a paradigm previously used in an ERP study by Gray and colleagues (2004). Subjects viewed personal semantic facts that they had provided prior to the scanning session. These were intermixed with non-self-relevant items from the same categories of personal information. Some items were presented in a different colour font (oddball items). Subjects performed a simple vigilance task in which they responded when the presented word was shown in green rather than white font. This paradigm previously revealed increased attention, as measured with ERPs, when viewing both self-relevant and oddball items (increased P300 response). We expected to demonstrate increased activity in the CMS when subjects were passively viewing self-relevant stimuli relative to oddball and control stimuli. In addition, we expected to reveal activity in classical attention regions during the presentation of both self-relevant and oddball stimuli, mirroring the ERP findings of Gray and colleagues.
Experiment Two Results
Behavioral Results
Behavioral responses were not obtained from one subject due to equipment error. The results described in this section are from the remaining 22 participants. On average participants responded to 97.9% (±5%, s.d.) of oddball stimuli, with a mean reaction time of 614.23ms (±13.1ms, s.e.m.) indicating that participants were able to maintain strong vigilance throughout the experimental session.
fMRI Results
Contrasting ODDBALL trials against NEUTRAL trials revealed greater activity in lateral parietal (left, BA40 [−39 −47 52]; right, BA40 [60 −36 45]), inferior temporal (left [X Y Z]; right BA37 [45 −53 −15]) and in bilateral insular cortices [left [−36 15 0]; right [33 20 2]). Additional regions activated included left (BA9 [−56 7 27]) and right (BA9 [53 16 27]) dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC)/supplementary motor area (SMA) ([0 30 18] and [3 8 46] contiguous).
Contrasting ODDBALL trials against SELF trials revealed greater activity in M1 (BA4 [−50 −29 54]), ACC (BA24 [3 10 33]), bilateral posterior parietal cortex (left, BA40 [−39 −27 43]; right, BA40 [59 −39 32]) and bilateral insula (left, BA13 [42 9 0]; right, BA13 [−42 −3 −5]). These activations are detailed in Figure 5.
Figure 5.
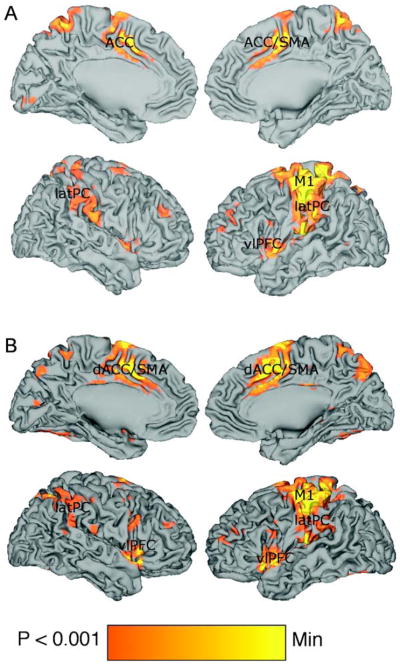
Brain activity during passive viewing of oddball stimuli relative to (A) neutral word stimuli and (B) self-relevant stimuli. Views of medial (top) and lateral (bottom) left and right hemispheres during both contrasts. ACC = anterior cingulate cortex; SMA = supplementary motor area; M1 = primary motor cortex; latPC = lateral parietal cortex; vlPFC = ventrolateral prefrontal cortex.
Contrasting SELF trials against NEUTRAL trials revealed greater activity in left (BA10 [−9 48 9]) and right (BA10 [9 50 10]) MPFC and in PCC (BA23 [−3, −25 29]), regions shown previously to be engaged during tasks that encourage self-referential processing. In addition to these regions, activity was also noted in left insular cortex (BA13 [−33 21 0]), lateral parietal cortex (left, BA40 [−36 −53 41]; right, BA40 [33 −62 39]), in DLPFC bilaterally (left, BA 9/44 [−53 16 32]; right [48 21 21]), SMA (BA8 [3 26 48]), ACC (BA25 [3 8 −8]) and bilateral caudate nucleus (left [−6 3 8]; right [9 9 2]).
Contrasting SELF trials against ODDBALL trials revealed activity in left MPFC (BA10 [−3 53 3]), dMPFC (BA9 [−9 57 30]), and PCC ([−13 −55 17]). Additional activations were observed in left inferior PFC (BA47 [−50 24 4]) and left posterolateral temporal cortex (BA21 [−59 −29 1]). These activations are detailed in Figure 6.
Figure 6.
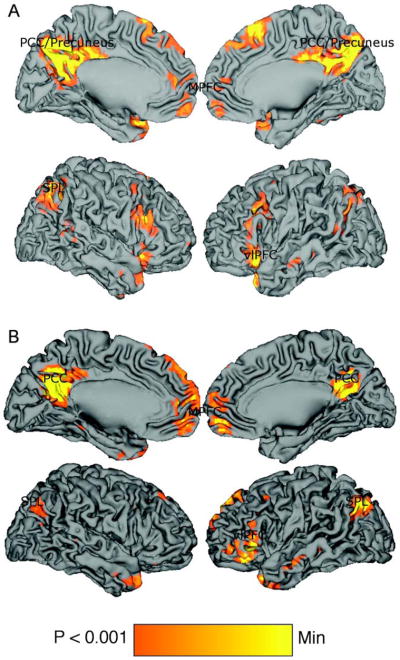
Brain activity during passive viewing of self-relevant stimuli relative to (A) neutral word stimuli and (B) oddball stimuli. Views of medial (top) and lateral (bottom) left and right hemispheres during both contrasts. MPFC = medial prefrontal cortex; PCC = posterior cingulate cortex; SPL = superior parietal lobule; vlPFC = ventrolateral prefrontal cortex.
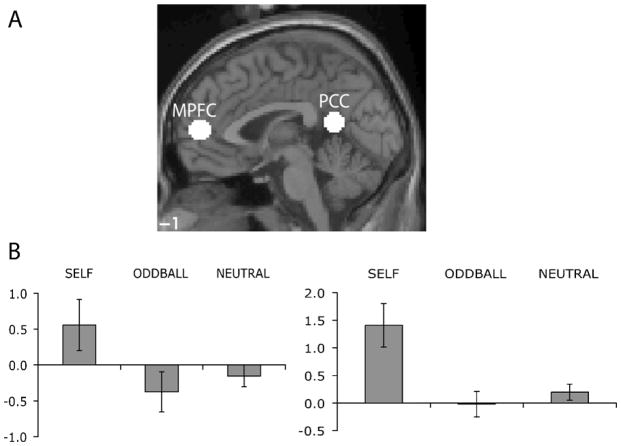
To investigate the nature of activity in these regions further, and to confirm the reproducibility of activations across differing task paradigms, we defined regions of interest based on a contrast of parametrically increasing self-relevance in our previous work (Moran et al., 2006). 8mm spheres were created around co-ordinates in MPFC (−6, 54, 9) and PCC (−6 51 15). Average parameter estimates across these regions and across subjects were submitted to separate one-way repeated measures ANOVAs with the factor CONDITION. Results from this region of interest analysis can be seen in Figure 7. A main effect of CONDITION in MPFC (F(2,44) = 6.98, p < .002) revealed that this region was reliably more active during SELF trials than during ODDBALL trials (planned comparison: F(1,22) = 11.437, p < .003), and during SELF than during NEUTRAL trials (planned comparison: F(1,22) = 8.169, p < .009), but that activity did not differ between ODDBALL and NEUTRAL trials (planned comparison: F < 1). A similar pattern of results in PCC (main effect of CONDITION: F(2,44) = 14.774, p < .0001) demonstrated that this region was significantly more active during SELF than ODDBALL trials (planned comparison: F(1,22) = 18.383, p < .0001), was significantly more active during SELF than NEUTRAL trials (planned comparison: F(1,22) = 17.814, p < .0001), but that activity did not differ between ODDBALL and NEUTRAL trials (planned comparison: F(1,22) = 368, p > 0.25). Interestingly, activity in both regions was higher during SELF trials than during rest, a finding which will be discussed in the context of the default mode literature.
Figure 7.
(A) Regions of MPFC and PCC were defined based on a contrast of increasing self-relevance in the report of Moran et al., 2006. (B) Extracting signal change from regions in MPFC (left) and PCC (right) revealed greater activity when viewing self-relevant stimuli than when viewing either neutral or oddball stimuli. Error bars represent s.e.m.
Experiment Two Discussion
Common activations assessed with a conjunction analysis across comparisons of SELF vs. NEUTRAL and SELF vs. ODDBALL trials were found in medial prefrontal (ventral and dorsal) and in posterior cingulate cortices. Activity in these regions has been noted across a number of functional imaging studies of self-referential processing, as reviewed in the meta-analysis by Northoff et al., (2006). Two factors not noted previously in the literature are that self-relevant material modulates CMS activity regardless of task demands (more specifically in the absence of any task), and that activity in vMPFC has deflected positively from a low-level fixation control condition (Gusnard and Raichle, 2001; Ingvar et al., 1979; Raichle et al., 2001; Shulman et al., 1997).
These findings indicate that CMS activity is not dependent upon explicit task demands. MPFC and PCC increased their activity in response to self-relevant information when subjects were instructed to view this information passively. Most strikingly, relative activations occurred here regardless of the comparison condition. The task and stimuli were identical in the SELF and NEUTRAL conditions (except for self-relevance), whereas the SELF and ODDBALL conditions differed further in that subjects were required to make a button press to green stimuli in the ODDBALL condition. These data support the notion that CMS are specifically involved in orienting to and manipulating self-relevant information. The fact that these activations are significant when comparing identical passive tasks (SELF vs. NEUTRAL) suggests that this responsiveness is not purely task-specific but is dependent upon the nature of incoming information. In previous self-referential processing studies activity is noted in these regions when subjects are asked to reflect upon the self-relevance of items such as trait adjectives, pictures, etc. These activations are noted regardless of the actual status of these items; PCC and MPFC respond when self-referencing whether the information is deemed self-relevant or not. In this study we have removed the demand for self-referencing and demonstrated that broadly equivalent stimuli produce very different activations depending on whether those stimuli are self-relevant. In a passive task such as this, it seems that the most pertinent explanation for these findings is that subjects are obligatorily self-referencing upon the presentation of self-relevant items. These effects in structures known to be involved in self-referencing are evidence that self-relevant information is able to grab attention regardless of current processing goals, and points towards the cortical midline structures as being the relevant neural substrate for this attention-grabbing effect.
The second striking fact about these findings is a positive deflection from a low-level baseline in PCC and MPFC. This finding is inconsistent with a very broad literature indicating that these regions typically decrease their activity when confronted with any task requiring external focus (Greicius et al., 2003; Greicius and Menon, 2004; Gusnard and Raichle, 2001; Raichle et al., 2001; Shulman et al., 1997; Wicker et al., 2003; but see Iacoboni et al., 2004 for increases in PCC and dMPFC while subjects watched social interactions). Wicker and colleagues go as far as to argue that “[b]y contrast, this [medial prefrontal] activity is greater during resting state than during both externally directed and internally directed attention.” (2003, p.224). This suggests that having subjects do anything other than rest (not just external tasks) would reduce activity in these regions. Passive viewing of self-relevant stimuli is undemanding, yet requires some external attention and the nature of the stimuli certainly encourages internal attention. Hence, during self-relevant items, we would expect to see lower activity relative to rest. How might positive going activation in both medial prefrontal and posterior cingulate cortices be best explained then? One possibility is that the nature of this study shifts functional activity during the control fixation task. Task demands in this study encouraged constant vigilance as subjects were expecting on each trial that there might be an oddball target. In a situation such as this, one might expect an effortful external focus of attention throughout the experimental session; that is, whatever processing occurs during typical passive rest conditions is attenuated as subjects continually monitor the external environment. Because of this, activity in default network regions likely decreased during the fixation trials and these regions appear to activate in response to self-relevant items.
General Discussion
The goals of the studies reported here were twofold: we attempted to extend the literature on self-referential processing by measuring brain activity when task demands did not require self-referential processing, and to characterize further how differences in item-to-item self-relevance affect neural activity. Results from these experiments argue that the main factor in activating CMS in experiments of self-related cognition is the degree to which subjects are self-referencing, whether that self-referencing is encouraged by task demands, or by the self-relevant nature of the stimuli themselves.
Recent theoretical advances suggest a functional distinction between dorsal and ventral aspects of MPFC (Northoff et al., 2004; Northoff et al., 2006). This position holds that ventral MPFC is engaged in the representation of stimuli as self-relevant, while dorsal MPFC is concerned with the evaluation of self-relevant stimuli. Activations in the present report were noted in ventral MPFC in experiment one, and in both dorsal (compared with oddball stimuli) and ventral MPFC (compared with both oddball and neutral stimuli) in experiment 2. Thus, it appears that both trait adjectives (in the context of explicit task demands) and personal semantic facts drive ventral MPFC in the representation of stimuli as self-relevant, whereas only personal semantic facts drive dorsal MPFC by their evaluation. These results, in the context of Northoff’s theoretical perspective, suggest that personal semantic facts encourage greater demands on the evaluative system than do trait adjectives, but that both types of stimuli warrant representation as self-relevant. Indeed, it is likely that personal semantic facts encourage other processes beyond those promoted by the processing of trait adjectives, such as autobiographical memory retrieval.
Recent functional imaging studies suggest a potential role for medial prefrontal cortex in maintaining attention to external stimuli (the “Gateway Hypothesis”, Burgess et al., 2005). It seems unlikely, given the present data and the consistency of findings linking MPFC with self-referential processing regardless of conceptual and perceptual domain, that this could be the case. Some have argued that MPFC activity attenuates whenever a goal-directed task of any kind is given (Raichle et al., 2001). The current data argue that MPFC activity is attenuated whenever the subject is not self-referencing: i.e., its activity did not differ between passive viewing of control stimuli and responding to green stimuli, but was attenuated during both, and not during a condition where the stimuli encouraged self-referential processing. We argue that baseline resting and self-referential processing then are potentially equivalent neurocognitive states; it seems that requiring self-referential processing or letting the subject decide for themselves what to think about alone will avoid attenuation of activity from baseline in MPFC. Further work to test this hypothesis might vary task difficulty while keeping stimuli equally self-referential between conditions. It appears from the current results, and many other behavioral studies before them (cf. Bargh, 1982), that subjects are obligatorily self-referencing in the presence of self-relevant information, and thus we would predict that MPFC would respond in both conditions, regardless of differences in task difficulty. Further work is needed, but a case is being built for the argument that MPFC activity serves to index access to the stream-of-consciousness.
More complex is the nature of PCC involvement. In experiment two, we showed that PCC activity mirrored that of MPFC. This region increased its activity in response to self-relevant information, and did not differ from baseline for either ODDBALL or NEUTRAL stimuli. Most, if not all (see Iacoboni et al., 2004) neuroimaging studies to date have reported task dependent decreases in PCC activity, such that its activity will attenuate when subjects are engaged in an external task. Again, we posit that the lack of negative deflection from baseline here represents a shift in activity during our fixation control task during this study. Since subjects were remaining in a state of constant vigilance, default mode network regions were less active during ‘baseline’. PCC activity has been noted most specifically during tasks that permit self-referential processing (for review see Northoff & Bermpohl, 2004; Northoff et al., 2006), and during memory tasks that encourage episodic or autobiographical retrieval (for review, see Cabeza and Nyberg, 2000). A recent review by Cavanna and Trimble (2006) of the anatomy, physiology and function of the precuneus and connected retrosplenial/posterior cingulate cortices noted that recent findings suggest a central role for the precuneus in tasks such as visuo-spatial imagery, episodic memory retrieval and self-processing operations. They argue further that the precuneus is involved in the interwoven network of the neural correlates of self-consciousness, and is engaged in self-related mental representations during rest. In an early PET study of focused (“taking a history”) and unfocused episodic memory retrieval the two regions commonly activated across both memory conditions were precuneus and medial inferior prefrontal cortex, suggesting that these activations “may reflect the time-linked components of both aspects of episodic memory, and [which] permit human beings to experience personal identity, consciousness, and self-awareness.” (Andreasen et al., 1995, p.1576). Further, Wagner and colleagues (2005) suggest that activity in PCC/precuneus may index differences in memory strength independent of emotional significance (e.g., remembering vs. knowing) and may function to shift attention towards the internally-generated representations that typically characterize episodic memories.
Taken together, these two strands of research converge nicely on the notion that these regions in medial parietal cortex are specifically engaged by retrieving and attending to personally salient episodes, in short, subserving autonoetic consciousness (Tulving, 1985). In other words, “explicit autonoetic consciousness is thought to emerge by retrieval of memory of personally experienced events (episodic memory)”, (Lou et al., 2004, p.6827). Lou and colleagues applied transcranial magnetic stimulation (TMS) to parietal cortex during memory retrieval. Speed and efficiency were affected only when the referent of retrieval was the self, and only when TMS was applied to parietal cortex. These results argue that posterior cingulate cortex activity reflects autonoetic consciousness and the manipulation of information and memories that are relevant to the self.
In this context, the interpretation of findings in our experiments is clear. Greater PCC involvement during SELF relative to ODDBALL and NEUTRAL trials (which did not differ from one another) in experiment two reflects the greater autobiographical memory retrieval and autonoetic consciousness processing encouraged by self-relevant material in the absence of specific task demands. Since trait adjectives are unlikely to encourage this type of processing unless task demands require it, there was no activity associated with self-relevance in the absence of self-referencing, during experiment one. Recent work by Johnson and colleagues (2006) suggests that activity in PCC during self-referential processing may be associated with experiential self-reflection, an interpretation entirely consistent with the pattern of findings from these two experiments.
The present experiments demonstrate specific involvement of medial prefrontal and posterior cingulate cortices in the processing of overtly self-relevant information in the absence of task demands. Bargh (1982; Ferguson and Bargh, 2004) has written persuasively and extensively on the nature of automatic attention to self-related stimuli. He assumes that “people develop automatic attention responses to self-relevant information” (1982, p.427). These findings in concert with other neuroimaging results suggest that Bargh’s automatic attention to self-relevant stimuli may be mediated by cortical midline structures that in part are responsible for ongoing attention to the stream of consciousness. Future research equating self-relevance but varying task difficulty across conditions should demonstrate involvement of these regions regardless of difficulty, strengthening the claim that self-referential processing and baseline resting are functionally equivalent neurocognitive states.
Acknowledgments
We thank T. Moran for her technical assistance. This work comprised part of JMM’s doctoral thesis at Dartmouth College, and JMM thanks committee members J.D.E. Gabrieli and P.J. Whalen for helpful comments throughout this work. This work was supported by the Dartmouth Brain Imaging Center.
References
- Anderson NH. Likeableness Ratings of 555 personality-trait words. Journal of Personality and Social Psychology. 1968;9:272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K. Remembering the past: Two facets of episodic memory explored with positron emission tomography. American Journal of Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Bargh JA. Attention and automaticity in the processing of self-relevant information. Journal of Personality and Social Psychology. 1982;43(3):425–436. [Google Scholar]
- Buckner RL, Petersen SE, Ojemann JG, Miezin FM, Squire LR, Raichle ME. Functional anatomical studies of explicit and implicit memory retrieval tasks. J Neurosci. 1995;15(1 Pt 1):12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Simons JS, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral PFC function. In: Duncan J, Phillips L, McLeod P, editors. Measuring the Mind: Speed, Control, and Age. Oxford University Press; Oxford: 2005. pp. 217–248. [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition: II. An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cohen J, Macwhinney B, Flatt M, Provost J. Psyscope - an interactive graphic system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behavior Research Methods Instruments & Computers. 1993;25(2):257–271. [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, et al. In search of the self: A positron emission tomography study. Psychological Science. 1999;10:26–34. [Google Scholar]
- d’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience. 2007;19:935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Ferguson MJ, Bargh JA. Liking is for doing: The effects of goal pursuit on automatic evaluation. Journal of Personality and Social Psychology. 2004;87:557–572. doi: 10.1037/0022-3514.87.5.557. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, et al. In search of the emotional self: An fMRI study using positive and negative emotional words. Am J Psychiatry. 2003;160:1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Lepage M, Graham SJ, Grady C, Keightley ML, et al. Distributed self in episodic memory: Neural correlates of successful retrieval of self-encoded positive and negative personality traits. Neuroimage. 2004;22:1596–1604. doi: 10.1016/j.neuroimage.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of Statistical Maps in Functional Neuroimaging Using the False Discovery Rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gray HM, Ambady N, Lowenthal WT, Deldin P. P300 as index of attention to self-relevant stimuli. Journal of Experimental Social Psychology. 2004;40:216–224. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: Uncoupled from deactivation but impacting activation. Journal of Cognitive Neuroscience,16. 2004:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Macrae CN, Kelley WM. What the social brain sciences can tell us about the self. Current Directions in Psychological Science. 2004;13(5):190–193. [Google Scholar]
- Heatherton TF, Wyland CW, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Soc Cogn Affect Neurosci. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Lieberman MD, et al. Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. NeuroImage. 2004;21(3):1167–1173. doi: 10.1016/j.neuroimage.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Ingvar DH. “Hyperfrontal” distribution of the cerebral grey matter flow in resting wakefulness; on the functional anatomy of the conscious state. Acta Neurol Scand. 1979;60:12–25. doi: 10.1111/j.1600-0404.1979.tb02947.x. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, et al. Dissociating medial frontal and posterior cingulate activity during self-reflection. Social Cognitive and Affective Neuroscience. 2006;1(1):56. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Ries ML, Hess TM, Carlsson CM, Gleason CE, Alexander AL, et al. Effect of Alzheimer disease risk on brain function during self-appraisal in healthy middle-aged adults. Arch Gen Psychiatry. 2007;64(10):1163–1171. doi: 10.1001/archpsyc.64.10.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Senior C, et al. Towards a functional neuroanatomy of self processing: effects of faces and words. Brain Res Cogn Brain Res. 2000;10(1–2):133–144. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, et al. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage. 2002;17(2):1080–1086. [PubMed] [Google Scholar]
- Lou HC, Luber B, et al. Parietal cortex and representation of the mental self. Proc Natl Acad Sci U S A. 2004;101(17):6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: An fMRI investigation. Neuroimage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Moran JM, Wig GS, Adams RB, Jr, Janata P, Kelley WM. Neural Correlates of Humor Detection and Appreciation. Neuroimage. 2004;21:1055–1060. doi: 10.1016/j.neuroimage.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical Evidence for Distinct Cognitive and Affective Components of Self. Journal of Cognitive Neuroscience. 2006;18:1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. “I know you are but what am I?!”: neural bases of self- and social knowledge retrieval in children and adults. J Cogn Neurosci. 2007;19(8):1323–1337. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Libezon I. Neural correlates of individual ratings of emotional salience: A trial-related fMRI study. Neuroimage. 2004;21:768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Kalat JW, Nebes R. Reliability and validity of some handedness questionnaire items. Neuropsychologia. 1974;12:43–47. doi: 10.1016/0028-3932(74)90025-6. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Moran JM, Scholz J, Gabrieli JDE. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Social Cognitive and Affective Neuroscience. 2006;1:229–234. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Self-appraisal decisions evoke dissociated dorsal-ventral aMPFC networks. Neuroimage. 2006;30:1050–1058. doi: 10.1016/j.neuroimage.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, et al. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. NeuroImage. 2004;22(2):941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. Common blood flow changes across visual tasks: Ii. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. In: Co-planar stereotaxic atlas of the human brain. Rayport M, editor. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–12. [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wicker B, Ruby P, Royet JP, Fonlupt P. A relation between rest and self in the brain. Brain Research and Review. 2003;43:224–230. doi: 10.1016/j.brainresrev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou T, Zhang J, Liu Z, Fan J, Zhu Y. In search of the Chinese self: An fMRI study. Science in China Series C. 2006;49(1):89–96. doi: 10.1007/s11427-004-5105-x. [DOI] [PubMed] [Google Scholar]
- Zysset S, Huber O, Ferstl E, von Cramon DY. The anterior frontomedian cortex and evaluative judgment: an fMRI study. Neuroimage. 2002;15:983–991. doi: 10.1006/nimg.2001.1008. [DOI] [PubMed] [Google Scholar]
- Zysset S, Huber O, Samson A, Ferstl EC, von Cramon DY. Functional specialization within the anterior medial prefrontal cortex: a functional magnetic resonance imaging study with human subjects. Neuroscience Letters. 2003;335:183–186. doi: 10.1016/s0304-3940(02)01196-5. [DOI] [PubMed] [Google Scholar]