Abstract
Introduction
Diabetes is one of the most common and fastest-growing comorbidities of pregnancy. Temporal trends in gestational diabetes mellitus (GDM) have not been examined at the state level. This study examines GDM prevalence trends overall and by age, state, and region for 19 states, and by race/ethnicity for 12 states. Sub-analysis assesses trends among GDM deliveries by insurance type and comorbid hypertension in pregnancy.
Methods
Using the Agency for Healthcare Research and Quality’s National and State Inpatient Databases, deliveries were identified using diagnosis-related group codes for GDM and comorbidities using ICD-9-CM diagnosis codes among all community hospitals. General linear regression with a log-link and binomial distribution was used in 2014 to assess annual change in GDM prevalence from 2000 through 2010.
Results
The age-standardized prevalence of GDM increased from 3.71 in 2000 to 5.77 per 100 deliveries in 2010 (relative increase, 56%). From 2000 through 2010, GDM deliveries increased significantly in all states (p < 0.01), with relative increases ranging from 36% to 88%. GDM among deliveries in 12 states reporting race and ethnicity increased among all groups (p < 0.01), with the highest relative increase in Hispanics (66%). Among GDM deliveries in 19 states, those with pre-pregnancy hypertension increased significantly from 2.5% to 4.1% (relative increase, 64%). The burden of GDM delivery payment shifted from private insurers (absolute decrease of 13.5 percentage points) to Medicaid/Medicare (13.2–percentage point increase).
Conclusions
Results suggest that GDM deliveries are increasing. The highest rates of increase are among Hispanics and among GDM deliveries complicated by pre-pregnancy hypertension.
Introduction
Diabetes is one of the most common and fastest-growing comorbidities of pregnancy.1,2 Gestational diabetes mellitus (GDM), defined as diabetes first diagnosed during pregnancy, has been associated with numerous adverse perinatal outcomes such as macrosomia, or larger-than-normal babies,3 which leads to difficult labor and delivery4 and maternal morbidity.5 The risk of developing GDM is increased in women with the following risk factors: age >25 years, non-white race, family history of diabetes,6 GDM in a previous pregnancy, chronic hypertension,7 high BMI, large abdominal circumference, high fasting glycemia in the first trimester of pregnancy, and the presence of polycystic ovary syndrome.8 Further, women who suffer from GDM as well as their offspring are also at higher risk for developing type 2 diabetes later in life.9 Consequently, pregnancies complicated by GDM need to be monitored closely for obstetric complications and adverse mother and infant outcomes.
In a previous study, analyses found that GDM rates differ by state; the variation was attributable to differences in obesity at the population level, age, race/ethnicity, hospital status, and health insurance status.10 Information on trends at the state level is needed to plan and focus healthcare services as well as to develop effective healthcare practices and policies for diabetes prevention and control. However, data on population-based, state-specific trends in GDM prevalence are limited. For states with available data, trends were assessed from 2000 through 2010 in GDM deliveries overall and by state, age, and race or ethnicity. Secondary analyses were conducted within the population of GDM deliveries to assess trends in GDM deliveries with comorbidities such as pre-eclampsia and pre-pregnancy hypertension, and funding of GDM deliveries by type of health insurance.
Methods
Study Sample
Data were used from the 2000–2010 State Inpatient Databases (SID), sponsored by the Agency for Healthcare Research and Quality to identify hospital discharges involving diabetes diagnosed during pregnancy.11 The databases contain information on hospital inpatient stays from all community hospitals in states participating in the Healthcare Cost and Utilization Project (HCUP) and are largely a census rather than a sample of those hospitals; they account for approximately 86% of hospitalizations nationally. Annual data collection by the databases includes the 19 participating states with data from 2000 through 2010: Arizona, California, Colorado, Florida, Hawaii, Iowa, Kentucky, Massachusetts, Maryland, Michigan, North Carolina, New Jersey, New York, Oregon, South Carolina, Utah, Washington, Wisconsin, and West Virginia.
Community hospitals are defined as short-term, nonfederal, general, and other hospitals, excluding hospital units of other institutions (e.g., prisons).12 Community hospitals (and HCUP data) include obstetrics–gynecology, ear–nose–throat, orthopedic, cancer, pediatric, public (e.g., county hospitals), and academic medical hospitals (e.g., university hospitals). Some states exclude hospitals that mainly focus on long-term care or psychiatric, alcoholism, or chemical-dependency treatment, although discharges from units of these types that are part of community hospitals are included. Although not all states include these types of hospitals, the numbers of deliveries from them are few; therefore, any differences in the population of deliveries would be minimal.
Hospital delivery discharge codes were identified using ICD-9-CM diagnosis codes or diagnosis-related group (DRG) codes. DRGs comprise a patient classification system that categorizes hospital stays into groups that are clinically similar with respect to resource use, including diagnosis and type of treatment or procedure. Each hospital stay has one DRG assigned to it. Delivery stays were identified by discharges having a DRG code of 767–768 and 774–775 (vaginal delivery) or 765–766 (cesarean [C]-section) during 2008–2010, or a DRG code 372–375 (vaginal delivery) or 370–371 (C-section) during 2000–2007.
Deliveries were identified with a GDM diagnosis by the presence of ICD-9-CM codes 648.8x listed anywhere on the discharge record. Cases that listed both codes for GDM and for pre-pregnancy diabetes were excluded (n=7,725; <1%).
Measures
Examined variables included maternal age; race/ethnicity; expected primary health insurance; and two associated comorbidities, pre-eclampsia and pre-pregnancy hypertension. Race/ethnicity categories were non-Hispanic (NH) white, NH black, Hispanic, and NH Asian/Pacific Islander. Expected primary health insurance payer categories included private; Medicaid/Medicare; uninsured; or other government insurance such as Worker’s Compensation, Civilian Health and Medical Program of the Uniformed Services, Civilian Health and Medical Program of the Department of Veterans Affairs, Title V, or other government program. Pre-eclampsia was defined by the presence of ICD-9-CM codes 642.3x–642.6x listed anywhere on the discharge record, and pre-pregnancy hypertension by ICD-9-CM codes 642.0x, 642.2x, 642.7x, 401.0x, 401.1x, 401.9x, 437.2x, or 402.xx–405.xx13 listed anywhere on the discharge record. Medicaid and Medicare were combined, though deliveries expected to be funded by Medicare comprised only about 1% of the total. Pre-pregnancy BMI was not available on maternal hospital discharges and therefore was not included. Data were not reported for California in 2002 or for Hawaii in 2005, and seven states did not report race or ethnicity during the entire study period (Iowa, Kentucky, North Carolina, Oregon, Utah, Washington, and West Virginia).
Statistical Analysis
Age-standardized rates of GDM were estimated for each state, region, and the selected subgroups, using the national number of deliveries from 2000 (i.e., the Nationwide Inpatient Sample [NIS] from HCUP) for age standardization. For the 12 states with race/ethnicity, age- and race- standardized rates were computed using data from the 2000 NIS. A subanalysis was also conducted to assess trends within the population of GDM deliveries. Those analyses included assessing trends among women with GDM in comorbidities such as pre-eclampsia and pre-pregnancy hypertension as well as changes in funding of GDM deliveries by insurance type. SAS, version 9.3, and SUDAAN, version 11.0.0, were used for data management and analyses to produce estimates. The number of deliveries was obtained by DRG codes from the HCUPnet online query system11 for California in 2002 and Hawaii in 2005, and, using PROC MI in SAS, all variables were imputed for those years based on data (e.g., race/ethnicity, age, GDM, pre-pregnancy diabetes status, insurance type, pre-eclampsia, pre-pregnancy hypertension) from other years within the same state. Less than 1% of data were missing for variables other than race/ethnicity for the rest of the states and years but, for consistency, those were also imputed. The one exception was among the 12 states reporting race and ethnicity: race and ethnicity were missing on average 26.2% per year and were imputed from available data in the same state. Potential bias due to the large proportion with missing race was assessed with a sensitivity analysis comparing complete case rates of GDM deliveries with imputed rates. General linear regression with a log-link and binomial distribution was used in 2014 to assess the annual change over 11 years from 2000 through 2010 and to test for a statistically significant change in prevalence over time. Because the change in total number of deliveries is informative about the change in GDM prevalence over time, statistically significant annual changes in total number of deliveries by characteristics assessed are presented. For example, if GDM prevalence is increasing significantly, knowing whether the total number of deliveries dropped, remained constant, or increased in relation to increase in GDM deliveries is more informative than only reporting change in GDM prevalence. This study was reviewed by the Human Subjects Coordinator at CDC and, as an analysis of secondary data without identifiers, was determined to be exempt from IRB review.
Results
Overall, the age-standardized prevalence of GDM deliveries in the 19 states increased significantly from 3.71 in 2000 to 5.77 per 100 deliveries in 2010 (relative change, 56%; annual change, p < 0.01) (Table 1). The greatest relative increase was seen in Utah (1.95 to 3.66 per 100 deliveries; relative increase, 88%); the smallest increase was seen in Maryland (4.26 to 5.80 per 100 deliveries; relative increase, 36%). In the last 2 years, four of the five states with the highest prevalence of GDM (≥6.00 per 100 births) were in the Western region, including California, Oregon, Washington, and Hawaii. The four regional trends of GDM prevalence increased significantly as well (p < 0.001). The Midwestern and Western regions increased more (relative increases of 69% and 64%, respectively) than the Northern and Southern regions (relative increases of 44% and 47%, respectively).
Table 1.
Prevalence of Gestational Diabetes Among Deliveries in 19 States, HCUP SID 2000–2010
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Absolute change |
Relative increase (%) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per 100 deliveries | 00–10 | 00–10 | |||||||||||
| Total 19 states | 3.71 | 4.03 | 4.41 | 4.29 | 4.52 | 4.74 | 5.20 | 5.50 | 5.49 | 5.67 | 5.77 | 2.06 | 56 |
| States by region | |||||||||||||
| North | 3.61 | 3.85 | 3.93 | 3.99 | 4.28 | 4.50 | 5.04 | 5.17 | 5.09 | 5.22 | 5.20 | 1.59 | 44 |
| Marylanda | 4.26 | 4.24 | 4.14 | 4.38 | 4.89 | 5.18 | 6.06 | 6.18 | 5.88 | 6.14 | 5.80 | 1.54 | 36 |
| Massachusettsa | 3.21 | 3.41 | 3.43 | 3.54 | 3.84 | 3.84 | 4.05 | 4.44 | 4.34 | 4.71 | 4.68 | 1.47 | 46 |
| New Jerseya | 3.41 | 3.62 | 3.74 | 3.93 | 4.04 | 4.57 | 5.27 | 5.14 | 4.96 | 5.07 | 4.92 | 1.51 | 44 |
| New Yorka | 3.68 | 4.02 | 4.15 | 4.00 | 4.34 | 4.44 | 4.88 | 5.05 | 5.09 | 5.08 | 5.30 | 1.62 | 44 |
| South | 3.86 | 4.16 | 4.60 | 4.44 | 4.78 | 4.84 | 5.39 | 5.56 | 5.64 | 5.46 | 5.69 | 1.83 | 47 |
| Floridaa | 3.66 | 4.05 | 4.79 | 4.44 | 4.70 | 4.76 | 5.21 | 5.42 | 5.32 | 5.24 | 5.25 | 1.59 | 43 |
| Kentucky | 3.63 | 4.01 | 4.24 | 3.86 | 4.34 | 4.54 | 4.97 | 5.34 | 5.84 | 5.99 | 6.40 | 2.77 | 76 |
| North Carolina | 4.22 | 4.27 | 4.34 | 4.50 | 4.92 | 4.85 | 5.59 | 5.80 | 5.81 | 5.48 | 5.89 | 1.67 | 40 |
| South Carolinaa | 4.01 | 4.38 | 4.75 | 4.57 | 5.07 | 5.29 | 5.90 | 5.86 | 6.30 | 5.94 | 6.18 | 2.17 | 54 |
| West Virginia | 3.95 | 4.68 | 4.94 | 4.95 | 5.28 | 5.44 | 6.40 | 5.61 | 5.65 | 5.04 | 5.83 | 1.88 | 48 |
| Midwest | 3.37 | 3.72 | 3.89 | 4.02 | 4.17 | 4.42 | 4.68 | 5.01 | 5.01 | 5.26 | 5.69 | 2.32 | 69 |
| Iowa | 3.37 | 3.66 | 3.85 | 3.91 | 4.04 | 4.15 | 4.63 | 4.84 | 4.93 | 5.38 | 5.76 | 2.39 | 71 |
| Michigana | 3.41 | 3.84 | 3.98 | 4.22 | 4.39 | 4.68 | 5.01 | 5.47 | 5.51 | 5.63 | 5.95 | 2.54 | 74 |
| Wisconsina | 3.28 | 3.52 | 3.71 | 3.72 | 3.85 | 4.13 | 4.12 | 4.32 | 4.22 | 4.59 | 5.23 | 1.95 | 59 |
| West | 3.79 | 4.14 | 4.36 | 4.52 | 4.69 | 4.95 | 5.37 | 5.81 | 5.82 | 6.19 | 6.22 | 2.43 | 64 |
| Arizonaa | 3.29 | 3.87 | 3.62 | 3.57 | 3.69 | 3.99 | 4.73 | 4.97 | 5.00 | 4.99 | 5.09 | 1.80 | 55 |
| Californiaa | 4.10 | 4.43 | 4.77 | 4.84 | 5.09 | 5.42 | 5.83 | 6.36 | 6.38 | 6.81 | 6.80 | 2.70 | 66 |
| Coloradoa | 2.69 | 2.96 | 3.87 | 4.19 | 3.59 | 3.44 | 3.99 | 4.32 | 4.03 | 4.28 | 4.25 | 1.56 | 58 |
| Hawaiia | 4.79 | 6.47 | 6.91 | 6.69 | 6.20 | 7.11 | 6.45 | 6.99 | 6.55 | 7.46 | 7.62 | 2.83 | 59 |
| Oregon | 3.63 | 3.71 | 4.17 | 3.72 | 3.99 | 4.19 | 4.53 | 5.19 | 5.24 | 6.05 | 6.51 | 2.88 | 79 |
| Utah | 1.95 | 2.07 | 2.20 | 2.17 | 2.37 | 2.69 | 3.06 | 3.15 | 3.17 | 3.53 | 3.66 | 1.71 | 88 |
| Washington | 3.86 | 4.31 | 4.77 | 4.92 | 5.32 | 5.60 | 5.82 | 6.07 | 6.05 | 6.37 | 6.24 | 2.38 | 62 |
Note: For California in 2002 and Hawaii in 2005, the number of deliveries was obtained from HCUPnet; all other variables for those two states for those 2 years were imputed from the other years’ data from those states.
The numerator used was the number of delivery discharges with a gestational diabetes mellitus diagnosis by the presence of ICD-9-CM codes 648.8x listed anywhere on the discharge record.
The denominator used was the number of delivery discharges having a diagnosis-related group (DRG) code of 767–768 and 774–775 (vaginal delivery) or 765–7766 (C-section) during 2008–2010 or a DRG code 372–375 (vaginal delivery) or 370–371 (C-section) during 2000–2007 listed on the discharge record.
All rates are age standardized to the 2000 populations of deliveries from HCUP NIS.
Indicates state had race/ethnicity data.
HCUP, Healthcare Cost and Utilization Project; NIS, Nationwide Inpatient Sample; SID, State Inpatient Databases.
GDM rates for all age groups in all 19 states increased significantly (p < 0.01) (Table 2). The greatest relative increase in GDM deliveries occurred among those aged 15–24 years (range, 59%–66%). Among age groups, the absolute increase in GDM deliveries followed a dose response, increasing with older age.
Table 2.
Prevalence of Gestational Diabetes Among Deliveries by Selected Characteristics in 19 States, HCUP SID 2000–2010
| 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Abs Chg | Relative increase | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per 100 births | |||||||||||||
| No. births (in 000’s) | 1993 | 1986 | 1948 | 2013 | 2034 | 2049 | 2116 | 2129 | 2050 | 2024 | 1961 | −32 | −1 |
| No. with gestational diabetes (in 000’s) | 75 | 82 | 85 | 89 | 96 | 101 | 113 | 120 | 116 | 119 | 119 | 44 | 59 |
| Age group (crude), years | |||||||||||||
| 15–19 | 0.97 | 1.05 | 1.46 | 1.14 | 1.20 | 1.25 | 1.38 | 1.56 | 1.47 | 1.50 | 1.61 | 0.64 | 66 |
| 20–24 | 1.97 | 2.14 | 2.40 | 2.27 | 2.39 | 2.50 | 2.82 | 3.04 | 2.98 | 3.04 | 3.13 | 1.16 | 59 |
| 25–29 | 3.52 | 3.83 | 4.01 | 4.06 | 4.27 | 4.43 | 4.83 | 5.13 | 5.07 | 5.27 | 5.40 | 1.88 | 53 |
| 30–34 | 4.94 | 5.36 | 5.85 | 5.74 | 6.11 | 6.45 | 7.07 | 7.42 | 7.45 | 7.65 | 7.65 | 2.71 | 55 |
| 35–39 | 6.81 | 7.34 | 8.17 | 7.87 | 8.22 | 8.58 | 9.45 | 9.91 | 10.01 | 10.44 | 10.68 | 3.87 | 57 |
| 40–44 | 9.05 | 9.78 | 10.65 | 10.18 | 10.87 | 11.42 | 12.19 | 12.83 | 13.22 | 13.49 | 13.85 | 4.80 | 53 |
| Race/ethnicitya | |||||||||||||
| Non-Hispanic white | |||||||||||||
| Crude | 3.45 | 3.74 | 3.92 | 4.02 | 4.20 | 4.42 | 4.79 | 5.01 | 5.02 | 5.22 | 5.30 | 1.85 | 54 |
| Age standardized | 3.44 | 3.74 | 4.20 | 4.03 | 4.20 | 4.42 | 4.79 | 5.03 | 5.01 | 5.20 | 5.29 | 1.85 | 54 |
| Hispanic | |||||||||||||
| Crude | 4.93 | 5.39 | 5.72 | 5.87 | 6.20 | 6.41 | 7.05 | 7.46 | 7.62 | 8.09 | 8.21 | 3.28 | 67 |
| Age standardized | 4.95 | 5.42 | 4.91 | 5.87 | 6.19 | 6.44 | 7.05 | 7.43 | 7.64 | 8.10 | 8.23 | 3.28 | 66 |
| Non-Hispanic black | |||||||||||||
| Crude | 3.76 | 4.18 | 4.59 | 4.55 | 4.74 | 4.99 | 5.34 | 5.85 | 5.47 | 5.53 | 5.75 | 1.99 | 53 |
| Age standardized | 3.78 | 4.12 | 4.39 | 4.52 | 4.76 | 4.98 | 5.35 | 5.84 | 5.48 | 5.56 | 5.76 | 1.98 | 53 |
| Non-Hispanic Asian/Pacific Islander | |||||||||||||
| Crude | 6.50 | 7.32 | 7.64 | 7.37 | 7.67 | 8.19 | 8.65 | 8.80 | 9.08 | 9.48 | 10.29 | 3.79 | 58 |
| Age standardized | 6.48 | 7.33 | 7.59 | 7.36 | 7.69 | 7.97 | 8.62 | 8.77 | 9.07 | 9.53 | 10.27 | 3.79 | 58 |
| Age and race standardizeda | 4.50 | 4.95 | 5.12 | 5.15 | 5.38 | 5.60 | 6.12 | 6.38 | 6.37 | 6.58 | 6.75 | 2.25 | 50 |
Note: All rates are age standardized to the 2000 populations of deliveries from HCUP NIS unless identified as “crude.”
Includes 12 states with race/ethnicity data each year.
HCUP, Healthcare Cost and Utilization Project; NIS, Nationwide Inpatient Sample; SID, State Inpatient Databases.
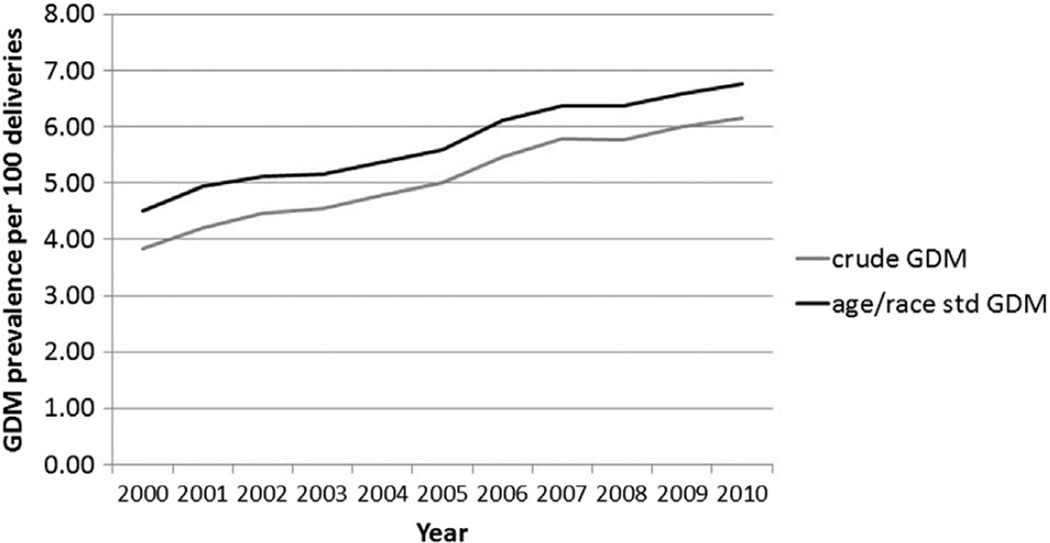
GDM among deliveries increased significantly (p < 0.01) among all race/ethnicity groups in the 12 states reporting race and ethnicity, with the highest relative increase in Hispanics (66%). Yet, throughout the study time period, NH Asians had the highest prevalence of GDM (range, 6.48%–10.27%). Age- and race-standardized GDM rates were consistently higher than crude GDM rates in these 12 states (Figure 1). Complete case rates of GDM deliveries were consistently lower than imputed rates for all race/ethnicity groups. This difference was nearly identical for NH whites, Hispanics, and NH Asians (within 9% on average). For NH blacks, the complete case rates were much closer to the imputed rates (within <1% on average).
Figure 1.
Crude and age- and race-standardized rates of gestational diabetes mellitus (GDM), 12 states: 2000–2010.
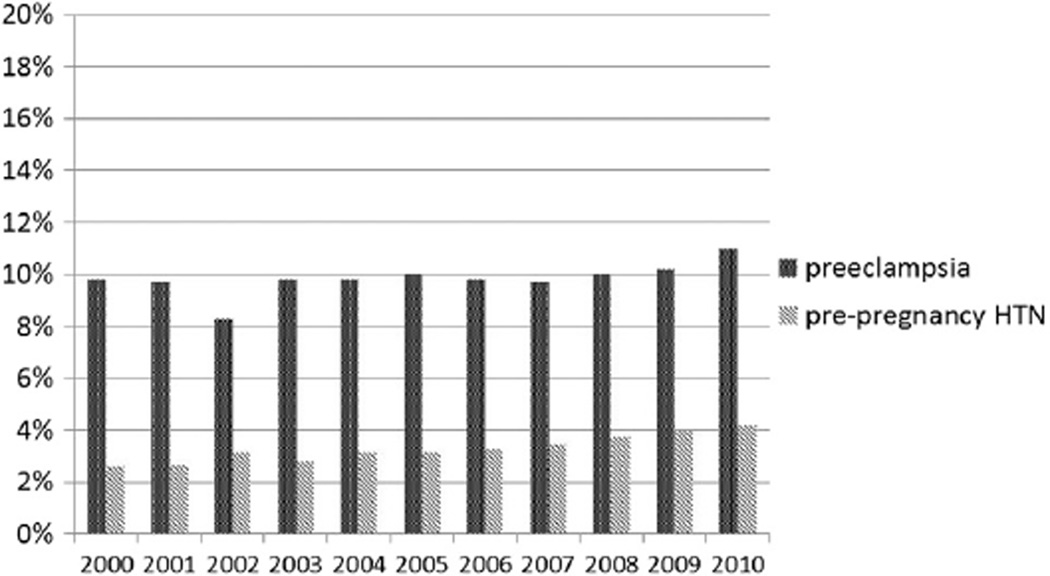
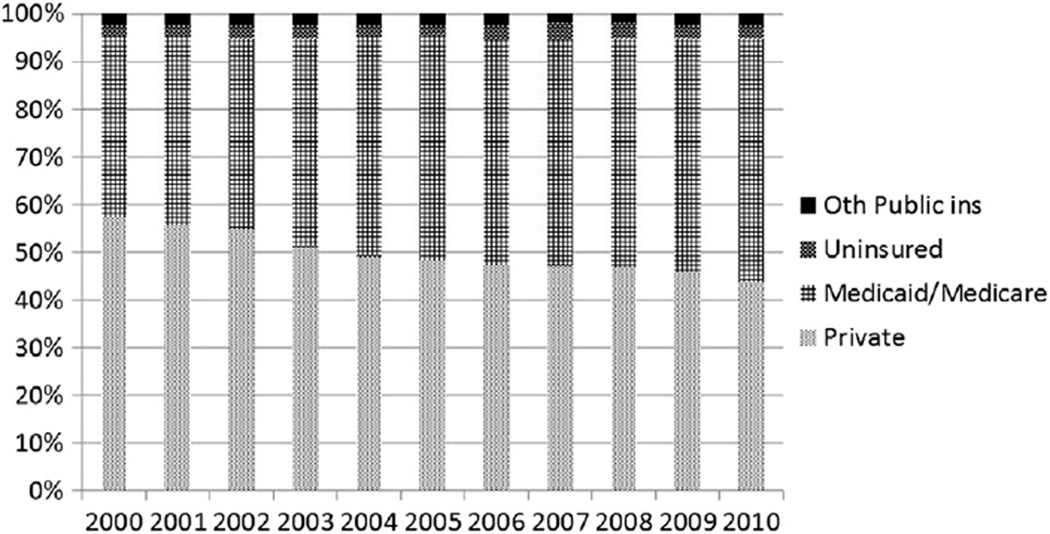
From 2000 through 2010, among GDM deliveries, the proportion with comorbidities increased significantly: pre-pregnancy hypertension from 2.5% to 4.1% (relative increase, 64%) and pre-eclampsia from 9.8% to 11.0% (relative increase, 12%) (Figure 2). The prevalence of Medicaid/Medicare funding of GDM deliveries increased from 37.6% to 50.8% (absolute increase, 13.2 percentage points) (p < 0.01), and the prevalence of private insurers funding GDM deliveries decreased from 57.5% to 44.0% (absolute decrease, 13.5 percentage points) (p < 0.01) (Figure 3).
Figure 2.
Proportion of gestational diabetes mellitus deliveries with hypertension, 19 states: 2000–2010.
Figure 3.
Proportion of gestational diabetes mellitus deliveries by expected primary payer, 19 states: 2000–2010.
The number of GDM deliveries increased significantly (p < 0.001) from 75,212 in 2000 to 119,229 in 2010 in the 19 states (relative increase, 59%). Over the 11-year period, significant increases (p < 0.05) in the total number of deliveries occurred among women aged 25–29 years (relative increase, 3%) and 40–44 years (relative increase, 19%); Hispanic (relative increase, 18%) and NH Asian/Pacific Islander women (relative increase, 32%); and deliveries with Medicare/Medicaid (relative increase, 31%). Conversely, statistically significant decreases occurred in the total number of deliveries among NH whites (relative decrease, 11%) and women with private insurance (relative decrease, 24%).
Discussion
The results of this study suggest that GDM delivery rates increased across all demographic groups (e.g., age, race/ethnicity). State and regional differences in GDM prevalence may reflect race and ethnicity differences in deliveries overall. Western states had the highest prevalence of GDM deliveries and also had the highest proportion of Asians.14 Throughout the study period, the highest rates of GDM were found among NH Asian and Pacific Islander women. Studies have shown that even at normal or low BMI, Asians have much higher risk of GDM compared with their NH white counterparts.15 Notably, the rate of GDM in NH Asian women is increasing more because of the much faster pace of increase in GDM deliveries (numerator) compared with that of the total deliveries (denominator), as the latter has also increased significantly.
Among the 12 states, with race/ethnicity, the age- and race-standardized rates were consistently higher than the crude rates, probably because of higher prevalence among older and non-white women. The highest relative increase in GDM by race/ethnicity was among Hispanics and may be related to increases in obesity prevalence during 1999–2008, which were greater among Mexican American women aged 20–39 years (from 30.6% to 39.6%) compared to black women (from 46.2% to 47.2%) and white women (from 28.4% to 34.0%).16 Also, the greatest absolute increase in GDM was in the West, the region with the highest proportion of Hispanics in 2010 (28.6%).17 The annual increase in number of deliveries (denominator) was statistically significant among Hispanic women, meaning that the increase in GDM among Hispanic women (numerator) was even greater than the increase in deliveries.
GDM deliveries have become more complicated as those with comorbidities such as pre-pregnancy hypertension and pre-eclampsia have increased. Pre-eclampsia is a leading cause of maternal and perinatal mortality and morbidity,18 and hypertensive disorders during pregnancy are major contributors to prematurity, small-for-gestational-age status, poor Apgar scores, C-sections, stillbirths, and early neonatal deaths.19 In addition, both the mother and infant are at increased risk for future cardiovascular disease and type 2 diabetes.20
GDM disproportionately burdens those with lower SES,21 and this may add to demands on publicly funded healthcare programs. Throughout the study period, the proportion of GDM deliveries funded by Medicaid/Medicare increased significantly. In addition, the annual increase in the number of deliveries funded by Medicaid/Medicare (denominator) was statistically significant; this means that the increase in GDM publicly funded deliveries (numerator) was even greater than the increase in the number of deliveries among women on Medicaid/Medicare. Starting in 1991, all state Medicaid programs were required to cover pregnant women with incomes <133% of the federal poverty level.22 Since 2000, there was a shift in GDM deliveries from being funded by private insurance to being funded by Medicaid/Medicare. The increase in GDM deliveries with comorbid conditions will likely impact the cost to insurers, and this may be an important area for further research.
This study’s strengths include data that were population-based, available at a state level, and reflected trends over an 11-year period. Although all states were not represented and, therefore, results could not be generalized to the U.S., each region of the country was represented by at least three states. We are aware of no data source that covers all states for state-specific estimates.
Limitations
This study was subject to several limitations. One limitation was inconsistent availability of race/ethnicity data both across and within states, meaning that GDM rates for race and ethnicity do not represent the 19 states in the entire study population. However, sensitivity analyses suggest conclusions would not have changed. Also, Indian Health Services hospitals are not included in the SID; thus, the sample of Native Americans’ deliveries in community hospitals was too small to report.
Variability in GDM rates may reflect reporting artifacts and the extent to which screening for GDM occurs, as rates of screening and reporting likely vary by type of health insurance and hospital characteristics. Further, screening recommendations for GDM changed during the study, and no data regarding screening practices were available.
No data on pre-pregnancy weight or BMI were available. One study found that for certain race/ethnicity groups, as many as 60% of GDM cases could be prevented if women entered pregnancy at a normal, healthy weight.23 In addition, women who are obese are four to eight times more likely to develop GDM.24 Therefore, the increasing prevalence of GDM in this study was likely strongly associated with rising levels of obesity during this period. Further, because ICD diagnosis codes were used, it was unclear whether increases could have been attributable in part to improved assessment or been driven by greater prevalence of underlying risk factors for GDM.
Finally, using ICD codes may have resulted in underestimation of GDM prevalence and complications such as hypertension in deliveries. One study found that using ICD codes for hypertension in deliveries had sensitivity as low as 58%.25 This study found that fewer than 2% of all deliveries were reported to be affected by hypertension, though one study reported that hypertension occurs in 5%–10% of pregnancies.26
Conclusions
Contributions of this study include the findings that GDM prevalence increased across demographic groups, and the proportion of GDM deliveries complicated by pre-pregnancy hypertension also increased. This clinical combination places children of mothers with GDM at risk for serious adverse outcomes. This study also found that the burden of funding GDM deliveries shifted from private insurance to public payers during the examined time period.
Effective diabetes prevention and control strategies for women of childbearing age can help to protect the health of women and their newborns. Structured lifestyle changes or pharmaceutical interventions can prevent or delay type 2 diabetes among women with a history of GDM,27 and these interventions can start before and during pregnancy.28 Breastfeeding also may mitigate the risk of developing type 2 diabetes for mothers, particularly those who are obese or who have GDM, and for their offspring as they grow into adulthood.29,30
Acknowledgments
Dr. Barbara Bardenheier is the guarantor of this work; as such, she had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This study was possible because of the statewide data collection efforts of the following organizations: Arizona Department of Health Services, Arkansas Department of Health, California Office of Statewide Health Planning and Development, Colorado Hospital Association, Florida Agency for Health Care Administration, Hawaii Health Information Corporation, Iowa Hospital Association, Kentucky Cabinet for Health and Family Services, Maine Health Data Organization, Maryland Health Services Cost Review Commission, Massachusetts Division of Health Care Finance and Policy, Michigan Health & Hospital Association, Nevada Department of Health and Human Services, New Jersey Department of Health, New York State Department of Health, North Carolina Department of Health and Human Services, Oregon Health Policy and Research, Oregon Association of Hospitals and Health Systems, Rhode Island Department of Health, South Dakota Association of Healthcare Organizations, Utah Department of Health, Vermont Association of Hospitals and Health Systems, Washington State Department of Health, and Wisconsin Department of Health Services.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of CDC.
Footnotes
No potential conflicts of interest relevant to this article were reported.
No financial disclosures were reported by the authors of this paper.
References
- 1.Lawrence JM, Contreras R, Chen W, Sacks DA. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999–2005. Diabetes Care. 2008;31(5):899–904. doi: 10.2337/dc07-2345. http://dx.doi.org/10.2337/dc07-2345. [DOI] [PubMed] [Google Scholar]
- 2.Correa A, Bardenheier B, Elixhauser A, Geiss LS, Gregg E. Trends in prevalence of diabetes among delivery hospitalizations, United States, 1993–2009. Matern Child Health J. 2015;19(3):635–642. doi: 10.1007/s10995-014-1553-5. http://dx.doi.org/10.1007/s10995-014-1553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitanchez D, Yzydorczyk C, Siddeek B, Boubred F, Benahmed M, Simeoni U. The offspring of the diabetic mother—short- and long-term implications. Best Pract Res Clin Obstet Gynaecol. 2015;29:256–269. doi: 10.1016/j.bpobgyn.2014.08.004. http://dx.doi.org/10.1016/j.bpobgyn.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Fadl HE, Ostlund IK, Magnuson AF, Hanson US. Maternal and neonatal outcomes and time trends of gestational diabetes mellitus in Sweden from 1991 to 2003. Diabet Med. 2010;27(4):436–441. doi: 10.1111/j.1464-5491.2010.02978.x. http://dx.doi.org/10.1111/j.1464-5491.2010.02978.x. [DOI] [PubMed] [Google Scholar]
- 5.Kessous R, Shoham-Vardi I, Pariente G, Sherf M, Sheiner E. An association between gestational diabetes mellitus and long-term maternal cardiovascular morbidity. Heart. 2013;99(15):1118–1121. doi: 10.1136/heartjnl-2013-303945. http://dx.doi.org/10.1136/heartjnl-2013-303945. [DOI] [PubMed] [Google Scholar]
- 6.Williams MA, Qiu C, Dempsey JC, Luthy DA. Familial aggregation of type 2 diabetes and chronic hypertension in women with gestational diabetes mellitus. J Reprod Med. 2003;48(12):955–962. [PubMed] [Google Scholar]
- 7.Chen P, Wang S, Ji J, et al. Risk factors and management of gestational diabetes. Cell Biochem Biophys. 2015;71(2):689–694. doi: 10.1007/s12013-014-0248-2. [DOI] [PubMed] [Google Scholar]
- 8.Popova PV, Grineva EN, Gerasimov AS, Kravchuk EN, Ryazantseva EM, Shelepova ES. The new combination of risk factors determining a high risk of gestational diabetes mellitus. Minerva Endocrinol. In press. [PubMed] [Google Scholar]
- 9.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. doi: 10.2337/diacare.25.10.1862. http://dx.doi.org/10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 10.Bardenheier BH, Elixhauser A, Imperatore G, et al. Variation in prevalence of gestational diabetes among hospital discharges for obstetric delivery across 23 States in the United States. Diabetes Care. 2013;36(5):1209–1214. doi: 10.2337/dc12-0901. http://dx.doi.org/10.2337/dc12-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.HCUPnet. U.S. DHHS; 2014. Agency for Healthcare Research and Quality. [DOI] [PubMed] [Google Scholar]
- 12.Agency for Healthcare Research and Quality. HCUP Databases. Healthcare Cost and Utilization Project (HCUP) 2007–2009. 2012 [PubMed] [Google Scholar]
- 13.Kuklina EV, Meikle SF, Jamieson DJ, et al. Severe obstetric morbidity in the United States: 1998–2005. Obstet Gynecol. 2009;113(2 Pt 1):293–299. doi: 10.1097/AOG.0b013e3181954e5b. http://dx.doi.org/10.1097/AOG.0b013e3181954e5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Census Bureau. Asian Population for the United States, Regions, and States, and for Puerto Rico: 2000 and 2010. 2012 [Google Scholar]
- 15.Gupta LS, Wu CC, Young S, Perlman SE. Prevalence of diabetes in New York City, 2002–2008: comparing foreign-born South Asians and other Asians with U.S.-born whites, blacks, and Hispanics. Diabetes Care. 2011;34(8):1791–1793. doi: 10.2337/dc11-0088. http://dx.doi.org/10.2337/dc11-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. http://dx.doi.org/10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Census Bureau. Hispanic or Latino Population for the United States, Regions, and States, and for Puerto Rico: 2000 and 2010. 2014 [Google Scholar]
- 18.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21(5):521–526. doi: 10.1038/ajh.2008.20. http://dx.doi.org/10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 19.Witlin AG, Saade GR, Mattar F, Sibai BM. Predictors of neonatal outcome in women with severe preeclampsia or eclampsia between 24 and 33 weeks’ gestation. Am J Obstet Gynecol. 2000;182(3):607–611. doi: 10.1067/mob.2000.104224. http://dx.doi.org/10.1067/mob.2000.104224. [DOI] [PubMed] [Google Scholar]
- 20.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53(6):944–951. doi: 10.1161/HYPERTENSIONAHA.109.130765. http://dx.doi.org/10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 21.Anna V, van der Ploeg HP, Cheung NW, Huxley RR, Bauman AE. Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diabetes Care. 2008;31(12):2288–2293. doi: 10.2337/dc08-1038. http://dx.doi.org/10.2337/dc08-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glied S, Jack K, Rachlin J. Women’s health insurance coverage 1980–2005. Womens Health Issues. 2008;18(1):7–16. doi: 10.1016/j.whi.2007.10.002. http://dx.doi.org/10.1016/j.whi.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Kim SY, Saraiva C, Curtis M, Wilson HG, Troyan J, Sharma AJ. Fraction of gestational diabetes mellitus attributable to overweight and obesity by race/ethnicity, California, 2007–2009. Am J Public Health. 2013;103(10):e65–e72. doi: 10.2105/AJPH.2013.301469. http://dx.doi.org/10.2105/AJPH.2013.301469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu SY, Kim SY, Bish CL. Prepregnancy obesity prevalence in the United States, 2004–2005. Matern Child Health J. 2009;13(5):614–620. doi: 10.1007/s10995-008-0388-3. http://dx.doi.org/10.1007/s10995-008-0388-3. [DOI] [PubMed] [Google Scholar]
- 25.Yasmeen S, Romano PS, Schembri ME, Keyzer JM, Gilbert WM. Accuracy of obstetric diagnoses and procedures in hospital discharge data. Am J Obstet Gynecol. 2006;194(4):992–1001. doi: 10.1016/j.ajog.2005.08.058. http://dx.doi.org/10.1016/j.ajog.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 26.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. http://dx.doi.org/10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 27.Ratner RE, Christophi CA, Metzger BE, et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93(12):4774–4779. doi: 10.1210/jc.2008-0772. http://dx.doi.org/10.1210/jc.2008-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrara A, Ehrlich SF. Strategies for diabetes prevention before and after pregnancy in women with GDM. Curr Diabetes Rev. 2011;7(2):75–83. doi: 10.2174/157339911794940738. http://dx.doi.org/10.2174/157339911794940738. [DOI] [PubMed] [Google Scholar]
- 29.Trout KK, Averbuch T, Barowski M. Promoting breastfeeding among obese women and women with gestational diabetes mellitus. Curr Diab Rep. 2011;11(1):7–12. doi: 10.1007/s11892-010-0159-6. http://dx.doi.org/10.1007/s11892-010-0159-6. [DOI] [PubMed] [Google Scholar]
- 30.Ziegler AG, Wallner M, Kaiser I, et al. Long-term protective effect of lactation on the development of type 2 diabetes in women with recent gestational diabetes mellitus. Diabetes. 2012;61(12):3167–3171. doi: 10.2337/db12-0393. http://dx.doi.org/10.2337/db12-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]