Highlights
-
•
We examined patterns of connectivity in human fetal brain networks.
-
•
The fetal brain demonstrates cerebral-cerebellar and cortical-subcortical connectivity.
-
•
Many forms of cerebral connectivity are present by the third trimester.
-
•
Default mode network connections were evident in fetuses older than 35 weeks.
-
•
Long-range functional connectivity is more prominent in older fetuses.
Keywords: Fetus, fMRI, Human, Prenatal, Resting-state, Connectome
Abstract
Formation of operational neural networks is one of the most significant accomplishments of human fetal brain growth. Recent advances in functional magnetic resonance imaging (fMRI) have made it possible to obtain information about brain function during fetal development. Specifically, resting-state fMRI and novel signal covariation approaches have opened up a new avenue for non-invasive assessment of neural functional connectivity (FC) before birth. Early studies in this area have unearthed new insights about principles of prenatal brain function. However, very little is known about the emergence and maturation of neural networks during fetal life. Here, we obtained cross-sectional rs-fMRI data from 39 fetuses between 24 and 38 weeks postconceptual age to examine patterns of connectivity across ten neural FC networks. We identified primitive forms of motor, visual, default mode, thalamic, and temporal networks in the human fetal brain. We discovered the first evidence of increased long-range, cerebral-cerebellar, cortical-subcortical, and intra-hemispheric FC with advancing fetal age. Continued aggregation of data about fundamental neural connectivity systems in utero is essential to establishing principles of connectomics at the beginning of human life. Normative data provides a vital context against which to compare instances of abnormal neurobiological development.
1. Introduction
One of the major achievements of human fetal brain development is the establishment of functional neural networks. Detailed understanding of normal processes of brain circuit formation is essential for discovering consequences of injury/insult, periods of vulnerability, and for early detection of clinically significant neural anomalies. The bulk of what is known about human brain connectional ontogeny comes from histological and magnetic resonance imaging (MRI) studies performed on post-mortem fetal brain specimens. These histological and MRI studies have shown that transient physical structures serve as scaffolding for axonal proliferation and cell migration (Kostovic and Jovanov-Milosevic, 2008) and that, in harmony with brain gyrification, long-range association pathways begin to be established during the second half of gestation (Kostović and Jovanov-Milosevic, 2006, Takahashi et al., 2012).
Until recently, quantification of neuroconnectivity during the fetal period has proven elusive for human in vivo brain research. However, recent advances in diffusion and resting state MRI have provided new means for non-invasive study of neural circuit formation in the perinatal period (Mailath-Pokorny et al., 2012). Tractography analyses of fetal brain diffusion tensor imaging data has demonstrated emergence of white matter pathways in the transient intermediate zone, splenium, and genu, consistent with in vitro findings (Kasprian et al., 2008). Resting-state functional MRI (rs-fMRI) applied to prematurely born human infants has shown that, as the preterm neonate grows, neural circuit functional activity becomes increasingly synchronized (Smyser et al., 2010) and that intrinsic connectivity networks (ICNs) are largely formed by 42 weeks postmenstrual age (Doria et al., 2010). However, preterm neonates are not ideal substitutes for learning about typical fetal brain development, because the underlying reason for preterm delivery and environmental factors cannot be disentangled from neurological observations in these cases. Important questions remain about large-scale neural organization, and establishment of ICNs prior to birth.
Recent investigations have established the utility of rs-fMRI for quantifying neural network development in utero. The first fetal rs-fMRI study was performed in pregnant women referred to MRI for suspected neurological anomalies discovered during fetal sonography. The investigators qualitatively compared functional connectivity (FC) networks in 16 fetuses that had healthy MRI results and minimal movement. They concluded that in the majority of cases temporal lobe networks were lateralized, whereas frontal and occipital networks were bilateral (Schöpf et al., 2012). Subsequently, two human fetal FC studies have been performed, assessing fetuses recruited as part of research studies, rather than as part of clinical follow-up. Statistical group-level comparisons of FC in 25 healthy fetuses demonstrated that cross-hemispheric connectivity increases with advancing postconceptual age, and that midline cross-hemispheric connectivity precedes lateral connectivity (Thomason et al., 2013). Graph theoretical analyses in 33 fetuses revealed that synchrony of activity across neural networks is increased in older, compared to younger, fetuses. In addition, connectivity between the posterior cingulate cortex (PCC) and other brain areas becomes more negative with increasing postconceptual age (Thomason et al., 2014). These studies provided the first indication that human fetuses possess basic forms of neural connectivity networks and that rs-fMRI may become instrumental for determining principals of human FC in utero.
Age-related changes in resting-state networks in utero remain unclear. We obtained cross-sectional rs-fMRI data from 39 fetuses between 24 and 38 weeks postconceptual age to examine patterns of connectivity across ten functionally integrated ICNs. Following prior work in neonates (Smyser et al., 2010), we measured fMRI covariation in sensorimotor, cingulate, occipital, prefrontal, temporal, and thalamic regions. Employing seed-based connectivity analysis (SCA), we interrogated qualitative and quantitative changes in ICNs associated with advancing fetal age. We predicted that homotopic and long-range connectivity would be strongest in older fetuses, across neural networks, and that within network covariance would be higher and between network covariance would be lower in older fetuses. The latter would be fitting with emergence of independently functioning ICNs.
2. Methods
2.1. Participants
Rs-fMRI data were collected from healthy pregnant women and their fetuses recruited from obstetrical clinics located within the Detroit Medical Center. Initial contact was made by physicians to potential participants in their care and followed up by one-on-one discussion with a member of the research team who explained study procedures and answered questions. Participants were informed that their participation in research was voluntary and not part of their prenatal care. They were assured that they were not selected on the basis of any medical findings, and it was explained that their choice to participate would not influence their future medical care. Exclusionary criteria included: presence of MRI contraindication, fetal central nervous system abnormality, and maternal size larger than would comfortably be accommodated in a 70-cm open-bore MRI system. Inclusionary criteria included: singleton pregnancy between 24 and 38 weeks postconceptual age, and maternal age ≥18 years. Written, informed consent was obtained prior to participation. All experimental procedures were approved by the Human Investigation Committee of Wayne State University.
Data were collected from a total of 39 pregnant women (median maternal age = 23 years, range = 18–37 years). The median gestational age (GA) of their fetuses was 32 weeks (range = 24 + 4 to 37 + 6, weeks + days) at the time of MRI. Seven fetal participants were excluded prior to group level analyses due to (a) having fewer than 100 fMRI volumes after removing high movement frames (n = 4), or (b) because they went on to be born preterm (n = 3), leaving a total of 32 participants. fMRI data from 22 of these participants has been reported previously (Thomason et al., 2014, Thomason et al., 2013).
2.2. Measures
Information about maternal health practices were obtained using a 45-item health questionnaire which assessed frequency of engaging in specific health behaviors related to diet, exercise, medical adherence, alcohol and tobacco use and exposure, and sleep (adapted from Jackson, 2006). Supplementary Table S1 provides a summary of questionnaire items. Health practices and birth outcomes (e.g., newborn weight, height, head circumference) were compared between younger ( weeks, SD ± 1.49), middle ( weeks, SD ± 1.97) and older ( weeks, SD ± 0.85) fetal groups by analysis of variance (ANOVA) implemented in SPSS version 21; p ≤ 0.05 was considered significant.
2.3. Imaging methods
Fetal MR exams were performed with a Siemens Verio 70-cm open-bore 3 Tesla scanner. An abdominal 4-Channel Siemens Flex Coil was placed around the maternal abdomen centered approximately at the fetal head. A T2 HASTE anatomical sequence (TR/TE, 3500/140 ms; 3-mm slice thickness) was repeated six times, twice in each orientation. Echo planar imaging (EPI) BOLD (TR/TE, 2000/30 ms; 4-mm slice thickness; axial) was performed for variable duration to accommodate readjustments to acquisition following fetal movement. The average number of BOLD fMRI time frames obtained per case was 349 (SD = 70). Specific absorption rates were extracted from fMRI scans for every subject.
2.4. Functional data preprocessing
BrainSuite (Shattuck and Leahy, 2002), Analyze 11.0 (AnalyzeDirect), and FSLview (Smith et al., 2004) were used to manually extract the fetal brain from surrounding tissue. Because the fetus is encased in an independently moving compartment (e.g., the mother), the fetal brain must be manually extracted before standard motion realignment procedures can be employed. As manual brain extraction is time intensive (requiring generation of masks via manual tracing) we extracted only frames during which fetal movement was minimal (mean duration = 74.41 s, SD = 53.632 s; min = 20 s, max = 338 s). Frame selection determinations were made after repeatedly viewing the timeseries as a movie in FSLview. Each segment during which fetal head movement was minimal was manually segmented from surrounding maternal and skull tissue, reoriented, realigned, and normalized to a 32-week fetal template (Serag et al., 2012) using Statistical Parametric Mapping version 8 (SPM8). The 32-week fetal template was selected as it was closest to the mean fetal GA. To correct for variations in normalization between segments, normalized images were then concatenated into one run, realigned, and a 6 mm full width half max (FWHM) Gaussian smoothing kernel was applied.
To evaluate possible covariation between fetal age and data quality, movement and number of analyzed time frames were compared in fetuses of different ages. Movement parameters were computed for both translational (Tx, Ty, Tz in mm) and rotational (Rpitch, Rroll, Ryaw in degrees) movement, including (a) mean movement, (b) the mean of the maximum frame-to-frame displacement across the data, or max excursion, and (c) the mean of the standard deviation for all frames after linear detrending, or root mean square (RMS). Thus, a total of 18 movement parameters were utilized to quantify fetal head movement. Additionally, the number of frames retained for all subjects was restricted to within 2 standard deviations (SD ± 50 frames) of the sample mean ( ). To test whether fetal age related to estimated differences in movement-related error or to number of frames analyzed ANOVA and bivariate. Pearson correlation were implemented in SPSS version 21; p ≤ 0.05 was considered significant.
2.5. Functional connectivity analyses
A seed-based connectivity approach implemented in CONN-fMRI toolbox ver.12.k (Whitfield-Gabrieli and Nieto Castanon, 2012) was utilized to compute connection values between spontaneous fMRI signal in a 6 mm radius “seed” (source) region and all other brain voxels. FC analyses were performed for 10 seed regions approximating anatomical areas previously tested in a study of preterm and term newborns (Smyser et al., 2010). Seed based connectivity analyses were performed in standardized space. Coordinates for seed regions are provided in Table 1, and illustrated in Fig. 1. Anatomical component correction (aCompCor) methodology, rather than global signal correction, was used to estimate and remove noise at the individual voxel level (Behzadi et al., 2007, Chai et al., 2012). In the present analysis we did not apply global signal correction because of the concern that it forces the presence of anticorrelated networks (Chang and Glover, 2009, Murphy et al., 2009, Weissenbacher et al., 2009), which could lead to false interpretation of observed effects. Principal components of the signals from white matter and cerebral spinal fluid, as well as translational and rotational movement parameters (and their first-order temporal derivatives) were removed by covariate regression analysis. A temporal band-pass filter of 0.01–0.1 Hz was applied to investigate low frequency correlations. Individual volume images resulting from seed-based connectivity analyses were submitted to random effects modeling in SPM8. One sample t-tests and linear regression were performed to obtain qualitative comparisons of networks in different fetal age groups and to quantify significant age-related changes in FC, respectively. Statistical tests assumed unequal variance. All neuroimaging results were considered significant at p ≤ 0.01.
Table 1.
Seed correlation maps were formed by correlating timecourse in each of these coordinate regions with all voxels in the brain.
| Intrinsic Connectivity Network (ICN) | 32-week fetal template coordinates, Serag et al. (2012) |
||
|---|---|---|---|
| X | Y | Z | |
| Motor leg | 12 | −6 | 26 |
| Motor hand | 24 | 0 | 20 |
| Motor face | 30 | 4 | 10 |
| Caudal posterior cingulate cortex | 4 | −20 | 0 |
| Dorsal anterior cingulate cortex | 2 | 24 | 4 |
| Cuneus | 6 | −38 | −8 |
| Medial prefrontal cortex | 2 | 36 | −10 |
| Lateral prefrontal cortex | 20 | 38 | −10 |
| Superior temporal gyrus | 32 | 0 | −4 |
| Dorsal thalamus | 4 | 0 | −2 |
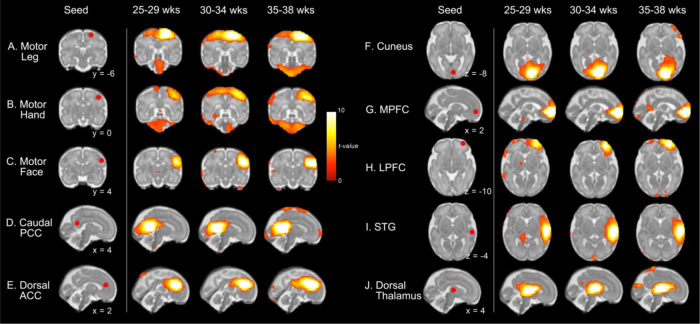
Fig. 1.
In utero whole-brain resting-state correlation maps corresponding to varied seed locations. Images are organized into columns corresponding to fetal age groups. Seed regions are shown in the first column on the left. Age-group 1-sample t-tests at p < 0.01 significance threshold are shown on representative coronal, sagittal, and axial slices overlaid on a 32-week fetal template (Serag et al., 2012). Abbreviations: posterior cingulate cortex, PCC; anterior cingulate cortex, ACC; medial prefrontal cortex, MPFC; lateral prefrontal cortex, LPFC; superior temporal gyrus, STG.
2.6. Analysis of age-related increases in long-range connectivity
We quantitatively examined the relationship between fetal age and amount of significant distant connectivity. Distant was defined as the furthest 2% of significant voxels (on average > 63 mm), specified individually for each of the 10 networks to accommodate their inherent unique spatial distributions. Sums of the number of distant voxels with t-values >2.462 (corresponding to p ≤ 0.01) were submitted to a between-groups ANOVA implemented in SPSS version 21; p ≤ 0.01 was considered significant.
3. Results
3.1. Participant characteristics
Participant and data characteristics across fetuses of different ages are summarized in Table 2. Fetal age groups did not differ in (1) self-reported health practices, F(2,28) = 0.047, p = 0.96, (2) number of retained fMRI time frames, , SD ± 50, F(2,29) = 0.902, p = 0.42, (3) specific absorption rates during fMRI, , SD ± 0.09, F(2,29) = 0.664, p = 0.52, nor (4) any of the 18 evaluated movement parameters (see supplementary Table S2). All fetuses included in analyses were delivered at term, and none were hospitalized following delivery.
Table 2.
Summary of participant and data characteristics.
| N (M:F) | GA at MRI (wks) | Birth Measures |
Frame total | |||||
|---|---|---|---|---|---|---|---|---|
| GA at birth (wks) | Weight (g) | Length (cm) | HC (cm) | 5 min Apgar | ||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Median (IQR) | Mean (SD) | ||
| Youngest | 11 (7:4) | 27.5 (1.5) | 38.7 (0.7) | 3107.7 (397.7) | 49.4 (2.6) | 33.9 (2.0) | 9 (0) | 193.8 (55.4) |
| Middle | 10 (6:4) | 32.8 (2.0) | 39.2 (1.1) | 3549.9 (508.9) | 51.0 (2.5) | 34.3 (1.4) | 9 (0) | 217.4 (40.2) |
| Oldest | 11 (6:5) | 36.1 (0.9) | 39.1 (1.1) | 3051.1 (604.8) | 48.5 (3.7) | 33.1 (2.1) | 9 (0) | 190.4 (51.6) |
| Overall | 32 (19:13) | 32.2 (3.9) | 39.0 (0.7) | 3226.4 (542.1) | 49.6 (3.1) | 33.8 (1.9) | 9 (0) | 200.0 (49.6) |
Abbreviations: male, M; female, F; standard deviation, SD; gestational age, GA; weeks, wks; grams, g; centimeters, cm; intraquartile range, IQR.
3.2. Increased neural network connectivity in older fetuses
Voxel-wise group regression revealed increased functional connectivity in older compared with younger fetuses in 7 of the 10 networks examined (networks A, B, C, F, H, I, J; all p < 0.01, cluster extent, k > 10). Motor, visual, auditory and thalamic networks showed overall increased FC with age, whereas midline connectivity networks (anterior and posterior cingulate and medial PFC) showed increased FC in younger fetuses.
3.3. Establishment of motor networks in utero
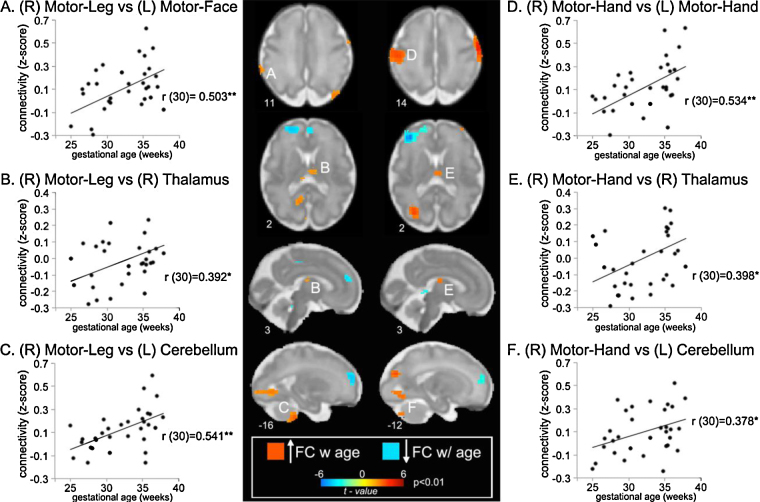
Patterns of motor, thalamic, and cerebellar connectivity varied in fetuses of different ages. Stronger FC was observed in older fetuses between motor cortices and thalamus and motor cortices and contralateral cerebellum, as illustrated in Fig. 2. In line with increased maturation of motor systems in utero, we also observed increased signal covariation between right and left primary motor cortices. This increased cross-hemispheric synchrony with increased age effect was strongest in the motor hand, compared to the motor face and motor leg networks.
Fig. 2.
Whole-brain fetal age regression analyses for Motor-Leg and Motor-Hand regions given on figure left and right side, respectively. Sagittal and axial slices at intersections of significant results for each regression are overlaid on 32-week fetal templates. Coordinates for each image are given in mm at the bottom left of each slice. Scatterplots depict functional connectivity (i.e., signal covariation) plotted as Fisher's transformed r values between specified, lettered brain areas and motor cortex across participants. With age, functional connectivity increased between bilateral motor regions, between motor cortices and ipsilateral thalamic regions, and between motor cortices and contralateral cerebellar regions. *p ≤ 0.05; **p ≤ 0.01.
3.4. Increased FC in default mode network (DMN) components in older fetuses
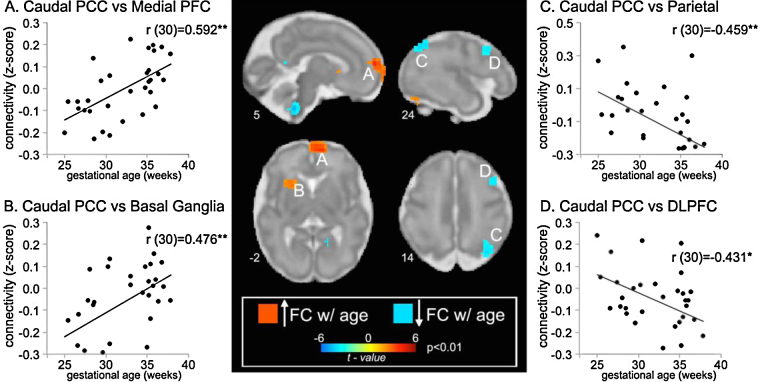
Qualitative comparisons of statistical results obtained in each fetal age group revealed significant caudal PCC to medial prefrontal connectivity was present in the oldest but not the younger fetal groups. This is portrayed in Fig. 1, which shows significant patterns of connectivity in each age group. The oldest fetal group demonstrated positive connectivity from the PCC seed to MPFC (Fig. 1, row D) and the reverse, MPFC seed to the PCC (Fig. 1, row G). These regions are key contributors to the DMN of the brain. Activity in the DMN has been shown to be reduced when individuals engage in attentionally demanding tasks, and activity in this network is negatively correlated with increased activity in regions important for executive function such as lateral prefrontal and parietal association cortices (Fox et al., 2005). Linear regression of fetal caudal PCC maps revealed that both positive connectivity to MPFC and negative connectivity to lateral prefrontal/parietal regions are increased in older fetuses (see Fig. 3).
Fig. 3.
Whole-brain fetal age regression analysis for the PCC seed region. Sagittal and axial slices at intersections of significant results are overlaid on 32-week fetal templates. Coordinates for each image are given in mm at the bottom left of each slice. Scatterplots depict functional connectivity (i.e., signal covariation) plotted as Fisher's transformed r values between specified, lettered brain areas and the caudal PCC across participants. With age, functional connectivity increased from PCC to medial prefrontal cortex and basal ganglia but decreased to parietal association areas and dorsal lateral prefrontal cortex. *p ≤ 0.05; **p ≤ 0.01.
3.5. Greater long-range connectivity in older fetuses
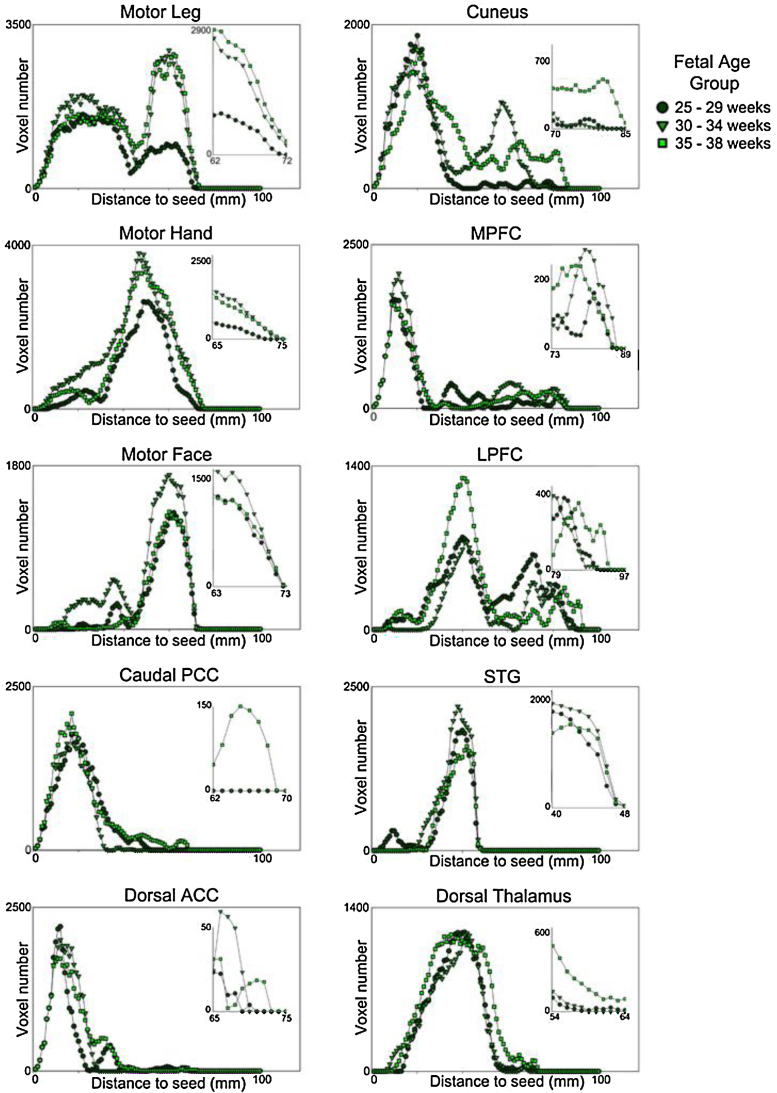
Older fetuses had significantly more long-range connectivity than younger fetuses, F(2,29) = 5.873, p = 0.008. Averages of total number of distant (furthest 2%) connected voxels revealed the youngest group had fewest ( ), the middle age group was intermediate ( ), and the oldest group had largest number of distant suprathreshold voxels ( ). This difference between groups showed some variation across networks, with strongest separation between age groups for the caudal PCC, cuneus, LPFC, motor-leg, and motor-hand networks, in that order. The number of significant distant voxels in the aforementioned networks was more than five times greater in older than younger fetal groups. Results are plotted individually for each network in Fig. 4. Given that these analyses were performed in standardized space, the observed age-group differences should not be attributed to variation in overall brain volume.
Fig. 4.
Number of suprathreshold voxels plotted against distance from seed coordinate. The relationship between distance to seed and number of significant voxels is shown for each of the three fetal age groups and for each network examined. Inset plots magnify select distant ranges to provide enhanced visualization of differences between groups.
4. Discussion
The primary goals of this study were to determine if typical resting state ICNs are present in the human fetal brain before birth and how connectivity in these networks varies with fetal age. Consistent with our hypotheses, we found that in older fetuses (1) long-range connectivity is stronger, (2) connectivity within ICNs is stronger, and (3) connectivity to areas outside of ICNs is reduced. For example, coactivation within motor, thalamic, and contralateral cerebellar regions increased with postconceptual age. These phenomena presumably reflect ongoing refinement within and between neural circuits, fitting with well described principals of neural segregation (Friston, 2002), and reproducing patterns observed in transitions from childhood to adulthood (Fair et al., 2007, Stevens et al., 2009).
We observed that older fetuses possess more significant long-range connections across networks. The principal of stronger long-range neural connections with advancing age has been demonstrated previously in neonates (Fransson et al., 2007, Gao et al., 2009), and across childhood and adolescence (Fair et al., 2007, Fair et al., 2009, Hagmann et al., 2010, Kelly et al., 2009, Supekar et al., 2009). Here, we show that this age related refinement in neural network architecture is present and observable beginning in the fetal period. Increased long-range signal covariance in older fetuses is fitting with prior observations that long-range association pathways form prenatally (Kostović and Jovanov-Milosevic, 2006, Takahashi et al., 2012). We advance these priors by demonstrating these effects in an in vivo model that will permit future examinations of health, injury, and disease etiology in fetal life.
Functional connections between regions most commonly cited as comprising the DMN, the PCC and medial PFC, were evident in fetuses older than 35 weeks postconceptual age. Coactivation of PCC and medial PFC became more synchronized with age, while coactivation from the PCC to areas of dorsal attention/executive networks became more negatively coupled with age. This is in agreement with previous graph theoretical resting-state fMRI in human fetuses showing that older (GA ≥ 31 weeks) but not younger fetuses (GA < 31 weeks) exhibit a module, or connectivity subnetwork, that comprises dorsal posterior and medial frontal brain regions (Thomason et al., 2014). That study also demonstrated that negative connectivity between the PCC and other brain regions increases with fetal age (Thomason et al., 2014). Together these unique approaches provide convergent support that regions of the DMN manifest distinctly coordinated activity prior to birth. Discovering coordinated DMN function in utero is also consistent with studies in term- and preterm-born infants where a ‘proto-default-mode network’ has been indicated (Fransson et al., 2007, Fransson et al., 2009).
Here, we discovered that temporal coherence in human fetal fMRI signals extends beyond synchrony in homotopic counterparts. Indeed, we observed several temporal coherence patterns: (1) subcortical to cortical (e.g., motor cortices to thalamic regions), (2) intra- (e.g., PCC to medial PFC) and inter-hemispheric (e.g., bilateral PFC and motor cortices), and (3) cerebral to cerebellar (e.g., motor cortices to contralateral cerebellum). Synchrony in fMRI signal can occur in the absence of direct anatomical connection (Greicius et al., 2009, Vincent et al., 2007). However, studies of adults with acute stroke (Lv et al., 2013), a child with a severed corpus callosum (Johnston et al., 2008), and combined diffusion tensor-FC studies (Greicius et al., 2009) indicate fMRI signal covariation is constrained by physical structures of the brain. Therefore, it has been suggested that resting-state signal correlations should be interpreted as reflecting histories of coactivation between brain regions (Dosenbach et al., 2007, Kelly et al., 2009, Power et al., 2010). In the human fetus this activity may represent active establishment of common function that emerges prior to the development of related cognitive or perceptual functions. This postulation is justified given extensive evidence that network dynamics can reshape the physical structures of neural networks (Katz and Shatz, 1996, Majewska and Sur, 2006). Here, we discovered many forms of network temporal coherence (e.g., cerebellar to cerebral, subcortical to cortical, homotopic) using fetal resting-state fMRI. Thus, spontaneous brain activity in the third trimester appears to recapitulate basic forms of human ICNs that may serve as scaffolding for development of these network structures.
A limitation to be considered in the present study is whether the seed regions selected for this study were ideal. There is limited precedence for coordinate selection in a fetal sample and a paucity of labeled fetal atlases or analyses tools available (Anderson and Thomason, 2013). Thus, for the present analysis, we adhered tightly to procedures successfully used in premature infants (Smyser et al., 2010) as these best approximate neuroanatomy of our study sample. Another limitation of our study is that we evaluated only 10 XYZ coordinates (i.e., seed regions) all located in the right hemisphere. This was an intentional constraint as we sought to restrict the total number of comparisons to reduce the possibility of introducing type I error (i.e., false positives). By comparison, prior resting-state analyses in preterm infants have tested 5–30 seed regions and have used more relaxed significance thresholds (Doria et al., 2010, Smyser et al., 2010). A final significant concern to be considered in the present study is potential error introduced by fetal motion. Recent studies of the effects of motion on resting-state fMRI have shown that even ‘micro-movement’, as small as 0.2 mm, can impact observed patterns of functional connectivity (Power et al., 2012, Satterthwaite et al., 2013, Van Dijk et al., 2012). Disconcertingly, these investigations show that increased movement can falsely inflate short-range connectivity and decrease long-range connectivity. To mitigate movement related error, we excluded high motion frames across subjects, resulting in similar overall movement across age groups. By intentionally removing equivalent frame numbers in older and younger fetal groups we were also able to assure that fetal participants were matched on total number of frames analyzed. By controlling these variables across groups we limited concern that quality of neuroimaging data may influence observed effects.
5. Conclusions
Through resting-state evaluation of healthy human fetuses we have identified the earliest forms of neural functional connectivity. Motor, visual, thalamic, DMN, frontal, and temporal networks likely represent immature forms of those described in older populations. We observed temporal coherence across (1) cerebral-cerebellar, (2) cortical-subcortical, (3) intra-hemispheric cortical, and (4) bilateral, cross-hemispheric structures, suggesting many forms of cerebral connectivity are present by the third trimester. We also observed that older fetuses possess significantly more long-range connections than younger fetuses. By evaluating resting-state fMRI in healthy human fetuses we have gained new information about operations in functional brain circuits in utero. Continued development and use of fetal resting-state fMRI is likely to result in major discoveries for clinical, perinatal and neurological sciences.
Conflict of interest statement
All authors declare no competing financial interests.
Funding
This research was supported, in part, by the Merrill Palmer Skillman Institute for Child and Family Development, by the Department of Pediatrics, Wayne State University (WSU) School of Medicine, and by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services. This project was also supported by WSU's Perinatology Virtual Discovery Grant (made possible by the W.K. Kellogg Foundation) and WSU's President's BRAIN Research Enhancement Program awards to MET. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgements
We thank Yashwanth Katkuri and Pavan Jella for their assistance in technical aspects of scan data acquisition, and Ingrid Haugen, Pooja Srivastava, Tommy Ricard, and Sonja Llancari for their assistance in data collection and participant care. We thank Jayant Sakhardande, Gregory H. Baldwin, and Berta Rihan for assistance in data management and analyses. We thank Brittany Olle, Lorraine Nikita, and Janine Bieda for assistance with participant recruitment and communication. We thank the pregnant mothers that chose to participate in this research project and acknowledge their expression of desire to help other mothers and babies to be born healthy. Additionally, we acknowledge the critical analysis provided by the Reviewers and thank them for their contribution to the work.
Footnotes
Available online 27 September 2014
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2014.09.001.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- AnalyzeDirect, Overland Park, KS.
- Anderson A.L., Thomason M.E. Functional plasticity before the cradle: a review of neural functional imaging in the human fetus. Neurosci. Biobehav. Rev. 2013;37(9):2220–2232. doi: 10.1016/j.neubiorev.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X.J., Castanon A.N., Ongur D., Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59(2):1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Glover G.H. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47(4):1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V., Beckmann C.F., Arichi T., Merchant N., Groppo M., Turkheimer F.E. Emergence of resting state networks in the preterm human brain. Proc. Natl. Acad. Sci. U.S.A. 2010;107(46):20015–20020. doi: 10.1073/pnas.1007921107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U.S.A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D., Dosenbach N.U., Church J.A., Miezin F.M. Functional brain networks develop from a “local to distributed” organization. PLoS Comput. Biol. 2009;5(5):1–14. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U.F., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. U.S.A. 2007;104(33):13507–13512. [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U.S.A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P., Skiold B., Engstrom M., Hallberg B., Mosskin M., Aden U. Spontaneous brain activity in the newborn brain during natural sleep – an fMRI study in infants born at full term. Pediatr. Res. 2009;66(3):301–305. doi: 10.1203/PDR.0b013e3181b1bd84. [DOI] [PubMed] [Google Scholar]
- Fransson P., Skiold B., Horsch S., Nordell A., Blennow M., Lagercrantz H. Resting-state networks in the infant brain. Proc. Natl. Acad. Sci. U.S.A. 2007;104(39):15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. Functional integration and inference in the brain. Prog. Neurobiol. 2002;68(2):113–143. doi: 10.1016/s0301-0082(02)00076-x. [DOI] [PubMed] [Google Scholar]
- Gao W., Zhu H., Giovanello K.S., Smith J.K., Shen D., Gilmore J.H. Evidence on the emergence of the brain's default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc. Natl. Acad. Sci. U.S.A. 2009;106(16):6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Supekar K., Menon V., Dougherty R.F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P., Sporns O., Madan N., Cammoun L., Pienaar R., Wedeen V.J. White matter maturation reshapes structural connectivity in the late developing human brain. Proc. Natl. Acad. Sci. U.S.A. 2010;107(44):19067–19072. doi: 10.1073/pnas.1009073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T. Relationships between perceived close social support and health practices within community samples of American women and men. J. Psychol. 2006;140(3):229–246. doi: 10.3200/JRLP.140.3.229-246. [DOI] [PubMed] [Google Scholar]
- Johnston J.M., Vaishnavi S.N., Smyth M.D., Zhang D., He B.J., Zempel J.M. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J. Neurosci. 2008;28(25):6453–6458. doi: 10.1523/JNEUROSCI.0573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprian G., Brugger P.C., Weber M., Krssak M., Krampl E., Herold C. In utero tractography of fetal white matter development. Neuroimage. 2008;43(2):213–224. doi: 10.1016/j.neuroimage.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Katz L.C., Shatz C.J. Synaptic activity and the construction of cortical circuits. Science. 1996;274(5290):1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kelly A.M., Di Martino A., Uddin L.Q., Shehzad Z., Gee D.G., Reiss P.T. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb. Cortex. 2009;19(3):640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kostovic I., Jovanov-Milosevic N. Subplate zone of the human brain: historical perspective and new concepts. Coll. Antropol. 2008;32(Suppl. 1):3–8. [PubMed] [Google Scholar]
- Kostović I., Jovanov-Milosevic N. The development of cerebral connections during the first 20–45 weeks’ gestation. Semin. Fetal Neonatal Med. 2006;11(6):415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Lv Y., Margulies D.S., Cameron Craddock R., Long X., Winter B., Gierhake D. Identifying the perfusion deficit in acute stroke with resting-state functional magnetic resonance imaging. Ann. Neurol. 2013;73(1):136–140. doi: 10.1002/ana.23763. [DOI] [PubMed] [Google Scholar]
- Mailath-Pokorny M., Kasprian G., Mitter C., Schopf V., Nemec U., Prayer D. Magnetic resonance methods in fetal neurology. Semin. Fetal Neonatal Med. 2012;17(5):278–284. doi: 10.1016/j.siny.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Majewska A.K., Sur M. Plasticity and specificity of cortical processing networks. Trends Neurosci. 2006;29(6):323–329. doi: 10.1016/j.tins.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Fair D.A., Schlaggar B.L., Petersen S.E. The development of human functional brain networks. Neuron. 2010;67(5):735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Ruparel K., Erus G., Elliott M.A., Eickhoff S.B. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuroimage. 2013;83:45–57. doi: 10.1016/j.neuroimage.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöpf V., Kasprian G., Brugger P.C., Prayer D. Watching the fetal brain at ‘rest’. Int. J. Dev. Neurosci. 2012:11–17. doi: 10.1016/j.ijdevneu.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Serag A., Aljabar P., Ball G., Counsell S.J., Boardman J.P., Rutherford M.A. Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. Neuroimage. 2012;59(3):2255–2265. doi: 10.1016/j.neuroimage.2011.09.062. [DOI] [PubMed] [Google Scholar]
- Shattuck D.W., Leahy R.M. BrainSuite: an automated cortical surface identification tool. Med. Image Anal. 2002;6(2):129–142. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smyser C.D., Inder T.E., Shimony J.S., Hill J.E., Degnan A.J., Snyder A.Z. Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M.C., Pearlson G.D., Calhoun V.D. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum. Brain Mapp. 2009;30(8):2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Musen M., Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7(7):15. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E., Folkerth R.D., Galaburda A.M., Grant P.E. Emerging cerebral connectivity in the human fetal brain: an MR tractography study. Cereb. Cortex. 2012;22(2):455–464. doi: 10.1093/cercor/bhr126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Brown J.A., Dassanayake M.T., Shastri R., Marusak H.A., Hernandez-Andrade E. Intrinsic functional brain architecture derived from graph theoretical analysis in the human fetus. PLoS ONE. 2014 doi: 10.1371/journal.pone.0094423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Dassanayake M.T., Shen S., Katkuri Y., Alexis M., Anderson A.L. Cross-hemispheric functional connectivity in the human fetal brain. Sci. Transl. Med. 2013;5(173) doi: 10.1126/scitranslmed.3004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J.L., Patel G.H., Fox M.D., Snyder A.Z., Baker J.T., Van Essen D.C. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A., Kasess C., Gerstl F., Lanzenberger R., Moser E., Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47(4):1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012 doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.