Abstract
Purpose
Several studies have shown substantially longer survival among persons with Down syndrome in recent decades. We examined survival patterns among Danish persons with Down syndrome by karyotype.
Methods
A national cohort of 3,530 persons with Down syndrome identified from the Danish Cytogenetic Register and a reference cohort of persons without Down syndrome randomly selected from the general population were followed from 1 April 1968 to 15 January 2009 by linkages to the Register of Causes of Death and the Civil Registration System.
Results
Overall, persons with Down syndrome had higher mortality than the reference cohort but to a lesser degree for persons with mosaic trisomy 21 than for persons with standard trisomy 21 or with Robertsonian translocations (hazard ratio 4.98 (95% confidence interval 3.51–7.08), 8.94 (8.32–9.60), and 10.23 (7.50–13.97), respectively). Among persons with Down syndrome born after April 1968, more recent birth cohorts had lower mortality rates than older birth cohorts, which was largely due to declining mortality among persons with Down syndrome who also had congenital heart defects.
Conclusion
Recent birth cohorts of persons with Down syndrome experienced declining mortality, likely due to treatment for congenital heart defects, and persons with mosaic trisomy 21 had better survival than persons with other Down syndrome karyotypes.
Keywords: congenital heart defects, Down syndrome, mortality, mosaic trisomy 21, survival
Introduction
Down syndrome (DS) is the most common identifiable genetic cause of intellectual disability, occurring in ~1 in 700 births, although the prevalence at birth varies depending on the maternal age structure and utilization of prenatal screening and pregnancy termination in the population studied.1–3 In most cases (90–95%), DS is caused by an extra free chromosome 21 (standard trisomy 21), which usually results from nondisjunction during maternal meiosis. Other cytogenetic alterations that may produce DS include Robertsonian translocations (a centric fusion between two acrocentric chromosomes, most commonly chromosomes 14 and 21) and mosaic trisomy 21 (in which only a portion of cells have an extra chromosome 21), each occurring in 2–4% of DS cases.4,5 The phenotypic manifestations associated with mosaic trisomy 21 are similar to those of standard trisomy 21, although the phenotype tends to be milder.6 Some studies have suggested that children with mosaic trisomy 21 appear to have better intellectual development than those with standard trisomy 21.7,8
Several studies have shown substantially longer survival for persons with DS in recent decades,9–13 but in nearly all those studies, survival by DS karyotype was not examined. One recent study suggested that children with mosaic DS have better survival than those with standard trisomy 21.14 This finding was based on a small number of persons with mosaic DS from one metropolitan area in the United States. Because survival for children with congenital heart defects has improved in recent decades,15 the question arises as to what extent the recent improvement in survival for persons with DS reflects changes in the karyotypic composition of persons with DS or in improvements in survival for persons with DS and congenital heart defects. In addition, most of the previous studies did not include a comparison with survival in the general population. In this analysis, we used a Danish population-based follow-up study to examine survival among individuals with different DS karyotypes, as compared with that in the general population, as well as survival of persons with DS and congenital heart defects.
Materials and Methods
Study design
We identified a cohort of persons with DS using the Danish Cytogenetic Register, which includes information on persons with DS diagnosed in Denmark since 1961. Then a cohort of persons without DS was randomly selected from the general population, matched on birth year with the persons with DS in a 1:20 ratio of DS cases to noncases, using the Civil Registration System. The two cohorts were linked to a number of nationwide registers, including the Register of Causes of Death, the Medical Birth Register, and the National Hospital Register. Data linkages were based on the personal identification number, which was introduced in Denmark in April 1968, and is assigned to each resident. The personal identification number, which includes date of birth and a code for sex, is unique to each resident, allowing complete follow-up and linkage to all national civil registers, including data on death, migration, and hospitalization. This study was approved by the Danish Data Protection Agency.
Identification of persons with DS
The Danish Cytogenetic Register was founded in 1968 to collect information on constitutional chromosomal abnormalities in Denmark. The register is based on reports from cytogenetic laboratories throughout the country and provides virtually complete coverage of constitutional chromosomal abnormalities diagnosed in Denmark.16 The register also retrospectively collected data on all analyses performed since the introduction of the cytogenetic analysis in 1961.17 By December 2007, the Cytogenetic Register contained information on 3,551 individuals with a postnatal cytogenetic diagnosis of DS, a verified personal identification number (i.e., alive on 1 April 1968 or born later), and residence in Denmark at the time of the cytogenetic study.
A karyotype based on studies of peripheral blood is available for all persons with DS in this study. Cytogenetic studies reported as trisomy G in the 1960s and 1970s (before the routine use of banded karyotyping that permitted distinguishing the two G-group chromosomes) were accepted as trisomy 21. Persons who were studied with fluorescence in situ hybridization only were not included. All reported karyotypes were reviewed (by S.A.R., J.M.F., and H.H.) and additional data from the reporting cytogenetic laboratory were requested when indicated. After the review, five persons were reclassified as not having trisomy 21. Individuals with trisomy 21 and an additional cytogenetic aberration were excluded: XYY (n = 1), XXY (n = 2), XXX (n = 3), translocations (n = 7), inversions (n = 2), and deletion (n = 1). The final cohort consisted of 3,530 individuals (1,928 males and 1,602 females; birth years: 1878–2007). Of these persons, 1,266 (35.9%) were born before April 1968.
Sampling of reference cohort
Based on the initial cohort of persons with DS (n = 3,551), 71,020 persons without DS were randomly selected from the Civil Registration System,18 with a sampling frame of 20:1 and matching on birth year. With the subsequent exclusion of 21 cases from the initial DS cohort (see above), 420 matched referents were dropped, resulting in 70,600 individuals available for comparison in the reference cohort.
Data on death
Information on death was obtained by linkage to the Register of Causes of Death and the Civil Registration System.18,19 Up to three causes of death were recorded for each individual in the Register of Causes of Death, based on the 8th Revision of International Classification of Diseases (ICD8) from 1969 to 1993 or the 10th Revision of International Classification of Diseases (ICD10) since 1994. We used data on the primary cause of death, and in a few cases where “Down syndrome” was coded as the primary cause of death, we used data from the secondary cause of death. Data from the Register of Causes of Death were available between 1 January 1970 and 31 December 2006, and data on vital status and migration from the Civil Registration System were available between 1 April 1968 and 15 January 2009. In this study, we used available information on death over the period from 1 April 1968 to 15 January 2009, from the two sources. In cases of inconsistency between the two registers, we used information from the Civil Registration System.
Information on covariates
We included sex (female, male), birth cohort (before April 1968, 1968–1979, 1980–1989, 1990–1999, and 2000–2007), birth weight (≥2,500 and <2,500 g), congenital heart defects (ICD8: 746–747.4; ICD10: Q20–Q26) (yes, no), and gastrointestinal tract defects (ICD8: 750–751; ICD10: Q38–Q45) (yes, no) as covariates. The available information on these covariates was dependent on when the registers were established. Information on sex and birth year was available for all cohort members. Information on birth weight was obtained from the Medical Birth Register and was available for those born after 1973. Information on congenital malformations was obtained from the National Hospital Register and was available for those born after 1977.
Statistical analysis
We estimated survival probabilities for persons with each of the three DS karyotypes as well as for the reference cohort using the Kaplan–Meier product-limit method. We used Cox proportional hazards regression models to estimate mortality hazard rate ratios for the cohort of persons with DS and for the reference cohort. Cohort members born after 1 April 1968 were followed from birth until time of death, emigration, or the end of follow-up (15 January 2009), whichever came first. For persons born before April 1968, follow-up was left-truncated, starting on 1 April 1968. The Cox regression models were stratified on birth year.
We examined associations of the covariates with mortality by restricting the Cox regression models to the cohort of persons with DS after stratification into mosaic and nonmosaic groups (the latter included standard trisomy 21 and Robertsonian translocation DS). The analyses on birth weight, congenital heart defects, and gastrointestinal tract defects were restricted to various birth periods defined by the availability of the information on these variables. We further checked for effect measure modification on mortality between congenital heart defects and birth cohort by including an interaction term in the regression model. Last, we examined causes of death in relation to karyotype and age at death (<20 and ≥20 years).
Results
Karyotype and congenital heart defects
Of 3,530 persons with DS, 3,272 (92.7%) individuals were classified as standard trisomy 21, 144 (4.1%) as DS resulting from a Robertsonian translocation, and 114 (3.2%) as mosaic trisomy 21. The median percentage of trisomy 21 cells in persons with mosaic DS was 60% (range: 2–95%), but this information was available in only 24 of 114 persons with mosaic DS. The karyotypic composition of persons with DS did not change over the study period (Table 1).
Table 1.
Distribution of persons with DS and congenital heart defects by karyotype and year of birth, Denmark
| Birth cohort | standard trisomy 21
|
robertsonian translocation ds
|
mosaic ds
|
total
|
||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Persons with DS | ||||||||
|
| ||||||||
| Before April 1968 | 1,171 | 92.5 | 53 | 4.2 | 42 | 3.3 | 1,266 | 100.0 |
|
| ||||||||
| 1968–1989 | 1,169 | 91.5 | 62 | 4.9 | 46 | 3.6 | 1,277 | 100.0 |
|
| ||||||||
| 1990–2007 | 932 | 94.4 | 29 | 2.9 | 26 | 2.6 | 987 | 100.0 |
|
| ||||||||
| Total | 3,272 | 92.7 | 144 | 4.1 | 114 | 3.2 | 3,530 | 100.0 |
|
| ||||||||
| Persons with DS and congenital heart defectsa | ||||||||
|
| ||||||||
| 1977–1989 | 274 | 45.1 | 16 | 44.4 | 5 | 18.5 | 295 | 44.0 |
|
| ||||||||
| 1990–2007 | 568 | 60.9 | 17 | 58.6 | 9 | 34.6 | 594 | 60.2 |
|
| ||||||||
| Total | 842 | 54.7 | 33 | 50.8 | 14 | 26.4 | 889 | 53.7 |
DS, Down syndrome.
Born after 1977 (percentage with congenital heart defects).
About 50% of persons with standard trisomy 21 and Robertsonian translocation DS had congenital heart defects, whereas about 25% of persons with mosaic DS had congenital heart defects. Younger cohorts of persons with standard trisomy 21 were more likely to have a diagnosis of congenital heart defects, and a similar tendency was also seen for younger cohorts of persons with Robertsonian translocation DS and mosaic DS (Table 1).
Survival
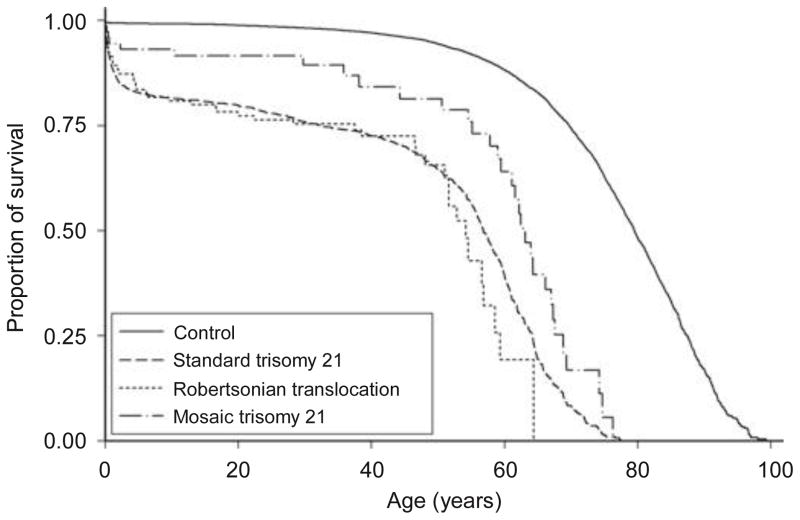
Overall, 1,073 persons with DS died between 1 April 1968 and 15 January 2009 (1,000 deaths for standard trisomy 21, 41 for Robertsonian translocation DS, and 32 for mosaic DS). The estimated 1- and 50-year survival probabilities were 0.89 (95% confidence interval 0.87–0.90) and 0.64 (0.62–0.67), respectively, for persons with standard trisomy 21, 0.91 (0.83–0.96) and 0.66 (0.53–0.75), respectively, for persons with Robertsonian translocation DS, 0.94 (0.86–0.98) and 0.81 (0.67–0.90), respectively, for persons with mosaic DS, whereas the corresponding estimates were 0.99 (0.99–0.99) and 0.94 (0.94–0.95), respectively, for the reference cohort (Figure 1).
Figure 1. Kaplan–meier survival curves for three karyotypes of persons with down syndrome and reference cohort, denmark, 1968–2009.
(Number of persons and deaths: 3,272 and 1,000, respectively, for persons with standard trisomy 21, 144 and 41, respectively, for persons with Robertsonian translocations, 114 and 32, respectively, for persons with mosaic trisomy 21, and 70,590 and 4,683, respectively, for the reference cohort)
Hazard ratio of mortality
Persons with DS had a higher mortality rate than the reference population; adjusted hazard ratio was 8.94 (8.32–9.60) for persons with standard trisomy 21, 10.23 (7.50–13.97) for persons with Robertsonian translocation DS, and 4.98 (3.51–7.08) for persons with mosaic DS. Restricting the analyses to persons who were entered into the cohort at birth (i.e., those born after 1 April 1968) increased these estimates (Table 2). Among those born after 1 April 1968, no significant effect measure modifications were detected between DS or reference group and birth year (P = 0.08 for standard trisomy 21, P = 0.12 for Robertsonian translocation DS, and P = 0.53 for mosaic DS), indicating a rather similar decline in mortality for both persons with DS and the general population in recent decades.
Table 2.
Mortality HRs by karyotype of persons with DS, as compared with reference cohort, Denmark, 1968–2009
| number of persons | number of deaths | crude hr | adjusted hr | 95% ci | |
|---|---|---|---|---|---|
| All persons | |||||
| Reference cohort | 70,590a | 4,683 | 1.00 | 1.00 | Reference |
| Standard trisomy 21 | 3,272 | 1,000 | 9.00 | 8.94 | 8.32–9.60 |
| Robertsonian translocation DS | 144 | 41 | 10.23 | 10.23 | 7.50–13.97 |
| Mosaic DS | 114 | 32 | 4.91 | 4.98 | 3.51–7.08 |
| Persons born after April 1968 | |||||
| Reference cohort | 45,246 | 613 | 1.00 | 1.00 | Reference |
| Standard trisomy 21 | 2,101 | 430 | 17.24 | 16.93 | 14.96–19.16 |
| Robertsonian translocation DS | 91 | 22 | 18.19 | 18.20 | 11.86–27.92 |
| Mosaic DS | 72 | 7 | 7.39 | 7.54 | 3.57–15.90 |
Cox regression with stratification on single year of birth, adjusted for sex.
CI, confidence interval; DS, Down syndrome; HR, hazard rate ratio.
Ten of 70,600 were excluded from the referent population: 7 emigrated, 2 died, and 1 was lost to follow-up before 1 April 1968.
Analyses of mortality by covariates
Analyses of mortality among persons with DS by covariate strata are shown in Table 3. Among persons with nonmosaic DS karyotypes, mortality declined from earlier to more recent birth cohorts, and those who were of low birth weight or who had congenital heart defects or gastrointestinal tract defects had higher mortality rates than those without the birth characteristic. Similar tendencies were seen for persons with mosaic DS, although the numbers were sparse. No significant difference in mortality was seen between males and females with DS.
Table 3.
Adjusted mortality HRs for covariates among persons with mosaic or nonmosaic DS, Denmark, 1968–2009
| coarivate | mosaic ds
|
nonmosaic ds
|
||||||
|---|---|---|---|---|---|---|---|---|
| number of persons | number of deaths | adjusted hr | 95% ci | number of persons | number of deaths | adjusted hr | 95% ci | |
| Sex | ||||||||
|
| ||||||||
| Female | 66 | 17 | 1.00 | Reference | 1,536 | 476 | 1.00 | Reference |
|
| ||||||||
| Male | 48 | 15 | 1.06 | 0.51–2.24 | 1,880 | 565 | 1.06 | 0.94–1.20 |
|
| ||||||||
| Birth cohort | ||||||||
|
| ||||||||
| Before April 1968 | 42 | 25 | 0.54 | 0.06–4.69 | 1,224 | 589 | 1.19 | 0.91–1.56 |
|
| ||||||||
| 1968–1979 | 22 | 5 | 1.00 | Reference | 754 | 236 | 1.00 | Reference |
|
| ||||||||
| 1980–1989 | 24 | 1 | 0.23 | 0.03–2.11 | 477 | 114 | 0.83 | 0.66–1.04 |
|
| ||||||||
| 1990–1999 | 19 | 1 | 0.31 | 0.03–2.78 | 616 | 70 | 0.41 | 0.31–0.53 |
|
| ||||||||
| 2000–2007 | 7 | 0 | – | 345 | 32 | 0.36 | 0.25–0.53 | |
|
| ||||||||
| P for trend | 0.217 | <0.001 | ||||||
|
| ||||||||
| Birth weighta | ||||||||
|
| ||||||||
| ≥2,500 g | 49 | 3 | 1.00 | Reference | 1,468 | 246 | 1.00 | Reference |
|
| ||||||||
| <2,500 g | 12 | 2 | 1.41 | 0.19–10.36 | 342 | 97 | 1.89 | 1.50–2.40 |
|
| ||||||||
| Congenital heart defectsb | ||||||||
|
| ||||||||
| Absence | 39 | 0 | – | NA | 729 | 54 | 1.00 | Reference |
|
| ||||||||
| Presence | 14 | 2 | – | NA | 875 | 211 | 4.67 | 3.44–6.32 |
|
| ||||||||
| Gastrointestinal tract defectsb | ||||||||
|
| ||||||||
| Absence | 49 | 1 | 1.00 | Reference | 1,469 | 233 | 1.00 | Reference |
|
| ||||||||
| Presence | 4 | 1 | 12.37 | 0.73–208.41 | 135 | 32 | 1.62 | 1.12–2.35 |
Cox regression, adjusted for sex and birth cohort.
CI, confidence interval; DS, Down syndrome; HR, hazard rate ratio; NA, not applicable.
Among those born after 1973.
Among those born after 1977.
The decline in mortality rates among persons with nonmosaic DS occurred only in persons with congenital heart defects (a significant effect measure modification on mortality was observed between congenital heart defects and birth year, P < 0.001) (Supplementary Table S1 online). Mortality in the first year of life revealed a similar trend (data not shown).
Causes of death
Causes of death were available for 983 persons with DS, with about one-third dying from congenital heart defects (Supplementary Table S2 online). The proportion of deaths due to congenital heart defects was greater among persons with standard trisomy 21 and Robertsonian translocation DS than among persons with mosaic DS, whereas the opposite was true for deaths due to neoplasm and diseases of the circulatory system.
Congenital heart defects were the main cause of death among persons with DS who died before the age of 20 years, whereas deaths due to diseases of the respiratory systems and the circulatory system were the main reasons among persons with DS who died after the age of 20 years.
Discussion
In this national cohort of persons with DS who were followed over a 40-year period, we observed that persons with DS had a higher mortality than the general population, with better survival for persons with mosaic DS than for persons with standard trisomy 21 or Robertsonian translocation DS. In accordance with the general population, more recent birth cohorts had lower mortality rates than earlier birth cohorts. The decline in mortality was in particular seen for persons with DS who were born with congenital heart defects. We observed no changes in the DS karyotypic composition over the study period, which has not been reported before.
Previous studies reported that persons with DS had a mortality of 5–11 times that of the general population,9,11,20,21 and our estimates support this. Many previous studies lacked information on mortality early in life, as our study does for those born before 1968. This biases the relative risk estimates toward lower values, because death in infancy is disproportionately more frequent among people with DS.22 As expected, restricting the analysis in our study to the cohort that was followed from birth increased the relative mortality rates (Table 1). Persons with Robertsonian translocation DS had a survival pattern similar to persons with standard trisomy 21, which is expected. Due to small sample size, its survival curve fluctuated around that of standard trisomy 21 (Figure 1). Persons with mosaic DS would be expected to have a lower mortality than those with other DS karyotypes, because these people tend to have milder phenotypic manifestations,6 and our data support this and are consistent with the findings in a previous study.14
Most previous studies,9,13,20,23–26 although not all,27 observed no statistically significant differences in mortality between males and females with DS. We found a 6% higher mortality in males but with confidence intervals that included no difference. Persons with DS who were of low birth weight or who had congenital heart or gastrointestinal tract defects had higher mortality.9,12,26 One of our novel findings is that all of the decline in DS mortality rate found in our study appears to have occurred among persons with congenital heart defects, and this indicates an effect of treatment for this group. We also found that persons with DS who died before the age of 20 years were most likely to have died from congenital heart defects. More recent cohorts of persons with DS were more likely to have a diagnosis of congenital heart defects, probably indicating more complete diagnoses of congenital heart defects (early in life) rather than increased prevalence.15 As we had limited information on the severity of the defects, we could not exclude the possibility that the improvement in survival of children with congenital heart defects might reflect the possibility that live births with DS in recent birth cohorts may exclude cases with more severe forms of congenital heart defects that were prenatally diagnosed and resulted in subsequent pregnancy terminations.3
The elderly persons with DS in our study were all individuals with DS who entered the cohort in April 1968. Cytogenetic diagnostics became available in the early 1960s, and our data included only persons with DS with cytogenetic verification. It is possible that elderly persons with DS who were functioning well in 1968 would not have been candidates for laboratory testing until they came into contact with the health-care system for other reasons. If so, we may also have oversampled elderly persons with DS with health problems who might have higher mortality at relatively younger ages as compared with elderly persons with DS who did not get karyotyped.
We believe that all persons with DS born after 1968 are very likely to have been diagnosed cytogenetically, and most of them were diagnosed within the first days of life. We started observation at the time of birth, not at the time of diagnosis, because we believe that all those who died early in life would be diagnosed if they had DS and the actual time of diagnosis might be influenced by comorbidities. To substantiate this assumption, we repeated all analyses by comparing children from the age of 1 and found similar results as those presented. We also found similar results when starting observation at the time of diagnosis rather than at the time of birth for those born after April 1968.
Our study had a number of strengths. We identified a large number of persons with DS from Denmark from the Danish Cytogenetic Register, and these persons were classified into groups with different karyotypes based on the results of cytogenetic testing. These persons were followed for up to 40 years, and we had virtually complete follow-up for all persons with DS in the country, due to the high-quality population-based registers on mortality and migration.
The Danish Cytogenetic Register provides a unique opportunity to study persons with a specific chromosome abnormality, such as DS, Klinefelter syndrome, Turner syndrome, and several other cytogenetic conditions.16,28 By linking a cohort of persons with a specific chromosome abnormality to other national registers, it is possible to investigate mortality and comorbidities, including cancer risk, among persons with these chromosome abnormalities.29–31 In addition, this approach can allow identification of new genetic regions that might contribute to the risk for other conditions, such as autism and schizophrenia.32,33
Results show a declining mortality rate for persons with DS over time that probably resulted from improvement in the treatment of heart malformations. It is important to understand the factors, including comorbidities, that affect survival among persons with DS. In addition, these data suggest that there is a need to study the role of healthcare and other social services needed for persons with DS as they age.
Supplementary Material
Acknowledgments
The study was supported by a cooperative agreement from the US Centers for Disease Control and Prevention (#5 U10 DD000230-06). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. We thank Claus Sværke from University of Aarhus and Jan Hansen from the Danish Cytogenetic Registry for data arrangement.
Footnotes
Supplementary material is linked to the online version of the paper at http://www.nature.com/gim
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Centers for Disease Control and Prevention (CDC) Improved national prevalence estimates for 18 selected major birth defects–United States, 1999–2001. MMWR Morb Mortal Wkly Rep. 2006;54:1301–1305. [PubMed] [Google Scholar]
- 2.Mégarbané A, Ravel A, Mircher C, et al. The 50th anniversary of the discovery of trisomy 21: the past, present, and future of research and treatment of Down syndrome. Genet Med. 2009;11:611–616. doi: 10.1097/GIM.0b013e3181b2e34c. [DOI] [PubMed] [Google Scholar]
- 3.Cocchi G, Gualdi S, Bower C, et al. International trends of Down syndrome 1993–2004: Births in relation to maternal age and terminations of pregnancies. Birth Defects Res Part A Clin Mol Teratol. 2010;88:474–479. doi: 10.1002/bdra.20666. [DOI] [PubMed] [Google Scholar]
- 4.Mikkelsen M, Fischer G, Stene J, Stene E, Petersen E. Incidence study of Down’s syndrome in Copenhagen, 1960–1971; with chromosome investigation. Ann Hum Genet. 1976;40:177–182. doi: 10.1111/j.1469-1809.1976.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 5.Mutton D, Alberman E, Hook EB. Cytogenetic and epidemiological findings in Down syndrome, England and Wales 1989 to 1993. National Down Syndrome Cytogenetic Register and the Association of Clinical Cytogeneticists. J Med Genet. 1996;33:387–394. doi: 10.1136/jmg.33.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modi D, Berde P, Bhartiya D. Down syndrome: a study of chromosomal mosaicism. Reprod Biomed Online. 2003;6:499–503. doi: 10.1016/s1472-6483(10)62174-8. [DOI] [PubMed] [Google Scholar]
- 7.Fishler K, Koch R, Donnell GN. Comparison of mental development in individuals with mosaic and trisomy 21 Down’s syndrome. Pediatrics. 1976;58:744–748. [PubMed] [Google Scholar]
- 8.Rosecrans CJ. The relationship of normal–21-trisomy mosaicism and intellectual development. Am J Ment Defic. 1968;72:562–566. [PubMed] [Google Scholar]
- 9.Day SM, Strauss DJ, Shavelle RM, Reynolds RJ. Mortality and causes of death in persons with Down syndrome in California. Dev Med Child Neurol. 2005;47:171–176. doi: 10.1017/s0012162205000319. [DOI] [PubMed] [Google Scholar]
- 10.Glasson EJ, Sullivan SG, Hussain R, Petterson BA, Montgomery PD, Bittles AH. The changing survival profile of people with Down’s syndrome: implications for genetic counselling. Clin Genet. 2002;62:390–393. doi: 10.1034/j.1399-0004.2002.620506.x. [DOI] [PubMed] [Google Scholar]
- 11.Hill DA, Gridley G, Cnattingius S, et al. Mortality and cancer incidence among individuals with Down syndrome. Arch Intern Med. 2003;163:705–711. doi: 10.1001/archinte.163.6.705. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen SA, Wong LY, Correa A, Gambrell D, Friedman JM. Survival in infants with Down syndrome, Metropolitan Atlanta, 1979–1998. J Pediatr. 2006;148:806–812. doi: 10.1016/j.jpeds.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down’s syndrome in the USA from 1983 to 1997: a population-based study. Lancet. 2002;359:1019–1025. doi: 10.1016/s0140-6736(02)08092-3. [DOI] [PubMed] [Google Scholar]
- 14.Shin M, Siffel C, Correa A. Survival of children with mosaic Down syndrome. Am J Med Genet A. 2010;152A:800–801. doi: 10.1002/ajmg.a.33295. [DOI] [PubMed] [Google Scholar]
- 15.Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122:2254–2263. doi: 10.1161/CIRCULATIONAHA.110.947002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen J. Topics in Human Genetics. Georg Thieme; Stuttgart: 1980. The Danish Cytogenetic Central Register: Organization and Results. [Google Scholar]
- 17.Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet. 2000;355:165–169. doi: 10.1016/S0140-6736(99)05264-2. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 19.Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health. 2011;39(7 suppl):26–29. doi: 10.1177/1403494811399958. [DOI] [PubMed] [Google Scholar]
- 20.Øster J, Mikkelsen M, Nielsen A. Mortality and life-table in Down’s syndrome. Acta Paediatr Scand. 1975;64:322–326. doi: 10.1111/j.1651-2227.1975.tb03842.x. [DOI] [PubMed] [Google Scholar]
- 21.Goldman SE, Urbano RC, Hodapp RM. Determining the amount, timing and causes of mortality among infants with Down syndrome. J Intellect Disabil Res. 2011;55:85–94. doi: 10.1111/j.1365-2788.2010.01349.x. [DOI] [PubMed] [Google Scholar]
- 22.Penrose LS. The incidence of mongolism in the general population. J Ment Sci. 1949;95:685– 688. doi: 10.1192/bjp.95.400.685. [DOI] [PubMed] [Google Scholar]
- 23.Baird PA, Sadovnick AD. Life expectancy in Down syndrome adults. Lancet. 1988;2:1354–1356. doi: 10.1016/s0140-6736(88)90881-1. [DOI] [PubMed] [Google Scholar]
- 24.Coppus AM, Evenhuis HM, Verberne GJ, et al. Survival in elderly persons with Down syndrome. J Am Geriatr Soc. 2008;56:2311–2316. doi: 10.1111/j.1532-5415.2008.01999.x. [DOI] [PubMed] [Google Scholar]
- 25.Dupont A, Vath M, Videbech P. Mortality and life expectancy of Down’s syndrome in Denmark. J Ment Defic Res. 1986;30 (Pt 2):111–120. doi: 10.1111/j.1365-2788.1986.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 26.Masaki M, Higurashi M, Iijima K, et al. Mortality and survival for Down syndrome in Japan. Am J Hum Genet. 1981;33:629–639. [PMC free article] [PubMed] [Google Scholar]
- 27.Glasson EJ, Sullivan SG, Hussain R, Petterson BA, Montgomery PD, Bittles AH. Comparative survival advantage of males with Down syndrome. Am J Hum Biol. 2003;15:192–195. doi: 10.1002/ajhb.10132. [DOI] [PubMed] [Google Scholar]
- 28.Videbech P, Nielsen J. Electronic data processing in the Danish cytogenetic central register and EDP problems of registers in general. Clin Genet. 1979;15:137–146. doi: 10.1111/j.1399-0004.1979.tb01752.x. [DOI] [PubMed] [Google Scholar]
- 29.Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab. 2006;91:3897–3902. doi: 10.1210/jc.2006-0558. [DOI] [PubMed] [Google Scholar]
- 30.Gravholt CH, Juul S, Naeraa RW, Hansen J. Morbidity in Turner syndrome. J Clin Epidemiol. 1998;51:147–158. doi: 10.1016/s0895-4356(97)00237-0. [DOI] [PubMed] [Google Scholar]
- 31.Hasle H, Olsen JH, Nielsen J, Hansen J, Friedrich U, Tommerup N. Occurrence of cancer in women with Turner syndrome. Br J Cancer. 1996;73:1156–1159. doi: 10.1038/bjc.1996.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauritsen M, Mors O, Mortensen PB, Ewald H. Infantile autism and associated autosomal chromosome abnormalities: a register-based study and a literature survey. J Child Psychol Psychiatry. 1999;40:335–345. [PubMed] [Google Scholar]
- 33.Mors O, Ewald H, Blackwood D, Muir W. Cytogenetic abnormalities on chromosome 18 associated with bipolar affective disorder or schizophrenia. Br J Psychiatry. 1997;170:278–280. doi: 10.1192/bjp.170.3.278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.