Abstract
Fms-like tyrosine kinase-3 ligand (Flt3L) uniquely binds the Flt3 (CD135) receptor expressed on hematopoietic stem cells (HSC), early progenitor cells, immature thymocytes, and steady state dendritic cells (DC) and induces their proliferation, differentiation, development and mobilization in the bone marrow, peripheral blood, and lymphoid organs. CDX-301 has an identical amino acid sequence and comparable biologic activity as the previously-tested rhuFlt3L which had discontinued clinical development over a decade ago. This Phase 1 trial assessed the safety, pharmacokinetic, pharmacodynamic and immunologic profile of CDX-301, explored alternate dosing regimens and examined the impact of rhuFlt3L on key immune cell subsets. Thirty healthy volunteers received CDX-301 (1–75 μg/kg/day) over 5–10 days. One event of Grade 3 community acquired pneumonia occurred. There were no other infections, DLTs, or SAEs. CDX-301 resulted in effective peripheral expansion of monocytes, hematopoietic stem and progenitor cells, and key subsets of myeloid DC and plasmacytoid DC, with no clear effect on regulatory T cells. These data from healthy volunteers support the potential for CDX-301, as monotherapy or in combination with other agents, in various indications including allogeneic HSC transplantation and immunotherapy, but the effects of CDX-301 will need to be investigated in each of these patient populations.
Introduction
Fms-like tyrosine kinase-3 ligand (Flt3L) uniquely binds Fms-like tyrosine kinase-3 (Flt3, CD135), which is expressed on hematopoietic stem cells (HSC), hematopoietic progenitor cells (HPC), immature thymocytes, and steady state dendritic cells (DC),1–3 resulting in the proliferation, differentiation, development and mobilization of these cells in the bone marrow, peripheral blood, and lymphoid organs. In earlier clinical trials of recombinant human (rhu) Flt3L in autologous hematopoietic stem cell transplantation (HSCT) for breast cancer,4,5 non-Hodgkin lymphoma, and ovarian cancer,6 rhuFlt3L administered daily × 14 with GM-CSF or G-CSF produced higher and more sustained levels of hematopoietic progenitor cells (CD34+ and colony-forming cells) in the apheresis product than G-CSF alone.
Mobilization of Flt3 receptor-expressing HSC, myeloid and lymphoid progenitor cells, DC, and especially immature thymocytes1 may be of particular importance in allogeneic HSCT, where full recovery of a functional immune system is slow and incomplete.7 Innate immunity, including neutrophils and NK cells, recovers more quickly. However, donor DC competence is somewhat delayed and B cell and, to a greater extent, T cell immune reconstitution is quite delayed, potentially accounting for increased opportunistic infection and malignant relapse. CD34+Flt3+ HSC can reconstitute lymphopoiesis in vivo in NOD/SCID mice,8 indicating that rhuFlt3L may overcome long lasting T cell deficiencies currently associated with HSCT.
RhuFlt3L increases both the CD11c+ DC and the CD11c− IL-3R+ DC precursors in the blood of healthy volunteers.9,10 In studies of rhuFlt3L as an adjuvant for innate or vaccine immunity,11–15 rhuFlt3L administered daily for 14 days with repeating monthly cycles was generally well tolerated with increases in circulating DC, however, neither enhanced immune or clinical responses were generally observed. RhuFlt3L-expanded DCs expressed low levels of costimulatory molecules required for inducing T cell activation, suggesting the need for additional DC-activating agents in order to enhance immune responses.16–18
Clinical evidence of potential utility in cancer therapy was reported by Fong et al. who used rhuFlt3L to expand DCs in vivo prior to leukapheresis, then loaded rhuFlt3L-expanded DCs with a carcinoembryonic antigen-derived peptide.19 After immunization with this cellular vaccine, two of 12 patients experienced dramatic tumor regression, one patient had a mixed response, and two had stable disease.
Thus, there is an abundance of pre-clinical and clinical data supporting the use of rhuFlt3L in stem cell mobilization for transplantation, cancer immunotherapy, and as a vaccine adjuvant, although additional questions remain to be answered. After a long pause, the clinical development of soluble rhuFlt3L (now designated CDX-301) has been reinitiated using a new manufacturing process including a proprietary Chinese hamster ovary cell production line and serum-free cell culture. CDX-301 was well-tolerated in toxicology studies in Cynomolgus monkeys and rats. This initial clinical study was performed to support the development of CDX-301 for these potential indications.
Materials and Methods
Clinical Study Design
This Phase 1 study was designed to broaden the biological characterization of rhuFlt3L in humans and to investigate mobilization using various doses and schedules of CDX-301. Study objectives were to evaluate the safety, pharmacokinetic, pharmacodynamic and immunologic profile of CDX-301. Specific pharmacodynamic objectives were to determine the effect of CDX-301 on circulating CD34+ stem cells, dendritic cells and dendritic cell subsets, regulatory T cells, NK cells and other hematopoietic/immune cells.
Using a standard 3+3 dose-escalating design, sequential cohorts of 3–6 healthy volunteers received CDX-301 by daily subcutaneous injection at a dose level of 1, 3, 10, 25 or 75 μg/kg for 5 days (Cohorts 1–5), 25 μg/kg for 7 days (Cohort 6), or 25 μg/kg for 10 days (Cohort 7). Toxicity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Dose-limiting toxicity (DLT) was defined as any treatment-related toxicity with severity ≥ Grade 2, excluding transient Grade 2 injection site reactions and transient asymptomatic Grade 2–3 hematologic changes attributed to the expected pharmacodynamic effects of CDX-301. The maximum tolerated dose was defined as the highest dose level in which <2 DLT were observed. Subjects were followed for safety and tolerability through 28 days following the last dose. The protocol (ClinicalTrials.gov identifier: NCT01465139) was approved by the Institutional Review Board (IRB) of The Rockefeller University and all participants provided informed consent.
Subjects
Male or female healthy volunteers, age 18 to 55 years, with body mass index 18–30, willing to use contraception and abstain from alcohol were enrolled. Exclusionary conditions included significant medical conditions; significant laboratory or electrocardiogram abnormalities; active or chronic infection including HIV or hepatitis B or C; syphilis acquired within 12 months; herpes zoster within 3 months; asthma requiring inhaled or oral medication within 5 years; clinically apparent or familial history autoimmune disease; history of tuberculosis, positive PPD, immunodeficiency, thrombosis, abnormally robust scarring reactions, or cancer. Subjects who had received immunosuppressive agents within a year; donated blood or received monoclonal antibody- or immunoglobulin-based fusion proteins within 8 weeks; started any new prescription medications within 6 weeks; or received any non-study vaccination or experimental therapy within 4 weeks were also ineligible.
Flow Cytometry of Hematopoietic Subsets
PBMCs were isolated from heparinized blood using ficoll-hypaque. PBMCs were stained with two cocktails ([reagents from BioLegend, unless otherwise indicated]; cocktail 1: CD19 [Invitrogen; clone SJ25-C1], CD3 [Invitrogen; clone S4.1], CD335 [clone 9E2], CD66b [clone G10F5], BDCA-1 [clone L161], BDCA-2 [Miltenyi; clone AC144], BDCA-3 [Miltenyi; clone AD5-14H12], CD34 [clone 581], CD14 [Invitrogen; clone TüK4] and Live/Dead Fixable Dead Cell Stain [Life Technologies]; cocktail 2: CD3 [Becton Dickinson; clone SK7], CD8 [Becton Dickinson; clone SK1, CD4 [clone OKT4], FoxP3 [clone 206D], CD25 [clone BC96], CD127 [clone A019D5], Live/Dead Fixable Dead Cell Stain). For surface staining, titrated antibodies were added to 2 million cells in 50 μl PBS for 20 min at 4°C. Intracellular staining for FoxP3 was conducted by incubating cells stained for cell surface markers with fixation/permeabilization solution (eBioscience) for 30 min at 4°C; cells were then stained in permeabilization buffer (eBioscience) with FoxP3 Ab or isotype control for 60 min at 4°C. Washed cells were fixed in 2% formaldehyde and stored at 4°C until analysis, which was performed using an LSR II flow cytometer (BD Biosciences). The whole sample was acquired and analysis was performed using Flow Jo 9.7 software (Tree Star). The gating strategy for analysis of the immune cell subsets is presented in Figure S1 (on-line only).
Immunogenicity and Pharmacokinetics
Serum samples were analyzed for the presence of anti-CDX-301 antibodies at day 21 and End of Treatment for all subjects. Additional samples were analyzed at day 14 for subjects receiving 75 μg/kg/day for 5 days and 25 μg/kg/day for 7 or 10 days. In a “bridging” format immunoassay, CDX-301 was coated directly in microtiter plate wells, blocked with albumin, incubated with the human serum samples, and detected with biotinylated-CDX-301 followed by a streptavidin-horseradish peroxidase conjugate and colorimetric substrate tetramethylbenzidine. A positive response was one above the 95% confidence interval for results from 30 naïve, normal human serum samples. Assay sensitivity was determined to be 31 ng/ml using purified polyclonal rabbit anti-Flt3L antibody.
Blood samples for pharmacokinetic analyses were obtained daily throughout treatment (prior to each dose); at 1, 3, 6, 9, and 12 hours after the first and last dose; and daily for at least four days following treatment. CDX-301 was quantified using an immunoassay in which mouse anti-human Flt3L IgG1 (mAb clone 40416, R&D Systems) was coated directly in microtiter plate wells, blocked with albumin, incubated with the serum samples, and detected with affinity purified and biotinylated goat anti-human Flt3L polyclonal antibody (R&D Systems) followed by peroxidase-conjugated streptavidin and colorimetric substrate tetramethylbenzidine. Concentrations over the range of 0.62 to 40 ng/ml were determined relative to CDX-301 standards.
Pharmacokinetic parameters were estimated from the CDX-301 concentrations using a non-compartmental analysis for extravascular administration (model 200, WinNonlin Version 5.3; Pharsight). The last three observed concentrations of CDX-301 were used to estimate the terminal phase disposition rate and additional time points were included if they improved the least squares correlation coefficient.
CDX-301
CDX-301 was purified from rhuFlt3L expressed in a Chinese hamster ovary cell line using anion exchange, hydrophobic interaction, and cation exchange chromatography. CDX-301 was fully characterized with respect to identity, purity and potency using appropriate GMP assays and testing was conducted to ensure stability.
Results
Dosing and Toxicity
Thirty subjects were enrolled and treated with CDX-301 (Table 1). Overall, CDX-301 was well-tolerated. All enrolled subjects completed the planned duration of dosing and 28-day safety follow up. A single subject receiving CDX-301 at 75 μg/kg/day for 5 days developed community acquired pneumonia on day 12. This subject had a remote history of community acquired pneumonia. This episode of pneumonia responded rapidly to antibiotic treatment, and he fully recovered within 2 weeks. Although the attribution to CDX-301 is unclear, the event was considered a DLT due to the temporal relationship to dosing. The cohort was expanded to a total of six subjects, and the study was completed with no additional infections or DLT. Additional treatment-related toxicity was infrequent. Transient lymphadenopathy (grade 1) was seen in six subjects (25 or 75 μg/kg/day cohorts) and single cases of diarrhea, injection site erythema, folliculitis, and dry mouth (all grade 1) were observed. No anti-CDX-301 antibodies were detected in any subject throughout treatment and follow up.
Table 1.
Treatment Cohorts in Phase I Clinical Study of CDX-301
| Cohort | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Dosing Duration | 5 days | 7 days | 10 days | ||||
| CDX-301 Dose Level | 1 μg/kg | 3 μg/kg | 10 μg/kg | 25 μg/kg | 75 μg/kg | 25 μg/kg | 25 μg/kg |
| Subjects Treated | 3 | 3 | 3 | 3 | 6 | 6 | 6 |
|
| |||||||
| Baseline Characteristics | |||||||
|
| |||||||
| Age, years (median [range]) | 34 (32, 49) | 32 (28, 34) | 34 (26, 38) | 21 (21, 35) | 31 (20, 42) | 34 (19, 54) | 45 (37, 48) |
| Male (n [%]) | 0 (0%) | 2 (67%) | 2 (67%) | 0 (0%) | 6 (100%) | 5 (83%) | 5 (83%) |
| Race (n [%]) | |||||||
| Black/African American | 2 (67%) | 2 (67%) | 1 (33%) | 1 (33%) | 2 (33%) | 2 (33%) | 2 (33%) |
| White | 0 (0%) | 1 (33%) | 1 (33%) | 2 (67%) | 2 (33%) | 0 (0%) | 4 (67%) |
| Asian | 0 (0%) | 0 (0%) | 1 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Other/Unknown | 1 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (33%) | 4 (67%) | 0 (0%) |
| Cell count (mean, SE) | |||||||
| WBC, K/μL | 5.5 (1.3) | 7.4 (2.1) | 4.6 (0.9) | 5.1 (0.4) | 5.3 (0.9) | 5.8 (0.3) | 6.1 (0.9) |
| Monocytes, K/μL | 0.23 (0.03) | 0.33 (0.03) | 0.23 (0.03) | 0.30 (0.00) | 0.33 (0.06) | 0.28 (0.05) | 0.37 (0.07) |
| CD34+, /mL | 551 (270) | 2325 (894) | 1314 (166) | 947 (189) | 2553 (580) | 2116 (602) | 2383 (424) |
| BDCA-1+CD14−, /mL | 6062 (1394) | 17548 (2019) | 17700 (6837) | 12729 (3605) | 13650 (2104) | 11122 (1768) | 10204 (860) |
| BDCA-3+CD14−, /mL | 590 (337) | 1677 (715) | 1553 (441) | 708 (263) | 1087 (184) | 1678 (260) | 995 (138) |
| BDCA-2+CD14−, /mL | 4008 (1107) | 2690 (1986) | 5815 (1502) | 5814 (1670) | 8101 (2329) | 6560 (805) | 5824 (502) |
| Treg, /mL | 3585 (1995) | 6131 (3451) | 4376 (1237) | 3479 (843) | 2959 (614) | 1574 (502) | 1794 (322) |
|
| |||||||
| Study Treatment and Tolerability | |||||||
|
| |||||||
| Completed CDX-301 treatment course | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 6 (100%) | 6 (100%) | 6 (100%) |
|
| |||||||
| Treatment-Related Adverse Events | None | None | None | Lymphadenopathy (Gr1, n=1) | Diarrhea (Gr1, n=1) Injection site erythema (Gr1, n=1) Lymphadenopathy (Gr1, n=3) Pneumonia (Gr3, n=1)* |
Dry mouth (Gr1, n=1) Folliculitis (Gr1, n=1) Lymphadenopathy (Gr1, n=1) |
Lymphadenopathy (Gr1, n=1) |
Gr, NCI-CTCAE severity grade
Event was considered serious and a dose-limiting toxicity.
Pharmacokinetics
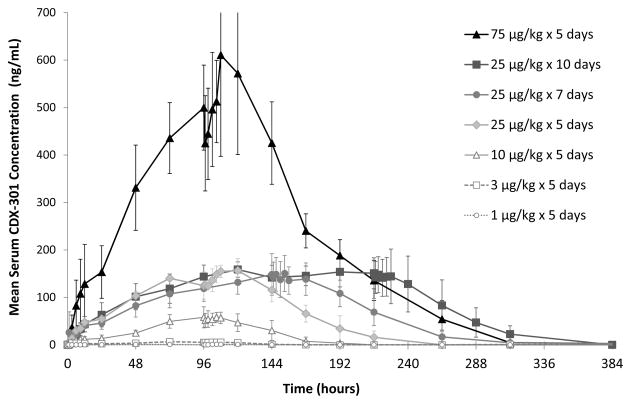
CDX-301 exhibited an absorption profile typical for subcutaneous administration. Mean times to maximum serum concentration (Tmax) ranged from 12.9 hours at 3 μg/kg/day to 24.1 hours at 25 μg/kg/day. Mean maximum serum concentrations (Cmax) on day 1 ranged from 3.7 to 161 ng/ml in subjects administered 3–75 μg/kg/day for 5 days and increased linearly with dose. Similarly, the area under the curve (AUC) values increased across the entire range in a dose-independent manner. The half-life of the terminal disposition phase was determined following the final dose administration and ranged from 12.3 hours at 10 μg/kg/day to 28 hours at 75 μg/kg/day. The mean serum concentrations of CDX-301 for each cohort are plotted as a function of time in Figure 1.
Figure 1.
CDX-301 serum concentrations are shown as a function of time following the first dose of CDX-301. CDX-301 concentrations were determined by immunoassay and mean values (± standard deviations) for each study day are plotted for the cohorts as indicated in the legend.
Expansion of Hematopoietic Cell Subsets
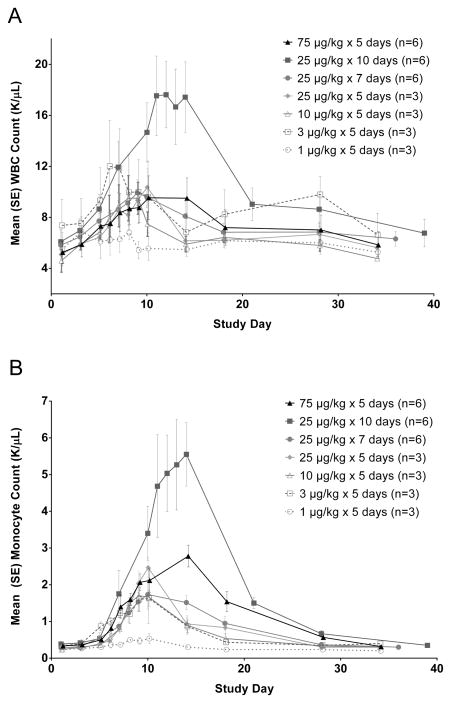
CDX-301 administration produced increases in peripheral blood white blood cells (WBC) which appears to be driven largely by increases in monocytes (Figure 2). Lymphocytes and neutrophils were not greatly affected by CDX-301 (data not shown), although modest increases in neutrophils were observed in the cohort administered 25 μg/kg/day for 10 days. In the lowest dose cohort (1 μg/kg/day × 5 days), increases in WBC and monocytes were minimal. In the other dose groups receiving 5–7 daily doses, the numbers of WBC and monocytes peaked around day 10 at similar numbers and subsequently declined to baseline over days 10–20. The cohort with an extended duration of dosing (10 days at 25 μg/kg/day) had significantly increased monocytes and WBC counts.
Figure 2.
CDX-301 increases WBC and monocyte counts in the peripheral blood. WBC (Figure 2A) and monocyte (Figure 2B) counts are plotted as mean values (± standard error) for the cohorts as indicated in the legend.
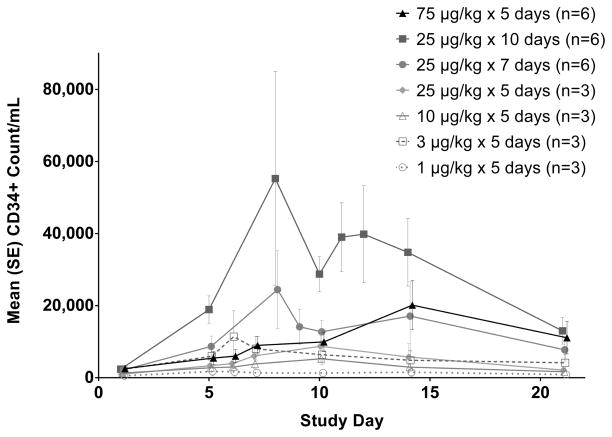
Similarly, the lowest dose of CDX-301 (1 μg/kg/day × 5 days) had minimal effect on circulating CD34+ cells, which include HSC and HPC (Figure 3). While there was noticeable inter-subject variability in CD34+ counts, all other dose cohorts had increases in the numbers of circulating CD34+ cells which peaked over days 8–14. Compared to the WBC and monocyte populations, the decline in numbers of CD34+ cells appeared somewhat slower, in some cases requiring greater than 20 days to return to baseline values. Dosing for 5 days achieved CD34+ cell counts ≥ 7000/ml, a level generally thought to be adequate for peripheral blood stem cell transplantation, but this was more consistent with the longer dosing regimens (Table 2).
Figure 3.
CDX-301 increases the number of CD34 high cells in the peripheral blood. Graphs show the kinetics of cell number per ml of blood over 21 days.. The absolute numbers per milliliter of blood were obtained by multiplying the number of cells (obtained by flow cytometry) by the total number of PBMCs per milliliter of blood. CD34 high cells numbers are plotted as mean values (± standard error) for the cohorts as indicated in the legend.
Table 2.
Peripheral blood CD34+ levels following CDX-301
| Cohort Number | CDX-301 Dose Regimen | Subjects Achieving CD34+ cell count ≥ 7000/ml N (%) | ||
|---|---|---|---|---|
| Day 5 | Day 7 | Day 10 | ||
| 1 | 1 μg/kg/day × 5 days | 0/3 (0) | 0/3 (0) | 0/3 (0) |
| 2 | 3 μg/kg/day × 5 days | 1/3 (33) | 1/3 (33) | 1/3 (33) |
| 3 | 10 μg/kg/day × 5 days | 0/3 (0) | 0/3 (0) | 0/3 (0) |
| 4 | 25 μg/kg/day × 5 days | 1/3 (33) | 1/3 (33) | 1/3 (33) |
| 5 | 75 μg/kg/day × 5 days | 2/6 (33) | 3/6 (50) | 3/6 (50) |
| 6 | 25 μg/kg/day × 7 days | 3/6 (50) | 4/6 (67)* | 4/6 (67) |
| 7 | 25 μg/kg/day × 10 days | 6/6 (100) | 6/6 (100)* | 6/6 (100) |
Day 8 (day 7 not available)
As previously noted (Figure 1), administration for 5 days at 75 μg/kg/day resulted in much greater CDX-301 exposure than did 10 days at 25 μg/kg/day; however, the latter produced greater CD34+ cell increases. This indicates that longer duration of dosing, rather than higher concentration of CDX-301 in the circulation, was responsible for the greater expansion of the CD34+ cells. CDX-301 administration for 10 days at 25 μg/kg/day resulted in a 23-fold increase from a mean baseline count of 2.4/μl to a peak of 55.3/μl.
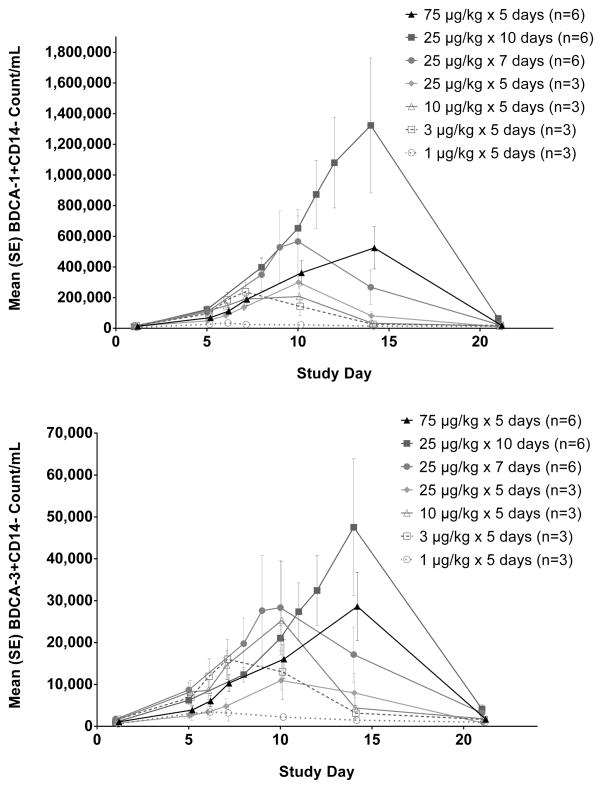
Similarly, the myeloid DC numbers resulting from 10 days of CDX-301 at 25 μg/kg/day exceeded those from 5 days at 75 μg/kg/day (Figure 4). CDX-301 administration for 10 days at 25 μg/kg/day resulted in a 130-fold increase in the blood DC antigen-1 (BDCA-1+) population from a mean baseline count of 10.2/μl to a peak level of 1323/μl. For the BDCA-3+ subset, 10 days of CDX-301 at 25 μg/kg/day resulted in a 48-fold increase from a mean baseline count of 0.99/μl to a peak level of 47.5/μl. Except for the lowest dose cohort (1 μg/kg/day × 5 days) that had minimal effects on myeloid DC numbers, all other dose cohorts produced large increases in circulating myeloid DC.
Figure 4.
CDX-301 increases the number of myeloid dendritic cells in the peripheral blood. Graphs show the kinetics of cell number per ml of blood over 21 days. The absolute numbers per milliliter of blood were obtained by multiplying the number of cells (obtained by flow cytometry) by the total number of PBMCs per milliliter of blood. The numbers of BDCA-1+ myeloid DCs in Figure 4A and BDCA-3 high myeloid DCs in Figure 4B are plotted as mean values (± standard error) for the cohorts as indicated in the legend.
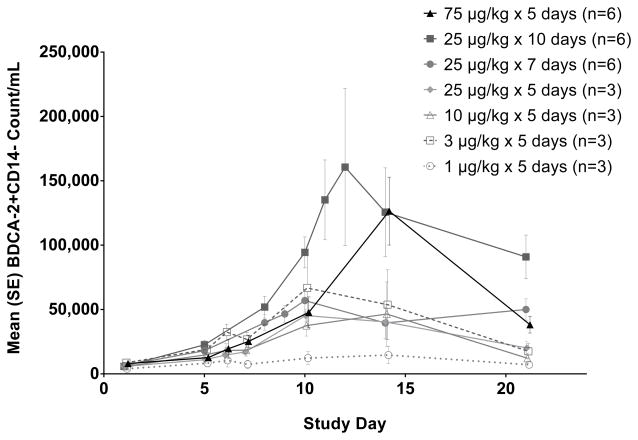
The results were similar for the expansion of the plasmacytoid BDCA-2+ subset (Figure 5). However in this case, the expansion from 10 days of CDX-301 at 25 μg/kg/day was more similar to that from 5 days of 75 μg/kg/day dosing than for the other subsets analyzed. Again, the lowest dose cohort showed only minimal changes in plasmacytoid DC. All other dose cohorts peaked over 10–14 days with approximately a 6 to 16-fold expansion of plasmacytoid DC.
Figure 5.
CDX-301 increases the number of plasmacytoid dendritic cells in the peripheral blood. Graphs show the kinetics of cell number per ml of blood over 21 days. The absolute numbers per milliliter of blood were obtained by multiplying the number of cells (obtained by flow cytometry) by the total number of PBMCs per milliliter of blood. The numbers of BDCA-2+ plasmacytoid DCs are plotted as mean values (± standard error) for the cohorts as indicated in the legend.
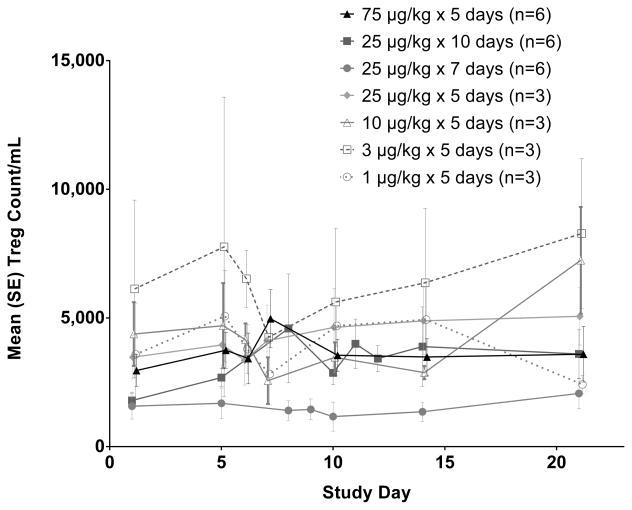
The effects of CDX-301 administration on regulatory T cells (Treg) in the peripheral blood were also examined (Figure 6), however, no clear trends or effects were observed in this study. Similarly, CDX-301 did not significantly alter the number of T- and B- lymphocytes or NK cells (Figure S2, on-line only).
Figure 6.
The CDX-301 dosing regimens studied did not markedly alter the numbers of regulatory T cells (Treg) in the peripheral blood. Graphs show the kinetics of cell number per ml of blood over 21 days. The absolute numbers per milliliter of blood were obtained by multiplying the number of cells (obtained by flow cytometry) by the total number of PBMCs per milliliter of blood. The numbers of Treg (CD3+ CD8- CD4+ CD25+ CD127− FoxP3+ cells) are plotted as mean values (± standard error) for the cohorts as indicated in the legend.
Discussion
Soon after the cloning of cDNA for human Flt3L in 1994,20 clinical development of a soluble, rhuFlt3L progressed into trials involving over 500 treated healthy volunteers and cancer patients. In these trials, rhuFlt3L was well-tolerated and biologically active, capable of expanding CD34+ HSC and HPC and myeloid and plasmacytoid DC in the peripheral blood. Following these initial successes, clinical development of rhuFlt3L stalled, but in vitro and in vivo research continued in a number of laboratories, expanding our understanding of this unique hematopoietic cell growth factor and cytokine. We have recently reinitiated the clinical development of rhuFlt3L (CDX-301) targeting hematopoietic stem cell transplantation and immunotherapy.
The Phase I study reported here evaluated daily subcutaneous dosing of CDX-301 for 5–10 days, instead of the 14-day regimens frequently used in earlier studies of rhuFlt3L. Consistent with prior studies,9 CDX-301 was well tolerated and expanded the expected hematopoietic subsets including CD34+ cells and DC. The results suggest that it may be possible to utilize shorter dosing regimens to effectively expand the CD34+ and key DC populations, including BDCA3+ DC, an important consideration for HSC transplantation, given that current G-CSF-based regimens utilize a 5 day dosing regimen and shorter regimen durations are generally more convenient for patients. Indeed, in the current study, 5 daily doses of CDX-301 alone at 25 or 75 μg/kg/day yielded CD34+ cells in numbers (≥7000/ml) sufficient for use in peripheral blood stem cell transplantation.
It has long been recognized that rhuFtl3L can interact synergistically with other hematopoietic growth factors21 and thus the combination of CDX-301 with other agents that mobilize hematopoietic cells may provide yet another approach to minimize the duration of CDX-301 administration while effectively expanding the targeted cell populations. In this regard, He and colleagues recently demonstrated that rhuFlt3L synergized with plerixafor to mobilize CD34+ cells and these effects were superior to G-CSF alone or in combination with plerixafor.22 Moreover, grafts mobilized by the rhuFlt3L plus plerixafor combination led to enhanced survival.
Interestingly, although the 75 μg/kg/day × 5 day dose regimen led to the greatest CDX-301 exposure (Figure 1), the 25 μg/kg/day × 10 day cohort had the greatest expansion of hematopoietic subsets. This may reflect the fact that many of the targeted Flt3 receptor-expressing cells are localized in the bone marrow where the rates of Flt3L penetration and mobilization of expanded cell populations might control the overall kinetics for the expanded cell populations in the periphery. In addition, the high serum concentrations of CDX-301 achieved by dose levels of 75 μg/kg/day may actually oversaturate the Flt3 receptors on the various hematopoietic subsets leading to a diminished expansion of the Flt3-expressing cells. The active form of Flt3L is a non-covalent homodimer23 which presumably can mediate coalescence of cell surface Flt3 receptors leading to intracellular signaling. The coalescence of Flt3 receptors by dimeric Flt3L may be competitively mitigated at very high Flt3L concentrations.
Studies in mice have demonstrated a positive regulatory feedback loop between DCs and Tregs at least partly mediated by Flt3L. A recent assessment of cryopreserved PBMC samples from an earlier trial in melanoma patients15 found that subcutaneous administration of rhuFlt3L at 20 μg/kg/day for 14 days increased the frequency and absolute number of CD4+CD25+FoxP3+ Treg cells in the peripheral blood.24 In the current study, no clear effects of rhuFlt3L administration on the numbers of Treg cells in peripheral blood were observed consistent with recent in vitro studies.25 The differences in the expansion of Treg cells between the studies may be due to the longer duration of daily dosing in the earlier study, or to other factors not yet identified. If confirmed, a shorter treatment duration may prove advantageous in immunotherapy regimens where an increase in Treg cells would be an undesirable effect of CDX-301 administration.
In summary, in this phase 1 clinical trial, we demonstrate that the currently clinically available rhFlt3L (CDX-301) was well tolerated and expanded hematopoietic cells including CD34+ cells and key DC subsets. We demonstrate using revised criteria for DC subset analyses and advanced multiparametric flow cytometry in humans, and using lower doses and shorter duration of CDX-301 in volunteers than in prior studies, that Flt3L efficiently expands monocytes, BDCA1+ and BDCA3+ DC with ascribed cross-presentation function, and plasmacytoid BDCA2+DC, in addition to HSC. We further demonstrate this occurs without an appreciable effect on Treg, NK cells, NK-T cells, B cells and CD4 and CD8 cells. However, variability was observed among individuals, and larger numbers of subjects would be required to identify smaller effects. The duration of dosing plays an important role in the expansion of the various populations and accordingly can be optimized for particular therapeutic indications. CDX-301 dosing regimens of shorter duration than the 14 daily doses used in earlier clinical studies of rhuFlt3L are suitable for further studies in a variety of clinical indications.
Supplementary Material
Acknowledgments
The authors are grateful to Ralph M. Steinman for his mentorship, wisdom and passion for clinical translation. We would also like to gratefully acknowledge the study volunteers; the Rockefeller CTSA, hospital and pharmacy staff; the support of Drs. James Krueger, Barry Coller and Michelle Lowes; and Renee Riggs for clinical trial management.
The study was funded by Celldex Therapeutics, Inc. The Rockefeller University Center for Clinical and Translational Science is supported, in part, by a Clinical and Translational Science Award (CTSA), and the National Center for Advancing Translational Sciences (NCATS), part of the National Institutes of Health. N.A. was supported in part by grant UL1TR000043/KL2TR000151 from the National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, the Dermatology Foundation, the Klarman Family Foundation and supported by NIAMS AR063461-01A1 (to N.A.). Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number U19AI111825. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. G.B. was supported by Iris and Junming Le Foundation.
Footnotes
Conflict of Interest
J.G., T.H., H.C.M., M.Y., T.D., and T.K. are employed by and hold stock options in Celldex Therapeutics, Inc. S.S. is on the board of directors and holds stock in Ariad. The remaining authors have no conflict of interest to disclose.
Supplementary information is available at Bone Marrow Transplantation’s website.
References
- 1.Bertho JM, Chapel A, Loilleux S, Frick J, Aigueperse J, Gorin NC, et al. CD135 (Flk2/Flt3) expression by human thymocytes delineates a possible role of FLT3-ligand in T-cell precursor proliferation and differentiation. Scandinavian journal of immunology. 2000;52:53–61. doi: 10.1046/j.1365-3083.2000.00746.x. [DOI] [PubMed] [Google Scholar]
- 2.Gabbianelli M, Pelosi E, Montesoro E, Valtieri M, Luchetti L, Samoggia P, et al. Multi-level effects of flt3 ligand on human hematopoiesis: expansion of putative stem cells and proliferation of granulomonocytic progenitors/monocytic precursors. Blood. 1995;86:1661–1670. [PubMed] [Google Scholar]
- 3.Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. The Journal of experimental medicine. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gasparetto C, Gasparetto M, Morse M, Rooney B, Vredenburgh JJ, Long GD, et al. Mobilization of dendritic cells from patients with breast cancer into peripheral blood stem cell leukapheresis samples using Flt-3-Ligand and G-CSF or GM-CSF. Cytokine. 2002;18:8–19. doi: 10.1006/cyto.2002.1009. [DOI] [PubMed] [Google Scholar]
- 5.Chao N, Litzow MR, Geller RB, Korbling M, Doroshow JH, Fay J, et al. Randomized Phase II Study of FLT3 Ligand (MOBIST™) in Combination With GM-CSF or G-CSF for Mobiilization of Peripheral Blood Progenitor Cells in Patients with Breast Cancer. American Society of Hematology Meeting Abstract. 1999;2954 [Google Scholar]
- 6.Stiff PJ, Beveridge RA, Vose J, Fay J, Schuster MW, Geller RB, et al. [2955] Randomized Phase II study of Flt3 Ligand (MOBIST™) in combination with GM-CSF or G-CSF for Mobilization of Peripheral Blood Progenitor Cells in Patients with Lymphoma or Ovarian Cancer. American Society of Hematology Meeting Abstract. 1999;2955 [Google Scholar]
- 7.Goldberg GL, Zakrzewski JL, Perales MA, van den Brink MR. Clinical strategies to enhance T cell reconstitution. Seminars in immunology. 2007;19:289–296. doi: 10.1016/j.smim.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sitnicka E, Buza-Vidas N, Larsson S, Nygren JM, Liuba K, Jacobsen SE. Human CD34+ hematopoietic stem cells capable of multilineage engrafting NOD/SCID mice express flt3: distinct flt3 and c-kit expression and response patterns on mouse and candidate human hematopoietic stem cells. Blood. 2003;102:881–886. doi: 10.1182/blood-2002-06-1694. [DOI] [PubMed] [Google Scholar]
- 9.Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski CR, Hoek J, et al. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood. 2000;96:878–884. [PubMed] [Google Scholar]
- 10.Pulendran B, Banchereau J, Burkeholder S, Kraus E, Guinet E, Chalouni C, et al. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. Journal of immunology. 2000;165:566–572. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 11.Freedman RS, Vadhan-Raj S, Butts C, Savary C, Melichar B, Verschraegen C, et al. Pilot study of Flt3 ligand comparing intraperitoneal with subcutaneous routes on hematologic and immunologic responses in patients with peritoneal carcinomatosis and mesotheliomas. Clin Cancer Res. 2003;9:5228–5237. [PubMed] [Google Scholar]
- 12.Higano CS, Vogelzang NJ, Sosman JA, Feng A, Caron D, Small EJ. Safety and biological activity of repeated doses of recombinant human Flt3 ligand in patients with bone scan-negative hormone-refractory prostate cancer. Clin Cancer Res. 2004;10:1219–1225. doi: 10.1158/1078-0432.ccr-1404-02. [DOI] [PubMed] [Google Scholar]
- 13.Marroquin CE, Westwood JA, Lapointe R, Mixon A, Wunderlich JR, Caron D, et al. Mobilization of dendritic cell precursors in patients with cancer by flt3 ligand allows the generation of higher yields of cultured dendritic cells. J Immunother. 2002;25:278–288. doi: 10.1097/01.CJI.0000016307.48397.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morse MA, Nair S, Fernandez-Casal M, Deng Y, St Peter M, Williams R, et al. Preoperative mobilization of circulating dendritic cells by Flt3 ligand administration to patients with metastatic colon cancer. J Clin Oncol. 2000;18:3883–3893. doi: 10.1200/JCO.2000.18.23.3883. [DOI] [PubMed] [Google Scholar]
- 15.Shackleton M, Davis ID, Hopkins W, Jackson H, Dimopoulos N, Tai T, et al. The impact of imiquimod, a Toll-like receptor-7 ligand (TLR7L), on the immunogenicity of melanoma peptide vaccination with adjuvant Flt3 ligand. Cancer Immun. 2004;4:9. [PubMed] [Google Scholar]
- 16.Marroquin CE, Westwood JA, Lapointe R, Mixon A, Wunderlich JR, Caron D, et al. Mobilization of dendritic cell precursors in patients with cancer by flt3 ligand allows the generation of higher yields of cultured dendritic cells. Journal of immunotherapy. 2002;25:278–288. doi: 10.1097/01.CJI.0000016307.48397.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosca PJ, Hobeika AC, Colling K, Clay TM, Thomas EK, Caron D, et al. Multiple signals are required for maturation of human dendritic cells mobilized in vivo with Flt3 ligand. J Leukoc Biol. 2002;72:546–553. [PubMed] [Google Scholar]
- 18.Brossart P, Grunebach F, Stuhler G, Reichardt VL, Mohle R, Kanz L, et al. Generation of functional human dendritic cells from adherent peripheral blood monocytes by CD40 ligation in the absence of granulocyte-macrophage colony-stimulating factor. Blood. 1998;92:4238–4247. [PubMed] [Google Scholar]
- 19.Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, et al. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci U S A. 2001;98:8809–8814. doi: 10.1073/pnas.141226398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyman SD, James L, Johnson L, Brasel K, de Vries P, Escobar SS, et al. Cloning of the human homologue of the murine flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood. 1994;83:2795–2801. [PubMed] [Google Scholar]
- 21.Jacobsen SE, Okkenhaug C, Myklebust J, Veiby OP, Lyman SD. The FLT3 ligand potently and directly stimulates the growth and expansion of primitive murine bone marrow progenitor cells in vitro: synergistic interactions with interleukin (IL) 11, IL-12, and other hematopoietic growth factors. The Journal of experimental medicine. 1995;181:1357–1363. doi: 10.1084/jem.181.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He S, Chu J, Vasu S, Deng Y, Yuan S, Zhang J, et al. FLT3L and plerixafor combination increases hematopoietic stem cell mobilization and leads to improved transplantation outcome. Biol Blood Marrow Transplant. 2014;20:309–313. doi: 10.1016/j.bbmt.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graddis TJ, Brasel K, Friend D, Srinivasan S, Wee S, Lyman SD, et al. Structure-function analysis of FLT3 ligand-FLT3 receptor interactions using a rapid functional screen. The Journal of biological chemistry. 1998;273:17626–17633. doi: 10.1074/jbc.273.28.17626. [DOI] [PubMed] [Google Scholar]
- 24.Klein O, Ebert LM, Zanker D, Woods K, Tan BS, Fucikova J, et al. Flt3 ligand expands CD4+ FoxP3+ regulatory T cells in human subjects. Eur J Immunol. 2013;43:533–539. doi: 10.1002/eji.201242603. [DOI] [PubMed] [Google Scholar]
- 25.Pletinckx K, Lutz MB. Dendritic cells generated with Flt3L and exposed to apoptotic cells lack induction of T cell anergy and Foxp3(+) regulatory T cell conversion in vitro. Immunobiology. 2014;219:230–240. doi: 10.1016/j.imbio.2013.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.