Abstract
Background
The New Hampshire Birth Conditions Program (NHBCP) is a population-based, active case ascertainment surveillance system that monitors the occurrence of 45 birth defects across the state. A 2008 law requires a new opt-out procedure whereby legal guardians can choose whether or not to have identifiable information retained in the NHBCP database. The purpose of this study was to determine the effects of implementing this opt-out legislation on data collection and surveillance of birth defects by the NHBCP.
Methods
Using surveillance data collected following implementation of the opt out legislation for the period January 1, 2007, through December 31, 2009, 2 opt-out groups were created: the identifiable information retained (IIR) group, consisting of families who did not choose to opt out, and the de-identified information retained group (DIIR), consisting of those who either chose to opt out or were treated as opt-out birth defect cases because their opt-out package was undeliverable. Descriptive statistics were calculated for each group, and chi-square or Fisher’s exact tests were used to compare the proportion of select sociodemographic and medical characteristics between the 2 opt-out groups.
Results
Of 776 infants, 120 (15.5%) fell into the DIIR group. Differences were observed by race/ethnicity (among non-Hispanic whites, 15% were in the DIIR group and among Hispanics, 33% were in the DIIR group; p=0.01) and by maternal age (among women 30–34 years of age, 11% were in the DIIR group, and among those 25 years of age or younger, 22% were in the DIIR group; p=0.05). Birth outcomes, payer source, county of residence, and common birth defect diagnoses did not differ between the opt-out groups.
Conclusion
This study demonstrated that there were significant differences in race/ethnicity and maternal age between parents who had de-identified information included in the NHBCP compared with those who did not choose to opt out. Although the surveillance of birth defects is not affected, the opportunities for certain types of research will be limited.
Keywords: opt-out, birth defects, surveillance, congenital malformations
Introduction
Surveillance of birth defects is important for identifying patterns in prevalence and potential risk factors. Birth defects occur among 3% of births, and are one of the leading causes of infant mortality in the United States.1 The causes of 65%–80% of birth defects are unknown2; however, certain risk factors have been linked with abnormal fetal development.3 Using data from birth defects surveillance systems, research studies may identify these risk factors, resulting in preventative action to decrease the occurrence of specific birth defects (for example, using folic acid supplementation to reduce the incidence of neural tube defects).3,4 Birth defect surveillance systems have been essential in comparing the prevalence of neural tube defects before and after fortification of enriched cereal grains with folic acid in the United States, and in identifying ethnic and racial disparities, resulting in targeted preconception folic acid awareness campaigns.4
In 2003, the New Hampshire Birth Conditions Program (NHBCP) was created. The NHBCP is a population-based, active case ascertainment surveillance system that monitors the occurrence of 45 birth defects among all newborns, stillborns, terminated fetuses (no gestational age limit), and infants up to 2 years of age. Although strict procedures are in place for protecting privacy and data confidentiality, a 2008 law (New Hampshire RSA 141:J) was passed requiring implementation of a new opt-out procedure that allows individuals the option to have identifiable information removed from the NHBCP database. This law applies to data collected from January 1, 2007 onwards. The purpose of this study was to assess the effects of the implementation of the opt-out legislation on data collection by the NHBCP and the surveillance of birth defects in New Hampshire.
Methods
Data Source
The NHBCP collects data from health care providers, health care facilities, clinics, laboratories, medical records departments, and state offices and agencies. Birth hospitals are visited at least annually for medical chart abstraction; cases confirmed through fetal pathology reports or clinical assessments also are included. The NHBCP birth defects list is based on guidance from the National Birth Defects Prevention Network and the Centers for Disease Control and Prevention (CDC). The NHBCP includes birth defect cases who meet the all of the following criteria: (1) offspring of a New Hampshire resident at time of birth; and (2) stillborn fetus, terminated fetus, or liveborn infant for whom a diagnosis is made no later than 2 years of age; and (3) infants or fetuses found by clinical assessment or autopsy to have a structural condition that meets the diagnostic criteria for a reportable birth condition.
As indicated in the legislation, the NHBCP is required to notify in writing the legal guardian or guardians of each individual with a birth defect diagnosis before retaining any identifiable information. During case abstraction, all information is collected on a paper form. Before information is entered into the electronic NHBCP database, and within a week of case abstraction, opt-out packages are mailed. If no response is obtained within 60 days of providing the notice, the NHBCP may retain identifiable information and enter all information into the database; however, the legal guardians can elect at any time to not participate in the NHBCP (Figure 1). If the mailed package is undeliverable and returned to sender, the birth defect case is retained; however, no identifiable information is entered into the NHBCP database (Figure 1). If legal guardians choose to opt-out, they are required to return the signed opt out form requesting their identifiable information not be retained.
Figure 1.
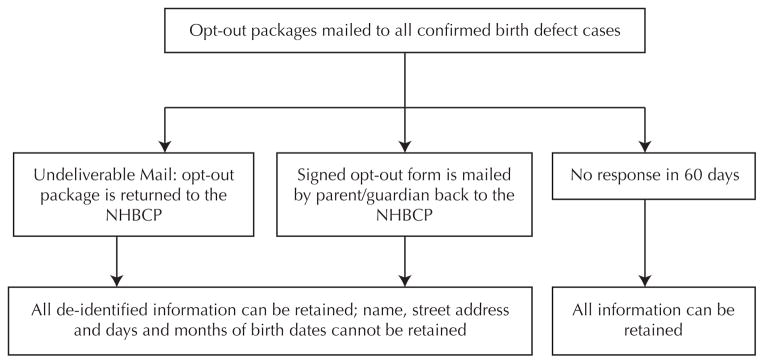
Flowchart Describing the Inclusion and Exclusion of Identifiable Information in the New Hampshire Birth Conditions Program (NHBCP) Depending on Opt-out Status
In addition to the birth defect diagnoses of offspring, information on other pregnancy outcomes, medical history, and demographics, as well as personal information, are collected. This information is collected from medical records and entered into the NHBCP database. The NHBCP obtains race/ethnicity data from birth certificates and uses the mother’s selection as a proxy for the baby. If the mother’s and father’s race differ, “more than one race listed” is selected. Personal identifiers and birth outcome data are also linked to birth certificate data for biannual data field checks to ensure accuracy and review missing fields such as maternal race and ethnicity, gestational age, maternal age, and paternal age. For those who choose to opt out or whose opt-out package is undeliverable, all information is retained within the NHBCP database with the exception of identifiable information such as names, street address, and day and month of birth.
Analytic Methods
Because the purpose of this study was to evaluate the effects of the opt-out procedure, NHBCP data following implementation of the opt-out legislation for the period January 1, 2007, through December 31, 2009, were used to create 2 opt-out groups for this time period: the identifiable information retained (IIR) group, consisting of birth defect cases for whom the legal guardian(s) did not choose to opt out, and the de-identifiable information retained (DIIR) group, consisting of those birth defect cases for whom their legal guardian(s) chose to opt out or who were treated as having opted out because the mailed opt-out package could not be delivered.
Data regarding sociodemographic and medical characteristics, including infant race/ethnicity, maternal age at delivery, infant year of birth, birth outcome, payer source, county of residence, and birth defect diagnosis were assessed. Descriptive statistics were calculated for each opt-out group, and chi-square tests, or Fisher’s exact tests, when the expected cell counts were less than 5, were used to compare characteristics between the 2 groups. Statistical significance was assessed at p < 0.05 using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
For the period 2007–2009, 776 infants were identified for inclusion by the NHBCP, of whom 120 (15.5%) fell into the DIIR group. Among the mothers of Hispanic and “other” ethnicities, larger proportions (33% and 29%, respectively) were in the DIIR group when compared to non-Hispanic white mothers at 15% (Table 1). For the different age categories, the largest proportion of mothers who were in the DIIR group were those younger than 25 years of age (Table 1). No significant differences by infant year of birth were observed; however, the higher number of birth defect cases among the DIIR group during the initial implementation of the law most likely was due to delayed mailings and, hence, a higher proportion of undeliverable opt-out packages (Table 1). Although there were no statistically significant differences by payer source, of those with private insurance, only 12% were in the DIIR group; whereas, of those with Medicaid or who self-paid, 20% and 19% (respectively) were in the DIIR group (Table 1). No substantial differences by birth outcome, county of residence (Table 1), or birth defect diagnoses (Table 2) were observed.
Table 1.
Sociodemographic and Medical Characteristics of Infants in the New Hampshire Birth Conditions Program for the Period 2007–2009a in the Identifiable Information Retained (IIR) Groupb and the De-identified Information Retained (DIIR) Groupc
| Characteristic | IIR Group (n=656)d | DIIR Group (n=120)d | P-valuee | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Infant Race/Ethnicity | n=590 | n=113 | 0.010 | ||
| Non-Hispanic white | 526 | 85 | 92 | 15 | |
| Non-Hispanic black or African American | 6 | 75 | 2 | 25 | |
| Hispanic | 22 | 67 | 11 | 33 | |
| Asian | 19 | 95 | 1 | 5 | |
| Other | 17 | 71 | 7 | 29 | |
| Maternal Age at Delivery | n=628 | n=116 | 0.049 | ||
| <20 years | 28 | 78 | 8 | 22 | |
| 20–24 years | 149 | 78 | 41 | 22 | |
| 25–29 years | 188 | 85 | 34 | 15 | |
| 30–34 years | 170 | 90 | 20 | 11 | |
| 35–39 years | 63 | 89 | 8 | 11 | |
| ≥40 years | 30 | 86 | 5 | 14 | |
| Infant Year of Birth | n=656 | n=120 | 0.542 | ||
| 2007 | 235 | 83 | 48 | 17 | |
| 2008 | 227 | 84 | 42 | 16 | |
| 2009 | 194 | 87 | 30 | 13 | |
| Birth Outcome | n=656 | n=120 | 0.077 | ||
| Livebirth | 632 | 84 | 119 | 16 | |
| Stillbirth | 7 | 86 | 1 | 14 | |
| Termination | 17 | 100 | 0 | 0 | |
| Payer Source | n=644 | n=117 | 0.096 | ||
| Private | 405 | 88 | 58 | 12 | |
| Medicaid | 225 | 80 | 56 | 20 | |
| Self-pay | 13 | 81 | 3 | 19 | |
| None | 1 | 100 | 0 | 0 | |
| County of Residence | n=628 | n=116 | 0.987 | ||
| Belknap | 31 | 84 | 6 | 16 | |
| Carroll | 6 | 75 | 2 | 25 | |
| Cheshire | 31 | 84 | 6 | 16 | |
| Coos | 20 | 87 | 3 | 13 | |
| Grafton | 34 | 83 | 7 | 17 | |
| Hillsborough | 218 | 84 | 43 | 16 | |
| Merrimack | 79 | 85 | 14 | 15 | |
| Rockingham | 123 | 87 | 19 | 13 | |
| Strafford | 53 | 83 | 12 | 17 | |
| Sullivan | 33 | 89 | 4 | 11 | |
Opt out legislation applies to NHBCP data collected from 2007 and onwards.
Identifiable information retained (IIR) group consists of parents that did not choose to opt out.
De-identified information retained (DIIR) group consists of parents that either chose to opt out, or were treated as an opt-out case since the mailed opt-out package was not delivered.
Due to missing data, each group may not sum to the total sample size.
Differences between the IIR group and DIIR group were assessed using chi-square or Fisher’s exact tests.
Table 2.
Birth Defectsa in the NHBCP from 2007–2009 in the Identifiable Information Retained (IIR) Groupb and the De-identified Information Retained (DIIR) Groupc
| Birth Defect | IIR Group (n=656) | DIIR Group (n=120) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Spina bifida | 7 | 100 | 0 | 0 |
| Microcephaly | 12 | 80 | 3 | 20 |
| Macrotia | 5 | 83 | 1 | 17 |
| Tetralogy of Fallot | 10 | 83 | 2 | 17 |
| Ventricular septal defects | 38 | 84 | 7 | 16 |
| Patent foramen ovale | 14 | 93 | 1 | 7 |
| Pulmonary valve stenosis | 10 | 83 | 2 | 17 |
| Hypoplastic left heart syndrome | 9 | 100 | 0 | 0 |
| Pulmonary atresia | 10 | 91 | 1 | 9 |
| Coarctation of the aorta | 4 | 80 | 1 | 20 |
| Orofacial clefts | 34 | 87 | 5 | 13 |
| Pyloric stenosis | 79 | 82 | 17 | 18 |
| Intestinal atresia | 8 | 80 | 2 | 20 |
| Intestinal aganglionosis | 6 | 100 | 0 | 0 |
| Hypospadias | 126 | 82 | 27 | 18 |
| Unilateral renal agenesis | 16 | 76 | 5 | 24 |
| Hydronephrosis | 75 | 83 | 15 | 17 |
| Hip dislocation | 20 | 87 | 3 | 13 |
| Limb deficiency | 7 | 78 | 2 | 22 |
| Diaphragmatic hernia | 7 | 100 | 0 | 0 |
| Gastroschisis | 6 | 75 | 2 | 25 |
| Trisomy 21 | 20 | 83 | 4 | 17 |
| Multiple defects | 95 | 86 | 16 | 14 |
Only displaying birth defects that had 5 or more cases, by infant.
Identifiable information retained (IIR) group consists of parents that did not choose to opt out.
De-identified information retained (DIIR) group consists of parents that either chose to opt out, or were treated as an opt-out case since the mailed opt-out package was not delivered.
Discussion
Results from this study demonstrated that certain factors could be associated with a family’s willingness to have their identifiable information included in a birth defect surveillance system. Key differences in some sociodemographic characteristics existed between the IIR group, consisting of families who did not choose to opt out of the NHBCP surveillance system, and the DIIR group, consisting of those who either did choose to opt out or were treated as opt-out birth defect cases because the mailed opt-out package was not deliverable. There were significant differences in infant race/ethnicity and maternal age at delivery, suggesting that there might have been socioeconomic differences influencing the decision to opt out, or that younger mothers and those of Hispanic ethnicity might have been more transient and harder to reach via mail. In a study that examined characteristics between mothers who had a baby born with a birth defect who either consented or did not consent to follow-up, significant differences were also observed by race and maternal age; however, these differences disappeared after adjustment for perinatal mortality.5 Although not statistically significant, in the current study, the differences observed by payer source, a proxy for socioeconomic status, also suggests that there might have been notable socioeconomic differences between the IIR and DIIR groups, as a greater proportion of those with Medicaid or who self-paid were more likely to opt out or have the opt-out package be undeliverable.
Within the DIIR group, we were not able to distinguish between the parents who chose to have their identifiable information removed from those parents who were considered to have opted out due to an undeliverable mail package as the reason why a case is in the DIIR group is not included in the NHBCP database. As a result, we were not able to assess differences between these 2 subgroups. Fortunately, the NHBCP is required to remove only identifiable information for the DIIR group regardless of whether the parent chose to opt out or the mail was undeliverable; if all data were to be removed for true opt-outs, for those classified as having opted out because of undeliverable mail, or for both of these sub-groups, there would be a considerable impact on accurate surveillance as prevalence rates for specific birth defects would be affected. Incomplete case inclusion in a given region would result in distorted prevalence estimates, resulting, in turn, in underestimation of the health care service needs of infants born with specific birth conditions.3 Additionally, differences in birth defect occurrence between the 2 opt-out groups could affect the accuracy and usefulness of the birth defect surveillance system; however, there did not appear to be substantial differences in the proportion of specific birth defects between the 2 groups. As it stands, the lack of identifiable information in the NHBCP will affect research studies if long-term follow-up is required, or if geospatial analysis is to be conducted for birth defect cluster analysis.6 Fortunately, researchers are still able to link infant records with maternal and paternal records; therefore, most risk factors for birth defects can be assessed in future studies.3
Public health surveillance is critical for monitoring diseases or conditions for their prevalence, potential risk factors, and health service requirements.7 To ensure appropriate evidence-based activities are created, complete and accurate surveillance data are necessary.8 Birth defects surveillance systems have access to data sources containing individual, health-related information under public health authority; however, as data protection and security concerns are increasing, state legislatures can mandate additional reporting guidelines and procedures.6,8 Privacy concerns and their effects on accurate surveillance are national issues, and not unique to New Hampshire.7 As is the case in New Hampshire, if opt-out procedures are mandated by law, it will be critical to improve these procedures to ensure surveillance is maximized for optimal public health benefit.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Update on overall prevalence of major birth defects—Atlanta, Georgia, 1978–2005. MMWR Morb Mortal Wkly Rep. 2008;57:1–5. [PubMed] [Google Scholar]
- 2.Nelson K, Holmes LB. Malformations due to presumed spontaneous mutations in newborn infants. NEJM. 1989;320:19–23. doi: 10.1056/NEJM198901053200104. [DOI] [PubMed] [Google Scholar]
- 3.Mai CT, Law DJ, Mason CA, et al. Collection, use, and protection of population-based birth defects surveillance data in the United States. Birth Defects Res A Clin Mol Teratol. 2007;79:811–814. doi: 10.1002/bdra.20420. [DOI] [PubMed] [Google Scholar]
- 4.Boulet SL, Yang Q, Mai C, et al. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res A Clin Mol Teratol. 2008;82:527–532. doi: 10.1002/bdra.20468. [DOI] [PubMed] [Google Scholar]
- 5.Law C, Robertson MO, Panny SR, Wulff LM. Characteristics influencing informed consent on a congenital malformations registry. Am J Public Health. 1988;78(5):572–573. doi: 10.2105/ajph.78.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayer R, Fairchild A. The limits of privacy: Surveillance and the control of disease. Health Care Anal. 2002;10:19–35. doi: 10.1023/A:1015698411824. [DOI] [PubMed] [Google Scholar]
- 7.Fairchild AL, Bayer R. Public health: Ethics and the conduct of public health surveillance. Science. 2004;303:631–632. doi: 10.1126/science.1094038. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) HIPAA privacy rule and public health guidance from CDC and the U.S. Department of Health and Human Services. MMWR Morb Mortal Wkly Rep. 2003;52(suppl):1–17. 19–20. [PubMed] [Google Scholar]


