Abstract
Latent sensitization is a rodent model of chronic pain that reproduces both its episodic nature and its sensitivity to stress. It is triggered by a wide variety of injuries ranging from injection of inflammatory agents to nerve damage. It follows a characteristic time course in which a hyperalgesic phase is followed by a phase of remission. The hyperalgesic phase lasts between a few days to several months, depending of the triggering injury. Injection of μ-opioid receptor inverse agonists (i.e., naloxone, naltrexone) during the remission phase induces reinstatement of hyperalgesia. This indicates that the remission phase does not represent a return to the normal state, but rather an altered state in which hyperalgesia is masked by constitutive activity of opioid receptors. Importantly, stress also triggers reinstatement. Here we describe in detail the procedures to induce and follow latent sensitization in its different phases in rats and mice.
Keywords: Chronic pain, constitutive activity, hyperalgesia, latent sensitization, neuropathic pain, opioid receptor, stress
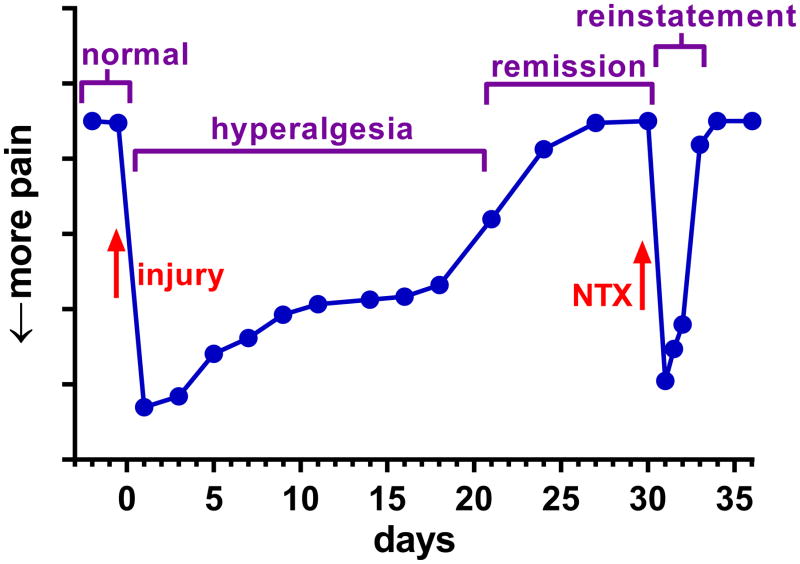
Many types of chronic pain, and in particular neuropathic pain, are episodic: periods of pain are interspaced with periods of remission which resemble the healthy state but can quickly give way to yet another pain bout. In particular, stress is a common trigger of pain episodes in these disorders. Recently, a rodent model of chronic pain has been developed that reproduces both its episodic nature and its sensitivity to stress. It has been called “latent pain sensitization” (Bessiere et al., 2007; Campillo et al., 2011) or “latent sensitization” (Lian et al., 2010); here we will use the later term abbreviated to “LS”. The diagram in Figure 1 illustrates the basic phases of LS:
Figure 1. Diagram of the phases of LS.
1) Tissue injury leads from the normal state into a period of hyperalgesia. 2) Pain subsides into a remission phase. 3) An injection of opioid inverse agonist (naltrexone, NTX) produces a temporary reinstatement of the hyperalgesia.
Tissue injury leads from the normal state to a period of hyperalgesia (increased responses to noxious stimuli) or allodynia (pain-like responses to a non-noxious stimulus). A variety of noxious stimuli can induce LS in rodent models of chronic pain, including a paw incision (Campillo et al., 2011; Li et al., 2001; Richebe et al., 2005; Rivat et al., 2007), complete Freund’s adjuvant (CFA) (Corder et al., 2013), carrageenan (Bessiere et al., 2007; Le Roy et al., 2011) and nerve injury (Solway et al., 2011).
The initial hyperalgesia eventually subsides into a period of remission. The length of the initial hyperalgesic phase depends on the injury, ranging from 7 days after paw incision (Li et al., 2001) to ~100 days in the cuff model of neuropathic pain (Yalcin et al., 2011).
However, the remission phase does not represent a return to the normal state as the hyperalgesia can be reinstated by a variety of stimuli or pharmacological agents. The most commonly used are antagonists of the μ-opioid receptor (MOR), such as naloxone or naltrexone (NTX), which “reinstate” the hyperalgesia for a period of time that is consistent with the pharmacokinetic half-life of the drug (2–4 hr). Strictly speaking, naloxone and naltrexone are inverse agonists of the MOR (see “Background Information”). NTX does not produce hyperalgesia in naïve animals. Remarkably, reinstatement by NTX can be repeated any number of times over a period of at least 5 months (Campillo et al., 2011; Corder et al., 2013). Other stimuli such as stress can similarly reinstate the painful state (Le Roy et al, 2011).
Basic Protocol 1: Complete Freund’s Adjuvant-induced Latent Sensitization in Mice
LS induced by injecting CFA in the hind paw of mice is a robust, well-characterized instance of this pain model, so we have chosen it as an example. This protocol can be adapted to study LS induced with other stimuli by simply changing the stimulus and paying attention to the different duration of the hyperalgesic phase. For mice, some groups have found that handling increases rather than decreases struggling and therefore avoid habituation. We have included here instructions for habituation (as performed at UCLA) in case the investigator decides to use it.
Materials List
Animals: mice (C57Bl/6J, Jax Mice, The Jackson laboratory, Bar Harbor, Maine). We have found other strains to be adequate. From experience, we have found that a sample size of 5–8/group/gender is sufficient to reduce error and obtain statistical significance. Mice are typically tested as young as 6 weeks and as old as 5 months, with females weighing ~20 g and males ~24 g.
Animal room with control of ambient temperature and relative humidity.
Standard polycarbonate cages (approximately 30 cm x 15 cm) to house mice (up to 5 mice/cage).
Chow (PicoLab Rodent Diet 20; 5053) and water bottles.
Complete Freund’s Adjuvant (CFA; Sigma, catalog number F5881)
Acrylic enclosures (10.16 cm x 10.16 cm) on top of an elevated mesh metal grid with stand (IITC, CA, Part number 410) large enough to test multiple mice. To reduce social visual cues from one mouse to another, the enclosures should not be translucent. Laminated card (white or black) can be fitted to the enclosures. See photo (Figure 2). Tall acrylic cylindrical tubes, 10 cm in diameter, can be used instead of square boxes.
Set of eight von Frey filaments (‘Touch-Test’, North Coast Medical, Inc., San Jose, CA, USA), in Log10 [10*Force (mg)] or (g) = 1.65 (0.008 g), 2.36 (0.02 g), 2.83 (0.07 g), 3.22 (0.61 g), 3.61 (0.4 g), 4.08 (1.0 g), 4.31 (2.0 g), 4.74 (6.0 g).
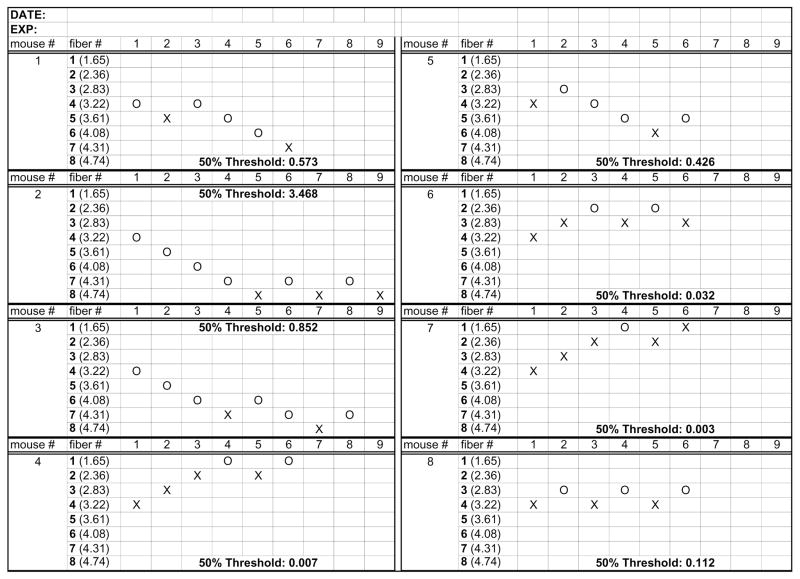
Up-and-Down scoring sheet (Figure 3).
Naltrexone hydrochloride or naloxone hydrochloride dihydrate (Sigma, catalog numbers N3136 and N7758, respectively).
Water, sterile.
50 μl Hamilton syringe (Hamilton Part number 7637-01), 30 G needle.
Isoflurane (Phoenix).
Vaporizer for isoflurane (Summit Anesthesia Support). An induction box connected to the vaporizer is required for induction of anesthesia.
Figure 2.
Acrylic enclosure for paw withdrawal responses to von Frey filaments in mice.
Figure 3. Sample scoring sheet for the Up-and-Down method.
Examples of the responses of 8 different mice. ‘X’ represents a paw withdrawal response and ‘O’ failure to respond. Numbers in parentheses are Log10 [10*Force (mg)]. Final ‘50% Threshold’ values were calculated using the algorithm in (Chaplan et al., 1994).
Mouse protocol
Habituation protocol (optional)
-
1)
Mice are handled for 5 min/day/per mouse for approximately one week prior to start of experiment. Avoid holding mice by the tail and train the mice to walk onto an outstretched hand.
-
2)
Three days before the first test, habituate mice to the testing room with dim lighting (30–50 Lux) while in home cages.
-
3)
Habituate mice to the testing apparatus for 2 days, 30 min daily.
Baseline measurements
-
4)
Allow the mice to acclimate in the acrylic enclosures atop the elevated grid for at least 30 min before testing. Unless the enclosure is greater than 10–12” high, put a cover on the enclosures so the mice can not jump out.
-
5)
Measure baseline paw withdrawal thresholds (PWTs) with von Frey filaments once a day for 2–3 days prior to CFA injection.
-
6)
Gently apply the von Frey filaments, between the openings of the grid, to the soft pad of the hind paw between the tori at the base of the digits. Use the up-and-down method (see Support Protocol: Up-and-Down Method).
-
7)
Avoid taking measurements while the animal is standing on its hind legs, grooming, or sleeping. If sleeping, very gently nudge the trunk with a pen. When testing multiple animals, move from animal to animal depending on the activity level.
-
8)
Return animals to their usual housing room after the measurements.
-
9)
Clean the rack and the area below it with deionized water between experiments.
CFA injection and hyperalgesia phase
-
10)
The final baseline measurements should be taken immediately before the CFA injection and averaged as “day 0”.
-
11)
Anesthetize the animals in a supine position with 1–2% isoflurane using the vaporizer and induction box.
-
12)
CFA can be used undiluted (100%) and injected subcutaneously in one hind paw (others inject as a 1:1 emulsion in water but this requires twice the injection volume). Amount of CFA is a critical parameter; we suggest 50 μl undiluted for rats and 5 μl undiluted for mice to yield a robust hyperalgesia that resolves fairly quickly. Mix CFA thoroughly before each injection (it tends to settle at the bottom of the bottle) and draw directly into the 50 μl Hamilton syringe with a 30 G needle.
-
13)
The needle is inserted at an oblique angle of ~ 20°, at the middle of the dorsal paw, near the base of the third toe (Figure 4). Slowly inject CFA over 1–2 seconds. Hold the needle in place for 5–15 s to allow pressure to dissipate and then withdraw gently.
-
14)
Measure PWTs on the following day and on selected subsequent days. Do not perform PWTs daily as this may lead to stress-induced hyperalgesia. We typically measure PWTs on days 1, 3, 5, 7, 10, 14, 21, 28 after the CFA injection. This timeline includes the initial phase of peak hyperalgesia (days 1–3), gradual resolution of initial hyperalgesia (days 5–21), and then the remission phase (day 21 onwards, Figure 1).
-
15)
PWTs should decrease dramatically on days 1 and 3, and then progressively return to baseline values, which should occur between days 14 and 28. Note that inflammation of the paw will develop quickly within the first few hours and days after CFA injection, and then persists for several weeks. During this time, animals may exhibit extended periods of paw elevation, thus precluding testing.
Figure 4.
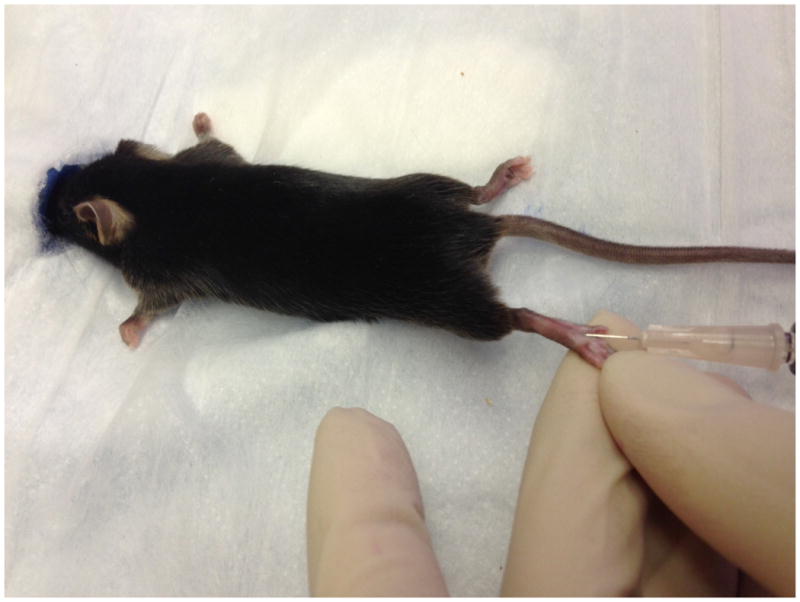
Injection of CFA in the hind paw of a mouse.
Naltrexone injection and the pain reinstatement phase
-
16)
Measure PWTs for that day, which will serve a baseline for the effect of naltrexone.
-
17)
Naltrexone is dissolved daily in sterile PBS and is injected subcutaneously (3 mg/kg in 300 μl) at the nape.
-
18)
Measure PWTs intermittently. For example, at 20, 40, 60 and 120 min, or 5, 15, 30, 45, 60, 90, 120, 180, and 240 min, and then again at 24 hr to ensure that there is a return to baseline.
-
19)
An alternative protocol with fewer PWT measures and more time in the home cage can be followed to reduce test/stress-induced hyperalgesia: Take a baseline PWT measure 24 hr prior to NTX injection. Habituate animals to testing room in their home cages for 15 min. Inject NTX and replace in home cage for 10 min. Then place into testing apparatus for an additional 10 min. Measure PWT’s at 20 and 60 minutes after injection.
Endpoint
-
20)
At the end of the experiment, the mice are euthanized with an overdose of isoflurane or pentobarbital and cervical dislocation. Tissues can be taken for physiological, biochemical, or molecular analyses.
Alternate Protocol 1: Complete Freund’s Adjuvant-Induced Latent Sensitization in Rats
The protocol in rats in basically the same as in mice. Note the different range of force of the von Frey filaments and the different needle used to inject naltrexone. If the same equipment is used for mice and rats, it is critically important to wash it thoroughly to eliminate all odor of the previous species, which can be a stressor. Habituation to handling and to the testing equipment reduces variability of the data collected in rats.
Materials List
Animals: Rats, Sprague-Dawley (Harlan, Indianapolis, IN, USA), male, 250–300 g.
Animal room with control of ambient temperature and relative humidity.
Standard polycarbonate cages adequate for rats (20 cm x 33 cm).
Chow and water bottles.
Complete Freund’s Adjuvant (CFA).
Elevated metal grid with acrylic enclosures
Set of eight von Frey filaments (‘Touch-Test’, North Coast Medical, Inc., San Jose, CA, USA): Log10 [10*Force (mg)] = 3.61, 3.84, 4.08, 4.31, 4.56, 4.74, 4.93, 5.18.
Up-and-Down scoring sheet (Figure 3).
Naltrexone or naloxone
Water, sterile.
50 μl Hamilton syringe, 26 G needle.
Isoflurane (Halocarbon Laboratories, River Edge, NJ, USA).
Vaporizer for isoflurane (Patterson Scientific). An induction box connected to the vaporizer is required)
Habituation period (recommended)
-
1)
Habituate rats to the testing apparatus for 2 days, 30 min daily.
Baseline measurements
-
2)
Allow rats to acclimate in the acrylic enclosures atop the elevated grid for at least 30 min before testing.
-
3)
Measure baseline paw withdrawal thresholds (PWTs) with von Frey filaments for 2–3 days prior to the CFA injection.
-
4)
Gently apply the von Frey filaments, between the openings of the grid, to the soft pad of the hind paw between the tori at the base of the digits. Use the up-and-down method (see Support Protocol: Up-and-Down Method).
-
5)
Avoid taking measurements while the animal is standing on its hind legs, grooming or sleeping. If sleeping, very gently nudge the trunk with a pen.
CFA injection and hyperalgesia phase
-
6)
The final baseline measurements should be collected just prior to CFA injection and recorded as “day 0”.
-
7)
Anesthetize the rats in the induction box with 5% isoflurane, supplied by the vaporizer.
-
8)
CFA is used undiluted (100%) and injected subcutaneously in one hind paw. Injection volume is a critical parameter: use 50 μl for rats. Mix CFA thoroughly before each injection and draw directly into the 1 ml syringe with a 25 G needle.
-
9)
The needle is inserted at an oblique angle from the heel in the middle of the paw. It is held in place for 15 s to allow pressure to dissipate and then withdrawn gently.
-
10)
On the following day, measure PWTs and on subsequent days as appropriate. Do not perform PWTs daily as this may lead to stress-induced hyperalgesia. We typically measure PWTs on days 1, 3, 5, 7, 14, 21, 28 after the CFA injection. This would assess the initial (days 1–7) and later (day 7–21) response followed by the full recovery phase (day 21 onwards).
-
11)
The PWTs should decrease dramatically on day 1 and progressively return to baseline values, which should occur between days 20 and 30.
Naltrexone injection and the pain reinstatement phase
-
12)
Measure PWTs for that day, which will serve a baseline for the effect of naltrexone.
-
13)
Naltrexone is dissolved daily in sterile saline and is injected subcutaneously (1 mg/kg in 300 μl) or intrathecally (1 μg or 2.6 nmol in 10 μl, plus 10 μl flush).
-
14)
Measure PWTs intermittently. For example, at 15, 30, 45, 60, 90 and 120 min.
Endpoint
-
15)
At the end of the experiment, the rats are euthanized with an overdose of pentobarbital.
Support Protocol: Up-and-Down Method of von Frey measurements
The Up-and-Down method using von Frey hairs has become the most common approach to the measurement of mechanical paw hypersensitivity after tissue or nerve injury. However, several other procedures are available to apply von Frey hairs, and several other stimulus paradigms can be used, such as the use of an electronic von Frey apparatus (Parada et al., 2003).
Materials
Set of eight von Frey filaments:
For mice: Log10 [10*Force (mg)] or (g)= 1.65 (0.008 g), 2.36 (0.02 g), 2.83 (0.07 g), 3.22 (0.61 g), 3.61 (0.4 g), 4.08 (1.0 g), 4.31 (2.0 g), 4.74 (6.0 g)
For rats: Log10 [10*Force (mg)] = 3.61 (0.4 g), 3.84 (0.6 g), 4.08 (1.0 g), 4.31 (2.0 g), 4.56 (4.0 g), 4.74 (6.0 g), 4.93 (8.0 g), 5.18 (15.0 g)
In house scoring sheet, such as the one used at UCLA (Figure 3).
Protocol
For the first trial, use the 3.22 (0.61g), filament for mice or the 4.31 (2.0g), filament for rats. Apply to the plantar hindpaw. The exact location depends on the pain model. In animal models of paw inflammation such as CFA, place the filament on the centermost region of the footpad, and apply sufficient pressure to cause a slight bend in the filament. Apply pressure gently, as a rapid increases in force can quickly sensitize peripheral nerves, yielding a false positive response.
Ensure that the amount of pressure applied to the paw remains constant for 4 seconds in the rat, 3 seconds in the mouse. If the paw withdraws briskly, then record as a positive response (mark an “X” on the appropriate column on the Up-and-Down scoring sheet) and then apply the next lowest von Frey filament. If the paw does not respond, then record as a negative response (mark an “O” in the appropriate column) and then apply the next highest von Frey filament. Four additional measures are recorded after the first change in response. Examples are mouse 1, 5 and 8 on the sample scoring sheet (Figure 3).
A positive response is defined as an abrupt lifting of the paw that is not due to normal walking or grooming.
A value is not recorded if: 1) the filament slips off of the paw before a withdrawal; 2) the filament engages the abdomen, some other sensitive area, or the wrong area of the paw; 3) the filament touches the wire mesh; or 4) the animal moves (walks or grooms). In this case, give the animal a 30 second break before retesting with the same fiber.
When all measurements have been taken, input the “X” and “O” values into the algorithm for the Up-and-Down method (Chaplan et al., 1994). Make sure to include all preceding O values to the first response on the score sheet and in the algorithm.
Rats not responding to any filament are assigned a maximum value of 15 g. For mice the reaction to the 4.74 fiber, the fiber of the highest gram force, is always taken as a positive response, see mouse number 2 and 3 in the sample scoring sheet (Figure 3).
Animals responding to all filaments are assigned a minimum value of 0.005 g for mice, such as mouse number 4 on the sample scoring sheet and 0.5 g for rat.
In the case of mice, another complex scenario is a positive response on the first 2 fibers. In this case, we have adapted the protocol for a total of 6 measures. This is to avoid repeated testing of a mouse that appears sensitized. See mouse number 4, 6 and 7 in the sample scoring sheet (Figure 3).
COMMENTARY
Background Information
A brief history of LS
LS was initially found to develop in the setting of opiate-induced hyperalgesia, triggered by the repeated administration of opiates like heroin, morphine (Celerier et al., 2000; Li et al., 2001), fentanyl (Bessiere et al., 2007; Rivat et al., 2007; Rivat et al., 2009) and remifentanil (Campillo et al., 2011). Opiate-induced and tissue injury-induced hyperalgesia are additive (Campillo et al., 2011) and share several characteristics: they are reinstated by naloxone (Campillo et al., 2011), seem to involve the activation of neurokinin 1 (NK1) receptors and of descending pain control pathways (Rivat et al., 2009), and can be blocked by opioid or NMDA receptor antagonists (Campillo et al., 2011; Le Roy et al., 2011; Rivat et al., 2007).
In neuropathic pain and other chronic pain disorders, pain episodes are often triggered by stress. LS models replicate this characteristic: forced swim stress and novel environment stress can produce reinstatement when given during the remission phase (Rivat et al., 2007), just like opioid inverse agonists.
Hyperalgesic priming (Joseph et al., 2010; Reichling and Levine, 2009) is a model similar to LS in that it is long-lasting (>3 weeks), the original hyperalgesia can be reinstated (in this case by pronociceptive agents such as prostaglandin E2), and is increased by stress. While priming takes place in peripheral afferent terminals, LS seems to be mediated centrally (Corder et al., 2013; Solway et al., 2011) although LS caused by nerve injury may be also mediated peripherally (Guan et al., 2010). Therefore, the relationship between hyperalgesic priming and LS remains unclear.
Mechanistically, both the hyperalgesia and reinstatement phases involve activation of NMDARs (Campillo et al., 2011; Corder et al., 2013; Rivat et al., 2007), whilst the remission phase involves activation of MORs (Corder et al., 2013) or KORs (Campillo et al., 2011). Although the location of opioid receptors involved in LS include the spinal cord, it is not known whether these are in dorsal horn neurons, central terminals of primary afferent, or both. Other questions to resolve are the mechanism underlying induction and maintenance of the prolonged activation of opioid receptors during the remission phase. The most obvious mechanism is the tonic release of opioid peptides in the dorsal horn. However, recent evidence suggests that remission involves constitutive signaling of MORs in the dorsal horn that silences LS and thus maintains an analgesic state (Corder et al., 2013).
μ-Opioid receptors
MORs are a member of the Class A of G protein-coupled receptors (GPCRs) that are Gi/o coupled and, for the most part, inhibit their cognate second messenger signaling pathways. This results in an inhibition of adenylyl cyclase and ion channels but activation of components of the MAP kinase cascade. As MORs bind morphine, the most clinically effective analgesic, ligand-induced signaling of MORs has been extensively studied. We know that when MOR binds its agonist it is phosphorylated by kinases such as GRK2/3, PKA, PKC, CaMKII and Src, which then recruit β-arrestin 1 or 2. The receptor is then internalized, rather than degraded, through a clathrin-dependent pathway and recycled through a Rab11 pathway.
In addition to such ligand-dependent signaling, MORs may signal in the absence of agonist (constitutive activity). This ligand-independent signaling state was first described for the δ-opioid receptor by Costa and Herz and relied on a pharmacological approach to detect negative intrinsic activity of GPCRs (Costa and Herz, 1989). Using an array of inverse agonists and neutral antagonists, constitutively active MORs have also been found (Wang et al., 1994). Unlike δ-opioid receptors, these receptors do not comprise a major proportion of the total receptor population under basal conditions (Vezzi et al., 2013). However, withdrawal from chronic morphine increases constitutively active MORs in rodents (Meye et al., 2012; Shoblock and Maidment, 2006; Shoblock and Maidment, 2007; Wang et al., 2007; Wang et al., 1994) and enhances the aversive effect of naloxone, a MOR inverse agonist, in the morphine dependent state (Shoblock and Maidment, 2006).
LS also increases constitutively active MORs to enhance endogenous analgesia and physical dependence (Corder et al., 2013). Our understanding of this signaling state is limited, but it was recently reported that constitutive activation of PKCα results in MOR phosphorylation at Ser363 (Illing et al., 2014), and that constitutively active receptors are rapidly internalized through a c-Src and β-arrestin-2 dependent mechanism (Lam et al., 2011; Walwyn et al., 2007). As the LS model involves a substantial increase in constitutively activity of MORs, it provides a unique opportunity to study this fascinating signaling state. A range of questions about the pathways and molecules that activate and maintain constitutive activity and the affected receptor populations, cell types and downstream signaling pathways remain to be determined. We posit that there must be intrinsic mechanisms to reverse LS; otherwise any individual exposes to a severe injury would be in a state of LS. Determination of such intrinsic mechanisms could yield clues leading to cures for chronic pain.
Inverse agonists and neutral antagonists
Constitutive activity of a receptor consists of an agonist-independent increase in signaling. Compounds that decrease this signal are called “inverse agonists”. There are also compounds that bind to the receptor without affecting their constitutive activity, yet they eliminate both receptor activation by agonists and the inhibitory effect of the inverse agonists; these compounds are called “neutral antagonists”. To establish the presence of constitutive activity, it is necessary to assess: 1) that an inverse agonist decreases the basal signaling of the receptor, and 2) that this effect of the inverse agonist disappears in the presence of a neutral antagonist. Constitutive activity of receptors other than MORs may contribute to pain remission (e.g. cannabinoid receptors), and the study of these receptors requires the availability of appropriate inverse agonists and neutral antagonists.
Critical Parameters
Injury-induced LS is robust and easily reproducible in the laboratory. This basic protocol of CFA-induced LS can be easily adapted to study other types of chronic pain by changing the insult that triggers the hyperalgesia.
Injuries used to elicit Latent Sensitization
Most tissue injuries that induce persistent pain (i.e. pain that last several days or more) have been found to induce LS. Stimuli that have been reported to induce LS in rodents include: plantar incision (Campillo et al., 2011; Corder et al., 2013; Li et al., 2001; Richebe et al., 2005; Rivat et al., 2007; Romero et al., 2011), CFA (Corder et al., 2013; Solway et al., 2011), carrageenan (Bessiere et al., 2007; Le Roy et al., 2011), visceral pain (Lian et al., 2010) and nerve injury (Solway et al., 2011). In addition, opiate drugs like morphine, fentanyl and remifentanil can induce LS by themselves and have synergistic effects with the injury stimuli listed above (Campillo et al., 2011; Celerier et al., 2000; Laulin et al., 2002; Li et al., 2001; Richebe et al., 2005; Rivat et al., 2009). The choice of the stimulus used in a particular LS study would depend largely on the questions and hypotheses being considered. The first step in such a study would be to confirm that the chosen injury produces a consistent, measurable indicator of hyperalgesia or allodynia. Not all injuries produce persistent pain and not all forms of pain elicit measureable behavioral responses. Conversely, of particular concern are models which produce a very long-lasting period of hyperalgesia. For example, some models of peripheral neuropathic pain produce hyperalgesia lasting 80 days, as in the cuff nerve injury model (Yalcin et al., 2011), or even longer, as in the spared nerve injury model (Decosterd and Woolf, 2000). Therefore, to study LS related to neuropathic pain, modifications must be made to reduce the severity of the nerve injury. We recommend a modified version of the spared nerve injury model, the CpxSx model (Shields et al., 2003; Solway et al., 2011), in which the common peroneal and sural branches of the sciatic nerve are cut and the tibial branch is left intact. CpxSx produced a hyperalgesic phase lasting about 28 days in mice (Solway et al., 2011) and 35 days in rats (Marvizon et al., manuscript in preparation).
Methods to measure hyperalgesia and allodynia
Whereas the method using von Frey filaments described here measures mechanical hypersensitivity, other methods can be substituted to measure heat or cold hypersensitivity. Thus, Solway et al. (2011) measured not only tactile hypersensitivity using von Frey filaments but also cold hypersensitivity upon topical application of a drop of acetone to the plantar paw skin. Other studies (Li et al., 2001) have measured heat hyperalgesia using paw withdrawal responses to radiant heat (Hargreaves et al., 1988). One study on LS to visceral pain (Lian et al., 2010) measured referred visceral hypersensitivity in rats by applying von Frey filaments to the lumbar dermatomes.
Drug administration to trigger reinstatement
In addition to inverse agonists of MORs, antagonists of other receptors can also trigger reinstatement. For example, nor-binaltorphimine, a kappa opioid receptor antagonist, produces reinstatement in LS induced by plantar incision (Campillo et al., 2011). Similarly, antagonists of Y1 or Y2 receptors for neuropeptide Y produced reinstatement to LS induced by nerve injury (CpxSx) or intraplantar CFA injection (Solway et al., 2011).
The route of drug administration is another important variable. Systemic injections of compounds that cross the blood-brain barrier can potentially affect receptors anywhere in the body. Since LS seems to be partially mediated by supraspinal mechanisms (De Felice et al., 2011; Le Roy et al., 2011; Rivat et al., 2009; Taylor and Corder, 2014), this leads to uncertainty about the site of action of the drug and the location of the receptors involved following systemic administration. To determine whether the receptors involved are located in the spinal cord, drugs can be injected intrathecally. However, the use of surgically-implanted, chronic intrathecal catheters could cause problems with interpretation of results, because the surgical procedure used to implant the catheter may causes an injury response leading to the development of LS. Hence, LS may be present in control animals that received a non-noxious stimulus (for example, saline instead of CFA) due to catheter implantation surgery. To avoid this problem, we recommend that intrathecal injections be conducted using an acute percutaneous method (Hylden and Wilcox, 1980). Alternatively, a control group of animals without intrathecal catheters could be included in the experiment.
Troubleshooting
Table 1 includes a list of potential problems and possible solutions. It is very important to establish a reliable baseline of responses before applying the injury stimulus that induces LS; otherwise it will be difficult to determine whether the thresholds of the animals have returned to baseline. PWT values greater than baseline (indicating the presence of analgesia) have been observed, but this effects tends to be small and thus requires a carefully established and reliable baseline. For these reasons, we recommend that baseline responses be assesses over multiple days, until stable. Another confound is stress, which can produce a reinstatement that is as robust as that produced by naltrexone. Indeed, the presence of humans (men in particular) can cause stress in rodents and change their behavioral responses to a noxious stimulus (Sorge et al., 2014). Therefore, testing should be completed by the same person in each experiments.
Table 1.
Troubleshooting
| Problem | Possible causes and solutions |
|---|---|
| The injurious stimulus does not produce hyperalgesia |
|
| The hyperalgesic phase is too short (<3 days) |
|
| The hyperalgesic phase is too long (>1 month) |
|
| Responses reach a plateau, but below the initial baseline |
|
| Baselines are different between groups of animals |
|
| Hyperalgesia is detected both ipsilateral and contralateral to the stimulus |
|
| Paw responses (to von Frey hairs or other measures) become higher than baseline. |
|
| Naltrexone or naloxone do not produce reinstatement |
|
| There is no return to baseline after injecting drug to produce reinstatement |
|
| Reinstatement occurs spontaneously or after saline injection |
|
| During the remission phase, responses drift up or down |
|
Some do not repeatedly habituate mice to the testing equipment, although this is almost always recommended in rats. Unlike rats, mice do not necessarily habituate and may become even more stressed by the handling.
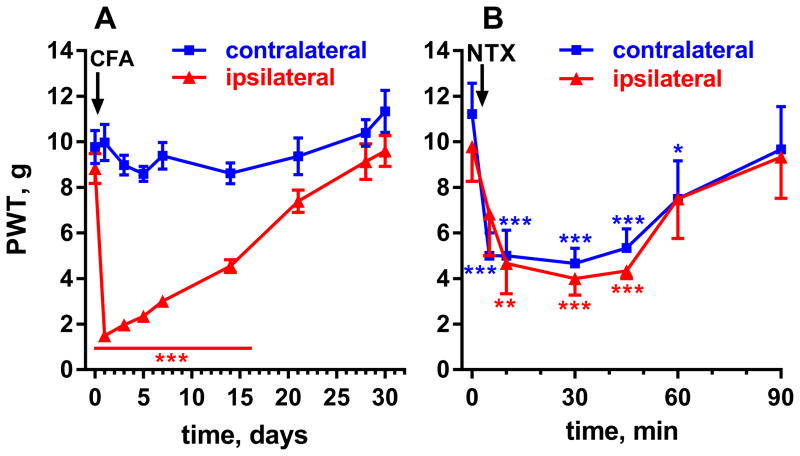
Anticipated Results
Figure 5 shows the results of a representative experiment in which LS was induced by injecting CFA (50 μl subcutaneous) in the hind paw of rats, as described above. Notice the robust hypersensitivity in the ipsilateral side, which resolved after 28 days (Figure 5A). Responses to von Frey filaments in the contralateral side remained at baseline. On day 30, rats were injected with naltrexone (1 mg/kg, s.c.). This resulted in reinstatement of hyperalgesia for about 1 hr in the ipsilateral side and the emergence of hyperalgesia in the contralateral side (Figure 5B).
Figure 5. Representative experiment showing CFA-induced LS in rats.
A. Rats (n=13) received an injection of CFA (50 μl, s.c.) in one hindpaw. Mechanical hypersensitivity, measured with von Frey hairs, developed ipsilaterally and resolved by day 28. Two-way ANOVA: p<0.0001 for time, side and interaction, p=0.0002 for subject matching. B. On day 30, six of the rats were injected with naltrexone (NTX, 1 mg/kg s.c.), which resulted in reinstatement of hypersensitivity lasting ~1 hr. Two-way ANOVA: p<0.0001 for time and subject matching, p=0.96 for side, p=0.65 for interaction. Holm-Sidak’s post-hoc tests: * p<0.05, ** p<0.01, *** p<0.001.
Time Considerations
When used as a model of chronic pain, LS experiments last weeks, and thus require maintenance of animals in a controlled environment for extended periods of time. This leads to higher animal costs and more complex institutional animal care protocols, which must be considered. However, once LS has been induced, animals require only standard care and occasional behavior measurements. With adequate planning to stagger measures, a single investigator can handle a large number of animals. However, the interval between each measurement and the time taken to test a batch of mice must be taken into consideration. The number of mice being tested in one batch cannot exceed the time interval to acquire one set of measurements. In this case, good record keeping and animal identification methods are essential.
Acknowledgments
Grant support: R01 DA033059 from the National Institute of Drug Abuse and I01 RX000378 from the Rehabilitation Research & Development Service, Department of Veterans Affairs to J.C.M.; R21 DA38248 and K02 DA19656 from the National Institute of Drug Abuse to B.K.T.
Footnotes
Conflict of Interest
The authors have no conflict of interests.
References
- Bessiere B, Richebe P, Laboureyras E, Laulin JP, Contarino A, Simonnet G. Nitrous oxide (N2O) prevents latent pain sensitization and long-term anxiety-like behavior in pain and opioid-experienced rats. Neuropharmacology. 2007;53:733–740. doi: 10.1016/j.neuropharm.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Campillo A, Cabanero D, Romero A, Garcia-Nogales P, Puig MM. Delayed postoperative latent pain sensitization revealed by the systemic administration of opioid antagonists in mice. Eur J Pharmacol. 2011;657:89–96. doi: 10.1016/j.ejphar.2011.01.059. [DOI] [PubMed] [Google Scholar]
- Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92:465–472. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, Wang ZJ, McCarson KE, Taylor BK. Constitutive μ-Opioid Receptor Activity Leads to Long-Term Endogenous Analgesia and Dependence. Science. 2013;341:1394–1399. doi: 10.1126/science.1239403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa T, Herz A. Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci U S A. 1989;86:7321–7325. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M, Sanoja R, Wang R, Vera-Portocarrero L, Oyarzo J, King T, Ossipov MH, Vanderah TW, Lai J, Dussor GO, Fields HL, Price TJ, Porreca F. Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. Pain. 2011;152:2701–2709. doi: 10.1016/j.pain.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Guan Y, Yuan F, Carteret AF, Raja SN. A partial L5 spinal nerve ligation induces a limited prolongation of mechanical allodynia in rats: an efficient model for studying mechanisms of neuropathic pain. Neurosci Lett. 2010;471:43–47. doi: 10.1016/j.neulet.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Illing S, Mann A, Schulz S. Heterologous regulation of agonist-independent μ-opioid receptor phosphorylation by protein kinase C. Br J Pharmacol. 2014;171:1330–1340. doi: 10.1111/bph.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Reichling DB, Levine JD. Shared Mechanisms for Opioid Tolerance and a Transition to Chronic Pain. J Neurosci. 2010;30:4660–4666. doi: 10.1523/JNEUROSCI.5530-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam H, Maga M, Pradhan A, Evans CJ, Maidment NT, Hales TG, Walwyn W. Analgesic tone conferred by constitutively active mu opioid receptors in mice lacking beta-arrestin 2. Mol Pain. 2011;7:24. doi: 10.1186/1744-8069-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laulin JP, Maurette P, Corcuff JB, Rivat C, Chauvin M, Simonnet G. The role of ketamine in preventing fentanyl-induced hyperalgesia and subsequent acute morphine tolerance. Anesth Analg. 2002;94:1263–1269. doi: 10.1097/00000539-200205000-00040. [DOI] [PubMed] [Google Scholar]
- Le Roy C, Laboureyras E, Gavello-Baudy S, Chateauraynaud J, Laulin JP, Simonnet G. Endogenous opioids released during non-nociceptive environmental stress induce latent pain sensitization via a NMDA-dependent process. Journal of Pain. 2011;12:1069–1079. doi: 10.1016/j.jpain.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Li X, Angst MS, Clark JD. Opioid-induced hyperalgesia and incisional pain. Anesth Analg. 2001;93:204–209. doi: 10.1097/00000539-200107000-00040. [DOI] [PubMed] [Google Scholar]
- Lian B, Vera-Portocarrero L, King T, Ossipov MH, Porreca F. Opioid-induced latent sensitization in a model of non-inflammatory viscerosomatic hypersensitivity. Brain Res. 2010;1358:64–70. doi: 10.1016/j.brainres.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meye FJ, van Zessen R, Smidt MP, Adan RA, Ramakers GM. Morphine withdrawal enhances constitutive mu-opioid receptor activity in the ventral tegmental area. J Neurosci. 2012;32:16120–16128. doi: 10.1523/JNEUROSCI.1572-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada CA, Vivancos GG, Tambeli CH, de Queiroz CF, Ferreira SH. Activation of presynaptic NMDA receptors coupled to NaV1.8-resistant sodium channel C-fibers causes retrograde mechanical nociceptor sensitization. Proc Natl Acad Sci USA. 2003;100:2923–2928. doi: 10.1073/pnas.252777799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richebe P, Rivat C, Laulin JP, Maurette P, Simonnet G. Ketamine improves the management of exaggerated postoperative pain observed in perioperative fentanyl-treated rats. Anesthesiology. 2005;102:421–428. doi: 10.1097/00000542-200502000-00028. [DOI] [PubMed] [Google Scholar]
- Rivat C, Laboureyras E, Laulin JP, Le Roy C, Richebe P, Simonnet G. Non-nociceptive environmental stress induces hyperalgesia, not analgesia, in pain and opioid-experienced rats. Neuropsychopharmacology. 2007;32:2217–2228. doi: 10.1038/sj.npp.1301340. [DOI] [PubMed] [Google Scholar]
- Rivat C, Vera-Portocarrero LP, Ibrahim MM, Mata HP, Stagg NJ, De Felice M, Porreca F, Malan TP. Spinal NK-1 receptor-expressing neurons and descending pathways support fentanyl-induced pain hypersensitivity in a rat model of postoperative pain. Eur J Neurosci. 2009;29:727–737. doi: 10.1111/j.1460-9568.2009.06616.x. [DOI] [PubMed] [Google Scholar]
- Romero A, Rojas S, Cabanero D, Gispert JD, Herance JR, Campillo A, Puig MM. A (1)(8)F-fluorodeoxyglucose MicroPET imaging study to assess changes in brain glucose metabolism in a rat model of surgery-induced latent pain sensitization. Anesthesiology. 2011;115:1072–1083. doi: 10.1097/ALN.0b013e31823425f2. [DOI] [PubMed] [Google Scholar]
- Shields SD, Eckert WA, 3rd, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. The journal of pain : official journal of the American Pain Society. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Maidment NT. Constitutively active micro opioid receptors mediate the enhanced conditioned aversive effect of naloxone in morphine-dependent mice. Neuropsychopharmacology. 2006;31:171–177. doi: 10.1038/sj.npp.1300782. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Maidment NT. Enkephalin release promotes homeostatic increases in constitutively active mu opioid receptors during morphine withdrawal. Neuroscience. 2007;149:642–649. doi: 10.1016/j.neuroscience.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Solway B, Bose SC, Corder G, Donahue RR, Taylor BK. Tonic inhibition of chronic pain by neuropeptide Y. Proc Natl Acad Sci U S A. 2011;108:7224–7229. doi: 10.1073/pnas.1017719108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014 doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Corder G. Endogenous Analgesia, Dependence, and Latent Pain Sensitization. Curr Top Behav Neurosci. 2014 doi: 10.1007/7854_2014_351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzi V, Onaran HO, Molinari P, Guerrini R, Balboni G, Calò G, Costa T. Ligands Raise the Constraint That Limits Constitutive Activation in G Protein-coupled Opioid Receptors. J Biol Chem. 2013;288:23964–23978. doi: 10.1074/jbc.M113.474452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walwyn W, Evans CJ, Hales TG. Beta-arrestin2 and c-Src regulate the constitutive activity and recycling of mu opioid receptors in dorsal root ganglion neurons. J Neurosci. 2007;27:5092–5104. doi: 10.1523/JNEUROSCI.1157-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sun X, Sadee W. Different effects of opioid antagonists on mu-, delta-, and kappa-opioid receptors with and without agonist pretreatment. J Pharmacol Exp Ther. 2007;321:544–552. doi: 10.1124/jpet.106.118810. [DOI] [PubMed] [Google Scholar]
- Wang Z, Bilsky EJ, Porreca F, Sadee W. Constitutive mu opioid receptor activation as a regulatory mechanism underlying narcotic tolerance and dependence. Life Sci. 1994;54:PL339–350. doi: 10.1016/0024-3205(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Bohren Y, Waltisperger E, Sage-Ciocca D, Yin JC, Freund-Mercier MJ, Barrot M. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.07.017. [DOI] [PubMed] [Google Scholar]