Abstract
Background
Dual-energy X-ray absorptiometry (DXA) techniques are limited in childhood chronic kidney disease (CKD) by the confounding effect of short stature and opposing parathyroid hormone effects on trabecular and cortical bone. Peripheral quantitative computed tomography (pQCT) is not subject to these limitations.
Methods
Lumbar spine (LS) and whole-body (WB) DXA and tibia pQCT scans were obtained in 88 stage 4–5 CKD and >650 healthy participants, ages 5–21 years. Sex- and race-specific Z-scores were generated for bone mineral density (BMD) and bone mineral content (BMC) by DXA, relative to age and adjusted for height Z-score (LS-BMD-Z and WB-BMC-Z), and compared to pQCT Z-scores for trabecular BMD (TrabBMD-Z) for age and cortical BMC (CortBMC-Z) for age and tibia length.
Results
LS-BMD-Z [0.50 (95% C.I. 0.28, 0.73), p<0.0001] and TrabBMD-Z [0.53 (0.27, 0.79), p<0.0001] were greater in CKD, and WB-BMC-Z [−0.36 (−0.53, −0.19), p<0.0001] and CortBMC-Z [−0.48 (−0.70, −0.27), p<0.0001] were lower, compared to reference participants. Z-scores were correlated at trabecular (LS-BMD-Z and TrabBMD-Z: R=0.36) and cortical (WB-BMC-Z and CortBMC-Z: R=0.64) sites in CKD; similar to correlations in reference participants.
Conclusions
Lumbar spine and whole-body DXA suggested greater trabecular BMD and lower cortical BMC in CKD, consistent with pQCT results; however, correlations were modest. Studies are needed to identify methods that predict fracture in childhood CKD.
Keywords: Pediatrics, Bone, Dual-energy X-ray absorptiometry, Peripheral quantitative computed tomography, Chronic kidney disease
Introduction
Bone strength is determined by bone mineral density (BMD) and bone quality, and peak bone mass accrued during childhood and adolescence is an essential determinant of life-long bone health [1]. Chronic kidney disease (CKD) compromises bone strength through effects on bone material properties, mineralization, turnover, microfracture repair, and trabecular and cortical architecture [2]. Alterations in bone density and quality during growth may lead to fractures, skeletal deformities, and chronic pain in CKD. Hence, establishing non-invasive methods to monitor bone disease and response to therapies in pediatric CKD is essential.
Dual-energy X-ray absorptiometry (DXA) is the most widely used method to assess bone health. DXA is inexpensive, fast, readily available, and delivers a low radiation dose. The International Society for Clinical Densitometry (ISCD) recommended the performance of posteroanterior lumbar spine (LS) and whole-body (WB) DXA in children with chronic diseases that may affect bone health [3]. However, these practice guidelines recommended against the use of DXA in children with CKD for two reasons. First, DXA results were poorly correlated with fracture risk in adults with CKD, and fracture discrimination in children has not been addressed. Second, the spectrum of abnormalities in bone turnover in CKD may differentially affect trabecular and cortical bone that are superimposed in the two-dimensional DXA image, yielding potentially misleading results [4]. Poor growth in CKD patients poses an additional challenge in the interpretation of DXA results. Because DXA is a two-dimensional projection technique that does not account for bone depth, DXA areal BMD (g/cm2) and bone mineral content (BMC, g) relative to age are systematically underestimated in children with low height Z-scores [5]. The ISCD guidelines proposed multiple methods to adjust DXA Z-scores for short stature, including generating Z-scores relative to height age, bone age, or height. However, these methods introduce biases related to differences in maturation between children with impaired growth and reference participants of the same height or height age [6]. More recently, Zemel et al. proposed a novel method that adjusts for height Z-score, effectively eliminating the association between DXA and height Z-scores in healthy children [7]. The impact of this approach has not been examined in disorders characterized by growth failure.
Peripheral quantitative computed tomography (pQCT) is a three-dimensional method that distinguishes between trabecular and cortical bone and provides volumetric measures of BMD (mg/cm3) that are not confounded by bone size. We recently used pQCT to demonstrate that pediatric CKD and secondary hyperparathyroidism were associated with significant reductions in cortical BMD and bone size, and with greater trabecular BMD in younger participants [8]. Yet, pQCT is not readily available for clinical applications.
The objectives of this study were to (1) characterize WB and LS DXA Z-scores in children with advanced CKD in this same cohort, compared to reference participants, to assess the impact of strategies to adjust DXA for growth failure and (2) examine the impact of those strategies on detection of cortical and trabecular bone findings seen on pQCT in the same participants. LS and WB DXA were chosen for analyses for two reasons: (1) the ISCD recommends using WB and LS DXA to evaluate bone health in children and (2) there is robust reference data available to the clinician in his or her office for analysis [3, 7, 9]. We hypothesized that adjustment for height Z-score would work in this population to minimize confounding by short stature. We also hypothesized that WB DXA Z-scores would capture cortical deficits identified on pQCT since WB BMC is approximately 80% cortical; while LS DXA Z-scores would not reflect CKD effects on trabecular bone given superimposed cortical bone in the vertebral body and spinous processes.
Methods
Study participants
The study population consisted of children and adolescents ages 5 to 21 years with pediatric onset of CKD at the Children’s Hospital of Philadelphia (CHOP) and Cincinnati Children’s Hospital Medical Center (CCHMC), enrolled in a larger study of bone health in CKD [8, 10, 11]. This DXA study is limited to the participants with advanced CKD (defined as an estimated glomerular filtration rate (eGFR) less than 30 ml/min/1.73 m2 or treatment with maintenance dialysis) and no history of renal transplantation. We excluded participants with a history of diseases known to affect bone health including neuromuscular disease, inflammatory bowel disease, sickle cell anemia, malignancy, and prior liver, bone marrow, or cardiac transplantation. A total of 88 children were eligible (58 at CHOP, 30 at CCHMC).
The study population was compared to a reference sample of 831 healthy children and adolescents ages 5 to 21 years (762 at CHOP, 69 at CCHMC). The reference participants were recruited from general pediatrics practices in the greater Philadelphia and Cincinnati areas and through general advertisements. Exclusion criteria for healthy reference participants were a history of illnesses or medication use that may affect growth, nutritional status, pubertal development, or bone accrual.
This study protocol was approved by the Institutional Review Boards at CHOP and CCHMC. Informed consent was obtained from study participants over age 18 and assent and parental consent from participants less than 18 years of age.
Anthropometry and physical maturity
Height was measured with a stadiometer (Hotain, Crymych, UK) and weight with a digital scale (Scaletonix, White Plains, NY). Tibia length was measured with a segmometer from the distal margin of the medial malleolus to the proximal border of the medial tibial condyle. Pubertal development stage was determined using a validated self-assessment questionnaire and classified by Tanner staging [12, 13]. Study participants and parents used the National Institute of Health classifications to describe their race, which was then categorized as black vs. non-black.
CKD disease characteristics and medications
Medical chart review, in addition to a participant and parent interview at the study visit, was used to obtain the medical history including the diagnosis of underlying renal disease, date of CKD diagnosis, current and prior medications, dialysis duration, and dialysis modality. Underlying disease was classified as (1) congenital anomalies of the kidney and urinary tract (CAKUT) including renal aplasia/hypoplasia/dysplasia, obstructive uropathy, and reflux nephropathy; (2) focal segmental glomerulosclerosis (FSGS); (3) systemic inflammatory disease (systemic lupus erythematosus and Wegener’s granulomatosis); and (4) other (cystinosis, renal ischemia, hemolytic uremic syndrome, tubulointerstitial nephritis, membranoproliferative glomerulonephritis Type I–III, idiopathic crescentic glomerulonephritis, membranous nephropathy, Alports syndrome, and Immunoglobulin A nephropathy).
Dual-energy x-ray absorptiometry
WB and LS DXA scans were performed using a Delphi/Discovery (Hologic, Bedford, MA, USA) densitometer with a fan beam in the array mode. LS DXA was analyzed with software version 12.3 and WB DXA with version 12.4. For LS DXA, DXA scans of the posteroanterior spine (lumbar vertebrae L1–L4) were obtained using standardized positioning techniques in the supine position to generate measures of areal BMD (g/cm2). WB BMC was obtained from the WB DXA scan excluding the head region, as recommended by the ISCD guidelines [14]. Calibration of the densitometers was verified by daily scanning of a LS phantom and weekly scans of a WB phantom. Based on the Bone Mineral Density in Childhood Study, in which CHOP and CCHMC participated, the in vivo percent coefficient of variation (%CV) was 0.85% for LS BMD and 1.06% for WB BMC [15].
Peripheral quantitative computed tomography
Scans of the left tibia were obtained by pQCT (Stratec XCT2000 12-detector unit, Orthometrix, Inc.) as previously described [8]. All scans were analyzed with Stratec software version 5.50 at CHOP. At the 3% metaphyseal site, scans were analyzed for trabecular volumetric BMD (mg/cm3). At the 38% diaphyseal site, scans were analyzed for cortical BMC (mg) as a summary measure of deficits in cortical BMD and dimensions. The manufacturer’s hydroxyapatite phantom was scanned daily for quality assurance. In our laboratory, the %CV was 1.4% for trabecular measures and 0.5% for cortical measures (unpublished data).
Laboratory studies
Non-fasting blood samples were collected during the study visit in all CKD participants. Serum creatinine (mg/dl) was measured by spectrophotometric enzymatic assay (Vitros, Johnson & Johnson Co., Rochester, NY) with the %CV of 1–5%. The eGFR (ml/min/1.73 m2) was calculated from height and serum creatinine using the pediatric estimating equations reported by the Prospective Cohort Study of Kidney Disease in Children [16]. Participants were categorized according to the National Kidney Foundation’s definition of CKD stages: Stage 4=eGFR 15 to 29; Stage 5=eGFR less than 15; and Stage 5D=on maintenance dialysis. Serum phosphorus was measured in the clinical laboratories using standard methods. Plasma parathyroid hormone (PTH) was measured by radioimmunoassay with 125I-labeled antibody (Scantibodies Clinical Laboratory), which quantifies intact PTH (iPTH). The intra-assay % CV for iPTH was 3–5% [17].
Statistical analysis
Sex- and age-specific Z-scores for height and body mass index (BMI) were calculated using the National Center for Health Statistics 2000 Center for Disease Control growth data [18].
WB DXA BMC, LS DXA BMD, and tibia pQCT trabecular BMD and cortical BMC outcomes were converted to sex-specific Z-scores for black and non-black groups using the power for the Box-Cox transformation, median, standard of deviation (LMS) method as previously described [19, 20]. The LMS method accounts for the nonlinearity, heteroskedasticity, and skew of bone data in growing children [21]. The Z-scores were generated using the LMS Chartmaker Program version 2.3. The CHOP reference sample (762 participants) was used to generate the LMS curves.
The pQCT Z-scores for trabecular BMD (tibia TrabBMD-Z) and DXA Z-scores for LS BMD (LS BMDageZ) and WB BMC (WB BMCageZ) were generated relative to age. The conventional age-based DXA Z-scores were then adjusted for height Z-score, generating WB BMChtZZ, and LS BMDhtZZ, using the same reference participants from CHOP used to generate LMS curves and the approach described by Zemel et al. [7]. This method uses prediction equations that adjust LS BMDageZ and WB BMCageZ for height Z-score, age, and the interactions between age and height Z-score. The advantage of this method is that in the peripubertal years, for example, children who have early (or delayed) puberty are often tall-for-age (or short-for-age) and this adjustment method captures some of the effect of pubertal timing. In addition, WB DXA BMC Z-scores were also generated relative to height alone (WB BMChtZ)—as advocated in prior studies [22–25]. Last, tibia pQCT cortical BMC is highly correlated with tibia length (R=0.88, p<0.0001). Therefore, the pQCT Z-scores for cortical BMC (tibia CortBMC-Z) were generated relative to tibia length, and further adjusted for age as previously described [8]. Analyses comparing CKD participants with the reference group were limited to those reference participants with complete imaging data for both pQCT and DXA (for WB BMC: 83 CKD participants, 728 reference participants; for LS BMD: 80 CKD participants, 707 reference participants; overall 88 CKD and 748 reference participants).
Stata 11.0 (Stata Corp., College Station, TX) was used for all statistical analyses. A p value of less than 0.05 was considered statistically significant and two-sided tests of hypotheses were used in all analyses. Group differences were assessed using Student’s t test, with adjustment for unequal variance as needed, or the Wilcoxon rank-sum test if the data were not normally distributed. Differences in proportions were assessed using the Chi-square test. Pearson pair-wise correlations were used to assess the correlation between pQCT and DXA Z-scores and between age and Z-scores in the CKD and reference participants. Analysis of variance (ANOVA) was used to assess the differences in DXA Z-scores across height Z-score categories, grouped by <−2, −2 to<−1, −1 to<1, and≥1. Bivariate linear regression models were used to assess differences in pQCT and DXA Z-scores between CKD participants and the reference group.
Results
Participant and disease characteristics
Table 1 summarizes CKD and reference group participants. Overall, pubertal maturation was delayed in CKD: within Tanner stages 2, 3, and 4, CKD participants were significantly older than the reference group (p<0.001), adjusted for sex and race. Participants with CKD had significantly lower height Z-scores (p<0.0001). On average, male CKD participants were 1.7 years (95% C.I. 1.2–2.1 years) older and females with CKD were 2.9 years (95% C.I. 2.1–3.7 years) older than reference participants of the same height. Weight Z-scores median (range) were −0.18 (−6.8 to 2.7). These low values were largely due to the short stature of the subjects. Participants’ BMI Z-scores median (range) were 0.08 (−3.58 to 2.58) as shown in Table 1.
Table 1.
Participant characteristics
| Variable | Reference participants | CKD participants |
|---|---|---|
| Number | 748 | 88 |
| Age, years | 11.3 | 14.5 |
| Median (range) | (5.0–22) | (5.7–20.8) |
| Sex, n (%) Male | 355 (47) | 59 (67) |
| Race, n (%) | ||
| White | 352 (47) | 61 (70) |
| Black | 320 (43) | 24 (27) |
| Asian | 18 (2) | 2 (2) |
| Other | 58 (8) | 1 (1) |
| Tanner, n (%) | ||
| Stage 1–2 | 370 (50) | 28 (32) |
| Stage 3 | 85 (11) | 17 (19) |
| Stage 4–5 | 288 (39) | 43 (49) |
| Height Z-score | 0.25 | −0.96 |
| Median (range) | (−2.59 to 3.26) | (−4.87 to 1.66) |
| BMI Z-score | 0.38 | 0.08 |
| Median (range) | (−2.17 to 2.99) | (−3.58 to 2.58) |
CKD chronic kidney disease; BMI body mass index (kg/m2 )
Table 2 summarizes disease characteristics of the CKD participants. There was substantial heterogeneity in cause and duration of disease seen in this cohort. In addition, there was a wide range of iPTH levels, ranging from 8–1,139 pg/ml, across CKD participants, representing the broad spectrum of bone disorders in severe CKD [26, 27].
Table 2.
Disease characteristics of CKD participants
| Variable | CKD participants |
|---|---|
| Number of participants | |
| CKD 4 | 32 |
| CKD 5 | 18 |
| CKD 5D | 38 |
| eGFR in CKD 4–5 | 19 |
| Median (IQR) | (14–25) |
| Age at diagnosis, years | 4.5 |
| Median (IQR) | (0.2–13.1) |
| Interval since diagnosis, years | 7.3 |
| Median (IQR) | (1.8–12.1) |
| Underlying renal disease, n (%) | |
| CAKUT | 44 (50) |
| FSGS | 24 (27) |
| Systemic inflammatory | 6 (7) |
| Other | 14 (16) |
| In CKD 5D: | |
| Duration of dialysis, months | 3.3 |
| Median (IQR) | (0.9–13.2) |
| Hemodialysis, n (%), vs. peritoneal dialysis | 26 (68) |
| Intact PTH (pg/ml)27,28 | |
| Median (Range) | |
| CKD 4 | 98 (8–663) |
| CKD 5 | 476 (56–1,046) |
| CKD 5D | 320 (9.5–1,139) |
| On medication at visit, n (%) | |
| Glucocorticoids | 6 (7) |
| Recombinant human growth hormone | 8 (9) |
| Calcitriol | 67 (76) |
| On medication ever, n (%) | |
| Glucocorticoids | 25 (29) |
| Recombinant human growth hormone | 24 (27) |
| Calcitriol | 75 (85) |
CKD chronic kidney disease; 5D patients on dialysis; eGFR estimated glomerular filtration rate (ml/min/1.73 m2 ); IQR intraquartile range; CAKUT congenital anomalies of the kidney and urinary tract; FSGS focal segmental glomerulosclerosis; PTH parathyroid hormone
Adjustment for growth failure
Figure 1 shows the associations between height Z-score category and DXA Z-scores relative to age (WB BMCageZ and LS BMDageZ), illustrating the significant extent to which short stature results in an underestimation of DXA BMC and BMD relative to age (ANOVA: WB BMCageZ p<0.0001; LS BMDageZ p <0.0001). In contrast, the pQCT measures (tibia TrabBMD-Z and CortBMC-Z) were not associated with height Z-score category (both p>0.13). The DXA measures adjusted for height Z-score (WB BMChtZZ and LS BMDhtZZ) or calculated relative to height (WB BMChtZ) were not associated with height Z-score category (all p>0.12).
Fig. 1.

Height Z-score and DXA Z-scores relative to age in CKD participants. a WB BMCageZ-scores significantly increased as height Z-score category increased. b LS BMDageZ-scores also significantly increased with height Z-score category. Abbreviations: DXA – dual-energy X-ray absorptiometry; CKD – chronic kidney disease; WB BMCageZ – DXA whole body less head bone mineral content Z-score for age; LS BMDageZ – DXA lumbar spine bone mineral density Z-score for age
Whole-body DXA and tibia cortical BMC
Figure 2a and Table 3 summarize the results in the sites used to assess cortical BMC (WB DXA and tibia diaphysis pQCT) in CKD compared to reference participants. WB BMCageZ was significantly lower in CKD participants, compared to the reference population. The WB DXA Z-score based on height, WB BMChtZ, was not significantly different from the reference group for CKD. The WB DXA Z-score adjusted for height Z-score, WB BMChtZZ, was significantly lower in CKD compared to reference participants; however, the deficit was less pronounced than observed for WB BMCageZ. In comparison, by pQCT, CKD participants had significantly lower tibia CortBMC-Z, compared to the reference group. Tibia CortBMC-Z was significantly associated with WB BMCageZ, WB BMChtZ, and WB BMChtZZ (R=0.56, 0.60, and 0.64, respectively, all p<0.0001). These correlations are similar to those observed in the healthy reference participants (R=0.53, 0.70, and 0.70, respectively, all p<0.0001). WB DXA Z-scores and pQCT tibia CortBMC Z-scores were not related to CKD participant’s age.
Fig. 2.
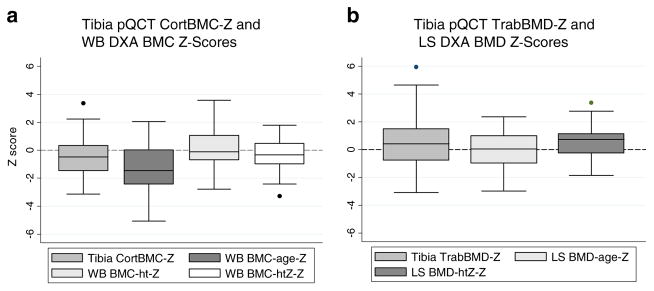
Tibia pQCT Z-scores and DXA Z-scores in CKD participants. a Tibia CortBMC-Z, WB BMCageZ, and WB BMChtZZ were significantly lower and WB BMChtZ was no different in CKD participants, compared to the reference participants. b Tibia TrabBMD-Z and LS BMDhtZZ were significantly greater and LS BMDageZ was no different in CKD participants, compared to the reference participants. Abbreviations: pQCT – peripheral quantitative computed tomography; DXA – dual-energy X-ray absorptiometry; CKD – chronic kidney disease; Tibia CortBMC-Z – pQCT tibia cortical bone mineral content Z-score for age and tibia length; WB BMCageZ – DXA whole body less head bone mineral content Z-score for age; WB BMChtZ – DXA whole body less head bone mineral content Z-score for height; WB BMChtZZ – DXA whole body less head bone mineral content Z-score for age, adjusted for height Z-score; Tibia TrabBMD-Z – pQCT tibia trabecular bone mineral density Z-score for age; LS BMDageZ – DXA lumbar spine bone mineral density Z-score for age; LS BMDhtZZ – DXA lumbar spine bone mineral density Z-score for age, adjusted for height Z-score
Table 3.
pQCT Z-scores and DXA Z-scores
| Imaging modality | Z-scores β (CI) | p value | Correlation coefficient DXA vs. pQCT (R) | p value | |
|---|---|---|---|---|---|
| Measures of cortical bone: tibia CortBMC-Z and WB DXA Z-scores in CKD participants (n=83), compared to the reference group (n=728) | |||||
| Tibia CortBMC-Z | −0.48 | (−0.70, −0.27) | <0.0001 | ||
| WB BMCageZ | −1.31 | (−1.56, −1.07) | <0.0001 | 0.56 | <0.0001 |
| WB BMChtZ | 0.13 | (−0.10, 0.36) | 0.28 | 0.60 | <0.0001 |
| WB BMChtZZ | −0.36 | (−0.53, −0.19) | <0.0001 | 0.64 | <0.0001 |
| Measures of trabecular bone: tibia TrabBMD-Z and LS DXA Z-scores in CKD participants (n=80), compared to the reference group (n=707) | |||||
| Tibia TrabBMD-Z | 0.53 | (0.27, 0.79) | <0.0001 | ||
| LS BMDageZ | −0.02 | (−0.26, 0.22) | 0.86 | 0.38 | <0.001 |
| LS BMChtZZ | 0.50 | (0.28, 0.73) | <0.0001 | 0.36 | <0.001 |
pQCT – peripheral quantitative computed tomography; DXA – dual-energy X-ray absorptiometry; CKD – chronic kidney disease; Tibia CortBMC-Z – pQCT tibia cortical bone mineral content Z-score for age, tibia length; WB BMCageZ – DXA whole body less head bone mineral content Z-score for age; WB BMChtZ – DXA whole body less head bone mineral content Z-score for height; WB BMChtZZ – DXA whole body less head bone mineral content Z-score for age, adjusted for height Z-score; Tibia TrabBMD-Z – pQCT tibia trabecular bone mineral density Z-score for age; LS BMDageZ – DXA lumbar spine bone mineral density Z-score for age; LS BMDhtZZ – DXA lumbar spine bone mineral density Z-score for age, adjusted for height Z-score
Lumbar spine DXA and tibia trabecular BMD
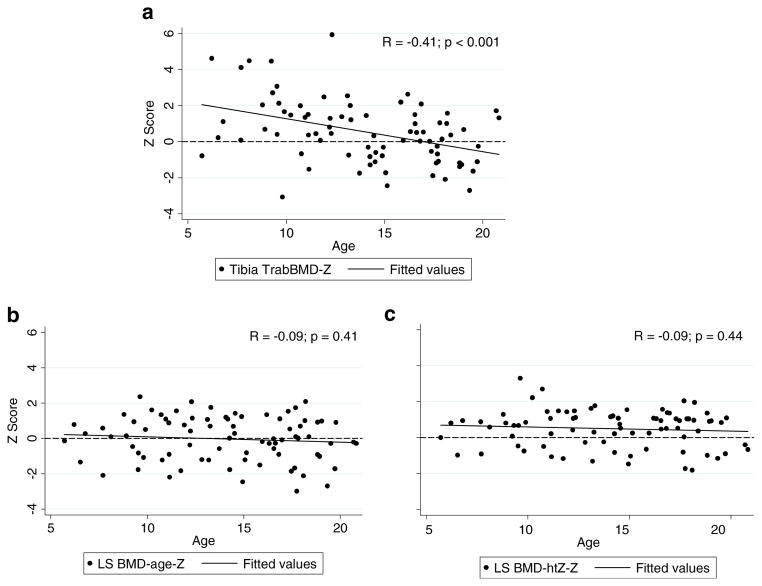
Figure 2b and Table 3 summarize the LS DXA Z-scores (LS BMCageZ and LS BMChtZZ) and tibia pQCT TrabBMD-Z-scores in CKD compared to reference participants. LS BMDageZ in CKD was not significantly different from the reference group. However, LS BMDhtZZ was significantly greater in CKD participants, compared to the reference participants. Tibia TrabBMD-Z-scores were significantly higher in CKD participants compared to the reference participants, and this difference was greater in the younger participants, as previously described [8] and illustrated in Fig. 3a. LS BMDageZ (Fig. 3b) and LS BMDhtZZ (Fig. 3c) were not significantly associated with age in CKD. The greater mean LS BMDhtZZ-scores in CKD compared to reference participants were observed in CKD participants of all ages (Fig. 3c). Tibia TrabBMD-Z was significantly associated with LS BMCageZ and LS BMChtZZ (R=0.38 and R=0.36, respectively, both p<0.001). These correlations are similar to those observed in the healthy reference participants (R=0.47 for both, p<0.0001).
Fig. 3.
Association of tibia pQCT TrabBMD-Z and LS DXA Z-scores with age in CKD participants. a There was a negative association between age and tibia TrabBMD-Z. Greater tibia TrabBMD-Z-scores were seen in younger children with CKD and TrabBMD-Z-scores were decreased in older children with CKD. b, c There was a lack of association of age with LS BMDageZ and LS BMDhtZZ. LS BMDhtZZ-scores remained elevated even in older children. Abbreviations: pQCT – peripheral quantitative computed tomography; DXA – dual-energy X-ray absorptiometry; CKD – chronic kidney disease; Tibia TrabBMD-Z – pQCT tibia trabecular bone mineral density Z-score for age; LS BMDageZ – DXA lumbar spine bone mineral density Z-score for age; LS BMDhtZZ – DXA lumbar spine bone mineral density Z-score for age, adjusted for height Z-score
Associations of DXA with laboratory parameters and medication data
Neither LS BMD nor WB BMC Z-scores, adjusted or unadjusted for height Z-score, were associated with iPTH levels (all p>0.1). Phosphorous levels were not associated with LS BMD (p>0.5) or WB BMC (p>0.06) Z-scores, adjusted or unadjusted for height Z-score. In this cohort, limited to severe CKD (eGFR<30 ml/min/1.73 m2), there were no associations between eGFR and WB BMC Z-scores (p>0.9) nor LS BMD Z-scores (p>0.8). In addition, WB BMC and LS BMD Z-scores did not differ according to CKD stage category (both p>0.2).
As shown in Table 2, 27% of participants had a history of recombinant human growth hormone (rhGH) therapy. These participants were significantly shorter than those without prior rhGH therapy, adjusted for CKD stage and duration of disease [β (95% CI) −0.84 (−1.5, −0.2)] consistent with growth failure as the indication for rhGH therapy. Hence, associations with DXA parameters were not assessed due to confounding by indication. Overall, 85% of participants had a history of calcitriol therapy. Given the cross-sectional nature of this study, and frequent changes in calcitriol therapies in advanced CKD, we did not examine associations between calcitriol therapy and bone outcomes.
A total of 29% of participants had a history of glucocorticoid therapy. Use of glucocorticoids was associated with significantly lower LS BMDhtZZ with a mean Z-score in non-users of 0.74 (95% CI 0.47, 1.0) compared to −0.07 (95% CI −0.47, 0.33) in users (p<0.01). Glucocorticoid use was not associated with WB BMChtZZ-score (p>0.1).
Discussion
Most studies of bone health in pediatric CKD used DXA and yielded inconsistent results, reporting decreased [28–32], normal [25, 33–35], or increased BMD [36, 37] compared to controls. These studies utilized varying methods to adjust for growth failure and different reference samples to generate Z-scores. These factors may partly explain the variability in results. Here, we demonstrated low WB DXA BMC Z-scores and normal LS DXA BMD Z-scores in severe CKD using conventional Z-scores relative to age, compared to a robust reference sample. As expected, adjustment for the significantly lower height Z-scores in the CKD participants resulted in marked attenuation of WB DXA BMC deficits (from a mean Z-score of −1.28 to −0.37), and increases in LS DXA BMD Z-scores (from a mean Z-score of −0.03 to 0.49) in CKD, compared to the reference participants.
These data demonstrate that it is essential to adjust DXA Z-scores for growth failure in CKD. However, the choice of method used to adjust for growth failure requires careful consideration of age and maturation effects. The WB BMC Z-score relative to height overcompensated for deficits, reflected by Z-scores, which were much higher (mean WB BMChtZ of 0.11) than by other DXA methods and pQCT. This is because the comparison of a child with CKD to a reference participant of the same height resulted in the comparison with a reference subject that was, on average, 2 years younger. This failure to consider differences in age led to a biased estimate of BMC, as demonstrated by Zemel et al. [7]. The advantage of the height Z-score adjustment method is that it does more than simply adjusting for bone size. Adjustment for height Z-score accounts for how short stature varies as a function of age and captures the effects of delayed maturation that parallel growth failure in CKD. In addition, the difference between the unadjusted, age-based Z-score and height Z-score adjusted DXA Z-score, which was, on average, approximately +1 SD for the WB and +0.5 SD for LS in this population, shows the degree to which a low DXA Z-score may be attributable to growth failure, rather than true bone deficits [7]. We propose that adjustment for age and height Z-score provides an unbiased estimate of DXA measures in CKD.
Prior pQCT studies of children with CKD, including this study, reported cortical deficits across all ages [8], and elevated trabecular BMD in younger children with CKD [8, 38–40]. The magnitude of cortical deficits captured by DXA, estimated as WB BMChtZZ (mean Z =−0.37) was comparable to those demonstrated by pQCT (mean Z=−0.51). Similarly, the magnitude of trabecular excess captured by DXA, estimated as LS BMDhtZZ (mean Z =+0.49) was comparable to those demonstrated by pQCT (mean Z=+ 0.53). However, LS DXA did not show the inverse association with age noted with pQCT TrabBMD (Fig. 3a). The higher tibia trabecular Z-scores seen in the younger children on pQCT may have been related to the metaphysis site and changes occurring adjacent to the growth plate in CKD [41–43]. This abnormal metaphyseal remodeling would not be seen in the spine. In addition, the mass of dense cortical bone in the spinous processes and shell of the vertebral body along with lower cortical volumetric BMD in CKD may be blunting LS DXA’s ability to detect the extremes in trabecular densities that occur in CKD [44]. These extremes in trabecular BMD are illustrated by the greater variability in pQCT TrabBMD Z-scores compared to DXA LS BMD Z-scores in Fig. 2b.
This study has four important limitations. First, the DXA and pQCT scans were not obtained at the same skeletal site. However, a study by Wren et al. compared spine DXA and quantitative computed tomography (QCT) and demonstrated that DXA over-diagnosed bone deficits in children with growth failure, similar to our results here [45]. Second, this study did not use fracture events as the gold-standard to define bone disease. As previously described [8], few of these participants had a history of fracture and most fractures occurred years prior to the study. Third, the lack of bone biopsy data prohibited assessment of pQCT and DXA methods to capture abnormalities in bone turnover. Fourth, associations with lab and medicine data are difficult to interpret given the cross-sectional nature of the study. Examination of changes in DXA over time as compared to variation in iPTH, for example, may reveal that DXA can and does correlate with biochemical parameters. In addition, longitudinal studies would alleviate the problem of confounding by indication, which is seen with assessment of the impact of growth hormone and calcitriol on DXA measures. However, this study has notable strengths. This is the first study to compare DXA and pQCT Z-scores in children or adults with CKD, and the first to include a robust concurrent reference population, facilitating adjustment for age, sex, race, and body size. This is also the first study to demonstrate the utility of the height Z-score adjustment method of DXA results in a chronic disease complicated by growth failure. It is also important to note that the correlation between DXA and pQCT in the CKD participants was similar to the correlation in the reference group, suggesting that DXA did not perform significantly worse in CKD.
In summary, conventional measures of DXA Z-scores relative to age resulted in a marked over-diagnosis of bone deficits in CKD. The height Z-score adjustment revealed trabecular and cortical patterns similar to those obtained with pQCT. Hence, we conclude whole-body DXA may prove useful as a clinical tool for the detection of cortical deficits, but this is dependent on the approach used to account for differences in growth and development in children with CKD. Correlations between pQCT and DXA were modest, suggesting that a single DXA assessment may not reflect bone health. Given the widespread availability of DXA, longitudinal studies are needed to determine if DXA is sensitive to changes related to CKD progression and treatments. Most importantly, fracture studies are needed to determine if DXA and/or pQCT reflect bone fragility in CKD.
Acknowledgments
Funding Sources NIH R01-DK060030, R01-HD040714, K24-DK076808, and UL1-RR-024134
Contributor Information
Lindsay M. Griffin, Email: griffinl@email.chop.edu, Department of Pediatrics, Children’s Hospital of Philadelphia, 3535 Market Street, Room 1564, Philadelphia, PA 19104, USA
Heidi J. Kalkwarf, Email: heidi.kalkwarf@cchmc.org, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA
Babette S. Zemel, Email: zemel@email.chop.edu, Department of Pediatrics, Children’s Hospital of Philadelphia, 3535 Market Street, Room 1564, Philadelphia, PA 19104, USA
Justine Shults, Email: jshults@mail.med.upenn.edu, Department of Biostatistics and Epidemiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA.
Rachel J. Wetzsteon, Email: wetzsteon@email.chop.edu, Department of Pediatrics, Children’s Hospital of Philadelphia, 3535 Market Street, Room 1564, Philadelphia, PA 19104, USA
C. Frederic Strife, Email: frederic.strife@cchmc.org, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.
Mary B. Leonard, Email: leonard@email.chop.edu, Department of Pediatrics, Children’s Hospital of Philadelphia, 3535 Market Street, Room 1564, Philadelphia, PA 19104, USA. Department of Biostatistics and Epidemiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA
References
- 1.NIH Osteoporosis Prevention, Diagnosis, and Therapy. NIH Consensus Statement. 2000;17(1):1–36. [PubMed] [Google Scholar]
- 2.Cunningham J, Sprague SM, Cannata-Andia J, Coco M, Cohen-Solal M, Fitzpatrick L, Goltzmann D, Lafage-Proust MH, Leonard M, Ott S, Rodriguez M, Stehman-Breen C, Stern P, Weisinger J. Osteoporosis in chronic kidney disease. Am J Kidney Dis. 2004;43(3):566–571. doi: 10.1053/j.ajkd.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Bishop N, Braillon P, Burnham J, Cimaz R, Davies J, Fewtrell M, Hogler W, Kennedy K, Makitie O, Mughal Z, Shaw N, Vogiatzi M, Ward K, Bianchi ML. Dual-energy X-ray absorptiometry assessment in children and adolescents with diseases that may affect the skeleton: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11(1):29–42. doi: 10.1016/j.jocd.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Parfitt AM. A structural approach to renal bone disease. J Bone Miner Res. 1998;13(8):1213–1220. doi: 10.1359/jbmr.1998.13.8.1213. [DOI] [PubMed] [Google Scholar]
- 5.Prentice A, Parsons TJ, Cole TJ. Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr. 1994;60(6):837–842. doi: 10.1093/ajcn/60.6.837. [DOI] [PubMed] [Google Scholar]
- 6.Saland JM, Goode ML, Haas DL, Romano TA, Seikaly MG. The prevalence of osteopenia in pediatric renal allograft recipients varies with the method of analysis. Am J Transplant. 2001;1(3):243–250. doi: 10.1034/j.1600-6143.2001.001003243.x. [DOI] [PubMed] [Google Scholar]
- 7.Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, Mahboubi S, Shepherd JA, Hangartner TN, Frederick MM, Winer KK, Kalkwarf HJ. Height adjustment in assessing dual-energy X-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95(3):1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wetzsteon RJ, Kalkwarf HJ, Shults J, Zemel BS, Foster BJ, Griffin L, Strife CF, Foerster DL, Jean-Pierre DK, Leonard MB. Volumetric bone mineral density and bone structure in childhood chronic kidney disease. J Bone Miner Res. 2011;26(9):2235–44. doi: 10.1002/jbmr.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Frederick MM, Huang X, Lu M, Mahboubi S, Hangartner T, Winer KK. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the Bone Mineral Density in Childhood Study. J Clin Endocrinol Metab. 2011;96:3160–3169. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuchman S, Kalkwarf HJ, Zemel BS, Shults J, Wetzsteon RJ, Foerster D, Strife CF, Leonard MB. Vitamin D deficiency and parathyroid hormone levels following renal transplantation in children and adolescents. Pediatr Nephrol. 2010;25(12):2509–2516. doi: 10.1007/s00467-010-1612-0. [DOI] [PubMed] [Google Scholar]
- 11.Foster BJ, Kalkwarf HJ, Shults J, Zemel BS, Wetzsteon RJ, Thayu M, Foerster DL, Leonard MB. Association of chronic kidney disease with muscle deficits in children. J Am Soc Nephrol. 2011;22 (2):377–386. doi: 10.1681/ASN.2010060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanner JM. Growth at adolescence. 2. Blackwell Scientific Publications; Oxford: 1962. [Google Scholar]
- 13.Tanner JM, Whitehouse RH, Marshall WA, Healy MJR, Goldstein H. Assessment of skeletal maturity and prediction of adult height (TW2 method) Academic Press; London: 1975. [Google Scholar]
- 14.Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, Lorenc RS, Tosi LL, Ward KA, Ward LM, Kalkwarf HJ. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd JA, Wang L, Fan B, Gilsanz V, Kalkwarf HJ, Lappe J, Lu Y, Hangartner T, Zemel BS, Frederick M, Oberfield S, Winer KK. Optimal monitoring time interval between DXA measures in children. J Bone Miner Res. 2011;26(11):2745–2752. doi: 10.1002/jbmr.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao P, Scheibel S, D’Amour P, John MR, Rao SD, Schmidt-Gayk H, Cantor TL. Development of a novel immunoradiometric assay exclusively for biologically active whole parathyroid hormone 1–84: implications for improvement of accurate assessment of parathyroid function. J Bone Miner Res. 2001;16(4):605–614. doi: 10.1359/jbmr.2001.16.4.605. [DOI] [PubMed] [Google Scholar]
- 18.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 19.Dubner SE, Shults J, Baldassano RN, Zemel BS, Thayu M, Burnham JM, Herskovitz RM, Howard KM, Leonard MB. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn’s disease. Gastroenterology. 2009;136(1):123–130. doi: 10.1053/j.gastro.2008.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wetzsteon RJ, Shults J, Zemel BS, Gupta PU, Burnham JM, Herskovitz RM, Howard KM, Leonard MB. Divergent effects of glucocorticoids on cortical and trabecular compartment BMD in childhood nephrotic syndrome. J Bone Miner Res. 2009;24(3):503–513. doi: 10.1359/JBMR.081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44(1):45–60. [PubMed] [Google Scholar]
- 22.Kelly TL, Wilson KE, Heymsfield SB. Dual-energy X-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9):e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez CP, Salusky IB, Kuizon BD, Ramirez JA, Gales B, Ettenger RB, Goodman WG. Bone disease in children and adolescents undergoing successful renal transplantation. Kidney Int. 1998;53(5):1358–1364. doi: 10.1046/j.1523-1755.1998.00866.x. [DOI] [PubMed] [Google Scholar]
- 24.Reusz GS, Szabo AJ, Peter F, Kenesei E, Sallay P, Latta K, Szabo A, Tulassay T. Bone metabolism and mineral density following renal transplantation. Arch Dis Child. 2000;83(2):146–151. doi: 10.1136/adc.83.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed SF, Russell S, Rashid R, Beattie TJ, Murphy AV, Ramage IJ, Maxwell H. Bone mineral content, corrected for height or bone area, measured by DXA is not reduced in children with chronic renal disease or in hypoparathyroidism. Pediatr Nephrol. 2005;20(10):1466–1472. doi: 10.1007/s00467-005-1973-y. [DOI] [PubMed] [Google Scholar]
- 26.KDOQI Clinical Practice Guidelines for bone metabolism and disease in children with chronic kidney disease. Am J of Kid Diseases. 2005;46(1):S1–S103. [Google Scholar]
- 27.KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int. 2009;76(113):S1. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 28.Andrade MC, Carvalhaes JT, Carvalho AB, Lazarretti-Castro M, Brandao C. Bone mineral density and bone histomorphometry in children on long-term dialysis. Pediatr Nephrol. 2007;22(10):1767–1772. doi: 10.1007/s00467-007-0546-7. [DOI] [PubMed] [Google Scholar]
- 29.Pluskiewicz W, Adamczyk P, Drozdzowska B, Szprynger K, Szczepanska M, Halaba Z, Karasek D. Skeletal status in children, adolescents and young adults with end-stage renal failure treated with hemo- or peritoneal dialysis. Osteoporos Int. 2002;13(5):353–357. doi: 10.1007/s001980200039. [DOI] [PubMed] [Google Scholar]
- 30.Pluskiewicz W, Adamczyk P, Drozdzowska B, Szprynger K, Szczepanska M, Halaba Z, Karasek D. Skeletal status in children and adolescents with chronic renal failure before onset of dialysis or on dialysis. Osteoporos Int. 2003;14(4):283–288. doi: 10.1007/s00198-002-1335-6. [DOI] [PubMed] [Google Scholar]
- 31.Pluskiewicz W, Adamczyk P, Drozdzowska B, Szprynger K, Szczepanska M, Halaba Z, Karasek D. Skeletal status in adolescents with end-stage renal failure: a longitudinal study. Osteoporos Int. 2005;16(3):289–295. doi: 10.1007/s00198-004-1672-8. [DOI] [PubMed] [Google Scholar]
- 32.Bakr AM. Bone mineral density and bone turnover markers in children with chronic renal failure. Pediatr Nephrol. 2004;19(12):1390–1393. doi: 10.1007/s00467-004-1670-2. [DOI] [PubMed] [Google Scholar]
- 33.Waller S, Ridout D, Rees L. Bone mineral density in children with chronic renal failure. Pediatr Nephrol. 2007;22(1):121–127. doi: 10.1007/s00467-006-0292-2. [DOI] [PubMed] [Google Scholar]
- 34.Van Dyck M, Gyssels A, Proesmans W, Nijs J, Eeckels R. Growth hormone treatment enhances bone mineralisation in children with chronic renal failure. Eur J Pediatr. 2001;160(6):359–363. doi: 10.1007/s004310100734. [DOI] [PubMed] [Google Scholar]
- 35.van der Sluis IM, Boot AM, Nauta J, Hop WC, de Jong MC, Lilien MR, Groothoff JW, van Wijk AE, Pols HA, Hokken-Koelega AC, de Muinck Keizer-Schrama SM. Bone density and body composition in chronic renal failure: effects of growth hormone treatment. Pediatr Nephrol. 2000;15(3–4):221–228. doi: 10.1007/s004670000470. [DOI] [PubMed] [Google Scholar]
- 36.Swolin-Eide D, Magnusson P, Hansson S. Bone mass, biochemical markers and growth in children with chronic kidney disease: a 1-year prospective study. Acta Paediatr. 2007;96(5):720–725. doi: 10.1111/j.1651-2227.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 37.Swolin-Eide D, Hansson S, Magnusson P. Children with chronic kidney disease: a 3-year prospective study of growth, bone mass and bone turnover. Acta Paediatr. 2009;98(2):367–373. doi: 10.1111/j.1651-2227.2008.01073.x. [DOI] [PubMed] [Google Scholar]
- 38.Behnke B, Altrogge H, Delling G, Kruse HP, Muller-Wiefel DE. Bone mineral density in pediatric patients after renal transplantation. Clin Nephrol. 1996;46(1):24–29. [PubMed] [Google Scholar]
- 39.Behnke B, Kemper MJ, Kruse HP, Muller-Wiefel DE. Bone mineral density in children with primary hyperoxaluria type I. Nephrol Dial Transplant. 2001;16(11):2236–2239. doi: 10.1093/ndt/16.11.2236. [DOI] [PubMed] [Google Scholar]
- 40.Lima EM, Goodman WG, Kuizon BD, Gales B, Emerick A, Goldin J, Salusky IB. Bone density measurements in pediatric patients with renal osteodystrophy. Pediatr Nephrol. 2003;18 (6):554–559. doi: 10.1007/s00467-002-1041-9. [DOI] [PubMed] [Google Scholar]
- 41.Mehls O, Ritz E, Krempien B, Willich E, Bommer J, Scharer K. Roentgenological signs in the skeleton of uremic children. An analysis of the anatomical principles underlying the roentgenological changes. Pediatr Radiol. 1973;1(3):183–190. doi: 10.1007/BF00974065. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez CP, He YZ. Effects of thyroparathyroidectomy, exogenous calcium, and short-term calcitriol therapy on the growth plate in renal failure. J Am Soc Nephrol. 2003;14(1):148–158. doi: 10.1097/01.asn.0000039565.56011.be. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez CP, He YZ, Leiferman E, Wilsman NJ. Bone elongation in rats with renal failure and mild or advanced secondary hyperparathyroidism. Kidney Int. 2004;65(5):1740–1748. doi: 10.1111/j.1523-1755.2004.00577.x. [DOI] [PubMed] [Google Scholar]
- 44.Leonard MB, Shults J, Zemel BS. DXA estimates of vertebral volumetric bone mineral density in children: potential advantages of paired posteroanterior and lateral scans. J Clin Densitom. 2006;9 (3):265–273. doi: 10.1016/j.jocd.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Wren TA, Liu X, Pitukcheewanont P, Gilsanz V. Bone densitometry in pediatric populations: discrepancies in the diagnosis of osteoporosis by DXA and CT. J Pediatr. 2005;146 (6):776–779. doi: 10.1016/j.jpeds.2005.01.028. [DOI] [PubMed] [Google Scholar]