Abstract
Growth hormone and its mediator, insulinlike growth factor 1 (IGF-1), are key determinants of growth in children and young adults. As patients with Fontan physiology often experience diminished longitudinal growth, we sought to describe IGF-1 levels in this population and to identify factors associated with IGF-1 deficiency. Forty-one Fontan subjects ≥5 years were evaluated in this cross-sectional study. Age- and gender-specific height Z scores were generated using national data. Laboratory testing included IGF-1 and brain natriuretic peptide (BNP) levels. IGF-1 levels were converted to age-, gender-, and Tanner stage–specific Z scores. BNP levels were log transformed to achieve a normal distribution (log-BNP). Medical records were reviewed for pertinent clinical variables. Predictors of IGF-1 Z score were assessed through the Student t test and Pearson’s correlation. Median age was 11.1 years (range 5.1 to 33.5 years), and time from Fontan was 8.2 years (1.1 to 26.7). Mean height Z score was −0.2 ± 0.9 with a mean IGF-1 Z score of −0.1 ± 1.3. There was no association between IGF-1 Z score and height Z score. Longer interval since Fontan (R = −0.32, p = 0.04), higher log-BNP (R = −0.40; p = 0.01), and lower indexed systemic flow on cardiac magnetic resonance (R = 0.55, p = 0.02) were associated with lower IGF-1 Z scores. In conclusion, in this cohort with Fontan physiology, higher BNP and lower systemic flow were associated with lower IGF-1 Z score. Longitudinal studies are needed to determine if these relations represent a mechanistic explanation for diminished growth in children with this physiology and with other forms of congenital heart disease.
The Fontan operation is the final stage of palliation for various forms of single ventricle heart disease. In the 4 decades since its initial description, life expectancy of patients who have undergone the Fontan operation has improved dramatically and attention has shifted toward the noncardiac sequelae of a circulation characterized by increased central venous pressure and diminished cardiac output. Longitudinal growth impairment is well recognized following the Fontan procedure.1–3 However, the underlying causes of impaired growth in this population are poorly understood. Growth hormone (GH) and its mediator, insulinlike growth factor 1 (IGF-1), are fundamentally important to linear growth in children whether they have congenital heart disease.4 The pituitary gland releases GH in response to a hypothalamic signal; GH stimulates the production of IGF-1 in the liver and locally within target organs. Both GH and IGF-1 act on the chondrocyte growth plate to stimulate longitudinal growth.5 Disruptions in this pathway have been implicated in growth impairment in other pediatric chronic diseases.6–9 Emerging evidence suggests that the GH/IGF-1 pathway may also be abnormal in children with congenital heart disease,10–12 but existing studies are limited by their small number of patients with single ventricle heart disease. The goals of this study were to describe IGF-1 levels in children and young adults after Fontan palliation, to correlate IGF-1 levels with growth parameters, and to identify risk factors for low IGF-1 levels to increase understanding of the underlying causes of growth failure in these patients.
Methods
Fifty Fontan participants aged ≥5 years were prospectively enrolled in a single-center cross-sectional study of growth, nutrition, body composition, and bone health from July 2011 through October 2013, as previously described.13 All Fontan patients followed by the center were eligible and were contacted for potential study participation. Exclusion criteria included the following: pregnancy, metal hardware that prevented dual-energy x-ray absorptiometry (as this was included in the original study protocol), Fontan baffie obstruction or single lung physiology, moderate-to-severe chronic kidney disease (estimated glomerular filtration rate <60 ml/min/1.73 m2), moderate-to-severe hepatic impairment (transaminases >2 times the upper limit of normal), and inability to complete the study procedures because of developmental delay. The data presented here exclude the 4 participants with protein-losing enteropathy (PLE), 2 with genetic syndromes potentially impacting growth, and an additional 3 with missing IGF-1 levels.
The study protocol was approved by the Institutional Review Board at The Children’s Hospital of Philadelphia. Informed consent was obtained from participants aged >18 years and assent as well as parental consent from those <18 years.
Pertinent variables including cardiac anatomy, ventricular morphology, presence of heterotaxy syndrome, date and type of Fontan procedure, and presence of a fenestration at initial operation were obtained through patient interviews and confirmed in the medical record. Oxygen saturation and hemoglobin were obtained from the medical record when available.
Anthropometric measures were obtained in light clothing with shoes removed. Weight (0.1 kg) was measured using a digital scale (Scaltronix, White Plains, New York). Height (0.1 cm) was measured using a stadiometer (Holtain Ltd, Croswell, Crymych, United Kingdom). Pubertal status (Tanner stage) was assessed with a validated self-assessment questionnaire.14
Serum brain natriuretic peptide (BNP) was measured using a chemiluminescent microparticle immunoassay. The lower limit of detection for our laboratory is 10 pg/ml; patients with levels <10 pg/ml were assigned a level of 10. IGF-1 was measured by Esoterix Laboratory Services (Calabasas Hills, California) using a radioimmunoassay. IGF-1 levels were converted to gender-, age-, and Tanner stage–adjusted Z scores using Esoterix laboratory’s reference data. Adult participants were considered to be 18 years old.
Echocardiography was performed on a Phillips IE33 machine (Phillips, Andover, Massachusetts) according to our standard imaging protocol. Ventricular function and atrioventricular valve regurgitation were qualitatively assessed. Cardiac magnetic resonance (CMR) studies were performed on a 1.5-T Avanto Magnetic Resonance Imaging scanner (Siemens Medical Solutions, Erlangen, Germany) with a 6-channel phased-array body coil using a standard imaging protocol. Phase-contrast velocity mapping was used to determine systemic blood flow (Qs) by measuring summed caval flow. Ventricular volumes and Qs were indexed to body surface area.
Continuous variables were expressed as means ± standard deviation or median (range). The 2000 National Center for Health Statistics growth data were used to calculate gender-specific Z scores for height and BMI, relative to age.15 Participants aged >20 years at the time of the study visit were assigned an age of 20 years for the generation of height Z scores. BMI Z scores were calculated in those aged <20 years. The distribution of BNP was highly skewed in favor of lesser values, so these values were normalized with natural logarithmic transformation (log-BNP). The associations between IGF-1 Z scores and Fontan clinical characteristics were examined using Pearson correlation for continuous variables and by comparisons of Z scores (Student t test) according to categorical variables such as the presence of a systemic right ventricle. Demographic and clinical characteristics were compared between those with IGF-1 Z scores greater than and less than the median Z score using Student t test and chi-square analysis. Of note, IGF-1 Z scores were not associated with lean body mass Z scores in our previous report in this cohort.13 Analyses were performed using Stata 12.0 (Stata Corp., College Station, Texas). A p value <0.05 was considered significant and 2-sided tests were used throughout.
Results
Demographic and anthropometric characteristics of the participants are summarized in Table 1. Height Z score did not vary with age or interval since the Fontan procedure. The disease-specific characteristics of the 41 Fontan participants are summarized in Table 2. CMR data were available on 17 participants and are summarized in Table 3. Median BNP level was 17.5 (range 10 to 247, interquartile range 17.9). Thirty-three of the participants (80%) had a BNP level <40, whereas 39 (95%) had a BNP level <100. Ten participants (24%) had a BNP level <10 pg/ml, the lower limits of detection for our laboratory.
Table 1.
Demographic and anthropometric characteristics (n = 41)
| Variable | Value |
|---|---|
| Age, median (range) (year) | 11.1 (5.1–33.5) |
| Interval from Fontan, median (range) (year) | 8.2 (1.1–26.7) |
| Male | 21 (51%) |
| White | 29 (71%) |
| Black | 5 (12%) |
| Other | 7 (17%) |
| Tanner stage 1–2 | 22 (54%) |
| Height Z-score, mean ± SD | −0.2 ± 0.9 |
| Range | −2.5 to 1.0 |
| Body mass index Z-score, mean ± SD* | 0.1 ± 0.9 |
| Range | −2.5 to 2.2 |
| Insulin-like growth factor-1 Z-score, mean ± SD | −0.1 ± 1.3 |
| Range | −2.7 to 3.0 |
SD, standard deviation.
participants < 20 years old, n = 34.
Table 2.
Fontan Clinical Characteristics
| Variable | Value | p value for association with IGF-1 Z-score |
|---|---|---|
| Anatomy | 0.71 | |
| Hypoplastic left heart syndrome | 13 (32%) | |
| Tricuspid atresia | 7 (17%) | |
| Unbalanced atrioventricular canal defect | 7 (17%) | |
| Other single ventricle variants | 6 (15%) | |
| Double outlet right ventricle | 6 (15%) | |
| Pulmonary atresia/intact ventricular septum | 2 (4%) | |
| Heterotaxy syndrome | 4 (10%) | 0.55 |
| Ventricular morphology | 0.09 | |
| Right ventricle | 17 (42%) | |
| Left ventricle | 14 (34%) | |
| Mixed | 10 (24%) | |
| Type of Fontan | 0.05 | |
| Extracardiac-conduit | 26 (63%) | |
| Lateral tunnel | 15 (37%) | |
| Fenestration* | 34 (83%) | 0.1 |
| Systemic oxygen saturation† | 0.95 (0.85–0.99) | 0.19 |
| Hemoglobin (g/dL)‡ | 14.4 (12.6–18.6) | 0.72 |
| Brain type natriuretic peptide, (pg/mL) median (range, interquartile range) | 17.5 (10–247;17.9) | 0.01 |
| Echocardiographic assessment§ | ||
| Normal or low normal ventricular function | 31 (84%) | 0.9 |
| Absent or mild atrioventricular valve regurgitation | 32 (86%) | 0.99 |
Results expressed as median (range) or n (%).
at time of initial operation.
n = 27.
n = 15.
n = 37.
Table 3.
Cardiac magnetic resonance imaging variables (n=17)
| Variable | Value | R value for association with IGF-1 Z-score | p value for association with IGF-1 Z-score |
|---|---|---|---|
| Ejection fraction (%) | 65 ± 10 | 0.01 | 0.97 |
| End-diastolic volume (cc/m2) | 82 ± 25 | 0.24 | 0.35 |
| End-systolic volume (cc/m2) | 30 ± 15 | 0.18 | 0.49 |
| Stroke volume (cc/m2) | 52 ± 15 | 0.23 | 0.37 |
| Summed caval flow (L/min/m2) | 2.4 ± 0.5 | 0.55 | 0.02 |
Results expressed as mean ± standard deviation.
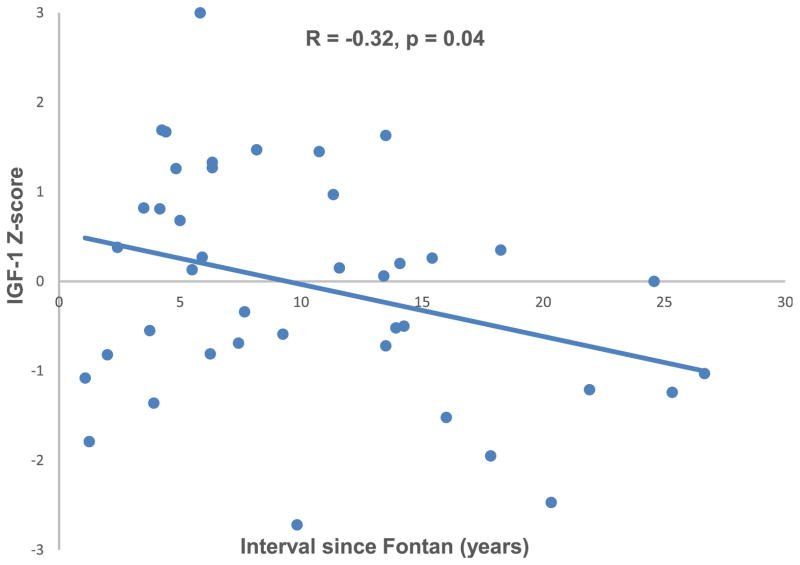
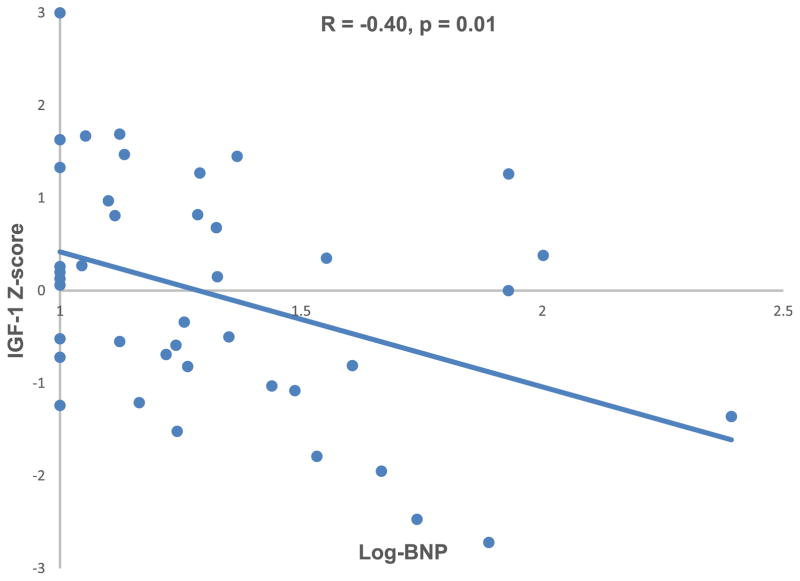
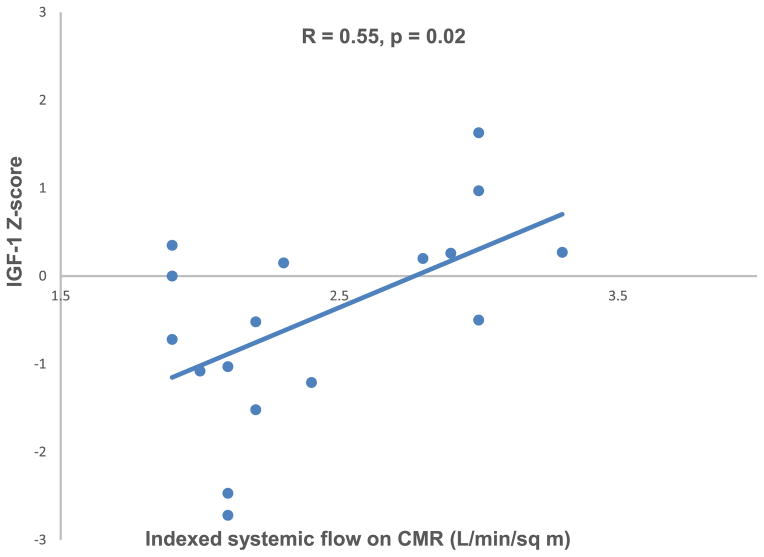
IGF-1 Z score was negatively associated with time from Fontan operation (R = −0.32, p = 0.04; Figure 1) and with log-transformed BNP level (R = −0.40, p = 0.01; Figure 2). IGF-1 Z score was positively associated with indexed Qs on CMR (R = 0.55, p = 0.02; Figure 3). There was no association between IGF-1 Z score and any other CMR variables (Table 3). IGF-1 Z score was not different based on cardiac anatomy, systemic ventricular morphology, presence of heterotaxy syndrome, Fontan type, history of a fenestration, qualitative ventricular function, or atrioventricular valve regurgitation on echocardiogram. There was no association between height Z score and IGF-1 Z score (R = 0.08; p = 0.6).
Figure 1.
IGF-1 Z score was inversely associated with interval since Fontan.
Figure 2.
IGF-1 Z score was inversely associated with log-BNP.
Figure 3.
IGF-1 Z score was positively associated with indexed systemic flow on cardiac magnetic resonance imaging.
More participants with HLHS had IGF-1 Z scores less than the median versus greater than the median (42% vs 23%, p = 0.02). There was a trend toward higher indexed Qs in participants with IGF-1 Z scores greater than the median (2.6 l/min/m2 vs 2.2 L/min/m2, p = 0.08). Otherwise demographic and clinical characteristics were similar between the 2 groups.
Discussion
In this cross-sectional evaluation of Fontan patients, we demonstrated a negative correlation between time from Fontan and IGF-1 Z score, a positive correlation between systemic output and IGF-1 Z score, and a negative correlation between serum BNP level and IGF-1 Z score. Taken together, these findings provide evidence of an additional noncardiac system that is adversely affected by Fontan physiology. The findings point to a disruption of the neuroendocrine growth axis and a downregulation of growth mediators as the “heart failure” burden associated with Fontan physiology progresses over time. This may have important implications for understanding the impact of heart failure on growth impairment in this population and may allow a more targeted approach to diagnosis and treatment.
Disruptions in the GH/IGF-1 pathway have been implicated in growth impairment in many pediatric chronic diseases6–9 and are particularly well described in Crohn disease. Patients with Crohn disease presumably have normal serum BNP levels, but studies have shown an association between high levels of inflammatory cytokines and low levels of IGF-1 in these patients. Treatment with anti-inflammatory agents has been associated with normalization of levels of IGF-1 and its binding protein (IGFBP-3),16 suggesting that therapies that treat the underlying disease process may have a normalizing effect on the neuroendocrine growth axis. Although investigation into the role of inflammation in the Fontan heart failure state is warranted, it would also be interesting to evaluate whether IGF-1 levels are responsive to therapies targeted at improving Fontan hemodynamics.
Although we did not find a relation between IGF-1 and growth in this cohort, a link between heart failure and growth factors has important implications for patients with Fontan physiology and for children with other forms of congenital heart disease. In support of this concept, Daymont et al17 demonstrated significant, simultaneous decreases in growth parameters before corrective or palliative surgery in a number of common forms of congenital heart disease, particularly those characterized by a large volume burden on the heart. In contrast, patients with malnutrition typically demonstrate sequential loss of growth percentiles—weight, followed by length, and finally head circumference. This data lend additional support to our findings that the neuroendocrine growth axis may be adversely affected by a heart failure state.
For infants with single ventricle heart disease, a mechanistic link between heart failure and growth mediators may be particularly important. In the Pediatric Heart Network’s Infant Single Ventricle study, high BNP levels were associated with lesser weight and length Z scores before the superior cavopulmonary connection and at 14 months.18 In a separate analysis, the investigators grouped patients according to height Z-score trajectory and BNP trajectory (“high” and “low” for both variables). They found that patients in the “high BNP–low height” trajectory group had worse neurodevelopmental outcomes at 14 months.19 Growth mediators were not evaluated in this study, but the data reported here support the hypothesis that abnormalities in growth factors may have mediated these associations in infants with “heart failure.”
Although we describe a potentially important relation between BNP and growth factors in the Fontan population, the general utility of BNP as a surveillance tool for “wellness” in older Fontan patients is unclear. The results of the Pediatric Heart Network’s cross-sectional study of children and adolescents with Fontan physiology did not support the routine use of BNP as a surveillance tool in asymptomatic patients, although the study did not assess the relation between BNP and growth parameters.20 However, other studies have suggested that serum BNP may be a useful test for clinical assessment of heart failure in infants and children with single ventricle physiology.21,22 N-terminal pro-BNP, the inactive fragment of prohormone of BNP, is a known marker of heart failure in acquired heart disease and has been shown to correlate with clinical parameters in adults with a variety of congenital lesions.23 If one considers the Fontan circulation to be a form of chronic, compensated heart failure in which the neuroendocrine growth axis is impaired, then further investigation into the serial assessment of BNP and IGF-levels in Fontan patients is warranted.
The primary limitations of this study are the small sample size and the cross-sectional design. Given the small sample size, there is notable spread in the scatterplots and the correlations are modest, despite their significance. Although we were able to demonstrate a relation between markers of heart failure and growth factors, we did not demonstrate a relation between IGF-1 and height Z score. Correlation of IGF-1 with height Z score may be evident in cohorts with greater growth deficits, such as Fontan patients with PLE. Given the nature of this study, we were not able to evaluate the relation between IGF-1 Z scores and heart failure in individual patients over time. Finally, only a subset of subjects underwent CMR, limiting our power to detect associations between CMR-derived variables and growth factors.
Acknowledgments
This study was funded by a Cardiac Center Grant and the Robert S. and Dolores Harrington Endowment in Pediatric Cardiology at the Children’s Hospital of Philadelphia.
Footnotes
Disclosures
Catherine M. Avitabile was supported by Grant T32 HL007915, Kevin K. Whitehead was supported by Grant K23 HL089647, and Mary B. Leonard was supported by Grant K24 DK076808 from the National Institutes of Health and are supported by Grants UL1 RR024134 and UL1 TR000003 from the Clinical and Translational Science Award. Other authors have no disclosures to report.
References
- 1.Cohen MI, Bush DM, Ferry RJ, Jr, Spray TL, Moshang T, Jr, Wernovsky G, Vetter VL. Somatic growth failure after the Fontan operation. Cardiol Young. 2000;10:447–457. doi: 10.1017/s1047951100008118. [DOI] [PubMed] [Google Scholar]
- 2.Cohen MS, Zak V, Atz AM, Printz BF, Pinto N, Lambert L, Pemberton V, Li JS, Margossian R, Dunbar-Masterson C, McCrindle BW. Anthropometric measures after Fontan procedure: implications for suboptimal functional outcome. Am Heart J. 2010;160:1092–1098. 1098 e1. doi: 10.1016/j.ahj.2010.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogt KN, Manlhiot C, Van Arsdell G, Russell JL, Mital S, McCrindle BW. Somatic growth in children with single ventricle physiology impact of physiologic state. J Am Coll Cardiol. 2007;50:1876–1883. doi: 10.1016/j.jacc.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 4.Wit JM, Camacho-Hubner C. Endocrine regulation of longitudinal bone growth. Endocr Dev. 2011;21:30–41. doi: 10.1159/000328119. [DOI] [PubMed] [Google Scholar]
- 5.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed SF, Farquharson C, McGrogan P, Russell RK. Pathophysiology and management of abnormal growth in children with chronic inflammatory bowel disease. World Rev Nutr Diet. 2013;106:142–148. doi: 10.1159/000342529. [DOI] [PubMed] [Google Scholar]
- 7.Ezri J, Marques-Vidal P, Nydegger A. Impact of disease and treatments on growth and puberty of pediatric patients with inflammatory bowel disease. Digestion. 2012;85:308–319. doi: 10.1159/000336766. [DOI] [PubMed] [Google Scholar]
- 8.Mahesh S, Kaskel F. Growth hormone axis in chronic kidney disease. Pediatr Nephrol. 2008;23:41–48. doi: 10.1007/s00467-007-0527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Switzer M, Rice J, Rice M, Hardin DS. Insulin-like growth factor-I levels predict weight, height and protein catabolism in children and adolescents with cystic fibrosis. J Pediatr Endocrinol Metab. 2009;22:417–424. doi: 10.1515/jpem.2009.22.5.417. [DOI] [PubMed] [Google Scholar]
- 10.Surmeli-Onay O, Cindik N, Kinik ST, Ozkan S, Bayraktar N, Tokel K. The effect of corrective surgery on serum IGF-1, IGFBP-3 levels and growth in children with congenital heart disease. J Pediatr Endocrinol Metab. 2011;24:483–487. doi: 10.1515/jpem.2011.061. [DOI] [PubMed] [Google Scholar]
- 11.Pons Leite H, Gilberto Henriques Vieira J, Brunow De Carvalho W, Chwals WJ. The role of insulin-like growth factor I, growth hormone, and plasma proteins in surgical outcome of children with congenital heart disease. Pediatr Crit Care Med. 2001;2:29–35. doi: 10.1097/00130478-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Soliman AT, Elawwa A, Khella A, Saeed S, Yassin H. Linear growth in relation to the circulating concentration of insulin-like growth factor-I in young children with acyanotic congenital heart disease with left to right shunts before versus after surgical intervention. Indian J Endocrinol Metab. 2012;16:791–795. doi: 10.4103/2230-8210.100678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avitabile CM, Leonard MB, Zemel BS, Brodsky JL, Lee D, Dodds K, Hayden-Rush C, Whitehead KK, Goldmuntz E, Paridon SM, Rychik J, Goldberg DJ. Lean mass deficits, vitamin D status, and exercise capacity in children and young adults after Fontan palliation. Heart. 2014;100:1702–1707. doi: 10.1136/heartjnl-2014-305723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris M, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adol. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 15.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Eivindson M, Gronbaek H, Skogstrand K, Thorsen P, Frystyk J, Flyvbjerg A, Dahlerup JF. The insulin-like growth factor (IGF) system and its relation to infliximab treatment in adult patients with Crohn’s disease. Scand J Gastroenterol. 2007;42:464–470. doi: 10.1080/00365520601010115. [DOI] [PubMed] [Google Scholar]
- 17.Daymont C, Neal A, Prosnitz A, Cohen MS. Growth in children with congenital heart disease. Pediatrics. 2013;131:e236–242. doi: 10.1542/peds.2012-1157. [DOI] [PubMed] [Google Scholar]
- 18.Butts RJ, Zak V, Hsu D, Cnota J, Colan SD, Hehir D, Kantor P, Levine JC, Margossian R, Richmond M, Szwast A, Williams D, Williams R, Atz AM. Factors associated with serum B-type natriuretic peptide in infants with single ventricles. Pediatr Cardiol. 2014;35:879–887. doi: 10.1007/s00246-014-0872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravishankar C, Zak V, Williams IA, Bellinger DC, Gaynor JW, Ghanayem NS, Krawczeski CD, Licht DJ, Mahony L, Newburger JW, Pemberton VL, Williams RV, Sananes R, Cook AL, Atz T, Khaikin S, Hsu DT. Association of impaired linear growth and worse neurodevelopmental outcome in infants with single ventricle physiology: a report from the pediatric heart network infant single ventricle trial. J Pediatr. 2013;162:250–256. e2. doi: 10.1016/j.jpeds.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atz AM, Zak V, Breitbart RE, Colan SD, Pasquali SK, Hsu DT, Lu M, Mahony L, Paridon SM, Puchalski MD, Geva T, McCrindle BW. Factors associated with serum brain natriuretic peptide levels after the Fontan procedure. Congenit Heart Dis. 2011;6:313–321. doi: 10.1111/j.1747-0803.2011.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah A, Feraco AM, Harmon C, Tacy T, Fineman JR, Bernstein HS. Usefulness of various plasma biomarkers for diagnosis of heart failure in children with single ventricle physiology. Am J Cardiol. 2009;104:1280–1284. doi: 10.1016/j.amjcard.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowenthal A, Camacho BV, Lowenthal S, Natal-Hernandez L, Liszewski W, Hills NK, Fineman JR, Bernstein HS. Usefulness of B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide as biomarkers for heart failure in young children with single ventricle congenital heart disease. Am J Cardiol. 2012;109:866–872. doi: 10.1016/j.amjcard.2011.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eindhoven JA, van den Bosch AE, Ruys TP, Opic P, Cuypers JA, McGhie JS, Witsenburg M, Boersma E, Roos-Hesselink JW. N-terminal pro-B-type natriuretic peptide and its relationship with cardiac function in adults with congenital heart disease. J Am Coll Cardiol. 2013;62:1203–1212. doi: 10.1016/j.jacc.2013.07.019. [DOI] [PubMed] [Google Scholar]