Abstract
Measures of muscle mass or size are often used as surrogates of forces acting on bone. However, chronic diseases may be associated with abnormal muscle force relative to muscle size. The muscle-bone unit was examined in 64 children and adolescents with new-onset Crohn’s disease (CD), 54 with chronic kidney disease (CKD), 51 treated with glucocorticoids for nephrotic syndrome (NS), and 264 healthy controls. Muscle torque was assessed by isometric ankle dynamometry. Calf muscle cross-sectional area (CSA) and tibia cortical section modulus (Zp) were assessed by quantitative CT. Log-linear regression was used to determine the relations among muscle CSA, muscle torque, and Zp, adjusted for tibia length, age, Tanner stage, sex, and race. Muscle CSA and muscle torque-relative-to-muscle CSA were significantly lower than controls in advanced CKD (CSA −8.7%, p = 0.01; torque −22.9%, p < 0.001) and moderate-to-severe CD (CSA −14.1%, p < 0.001; torque −7.6%, p = 0.05), but not in NS. Zp was 11.5% lower in advanced CKD (p = 0.005) compared to controls, and this deficit was attenuated to 6.7% (p = 0.05) with adjustment for muscle CSA. With additional adjustment for muscle torque and body weight, Zp was 5.9% lower and the difference with controls was no longer significant (p = 0.09). In participants with moderate-to-severe CD, Zp was 6.8% greater than predicted (p = 0.01) given muscle CSA and torque deficits (R2=0.92), likely due to acute muscle loss in newly diagnosed patients. Zp did not differ in NS, compared with controls. In conclusion, muscle torque relative to muscle CSA was significantly lower in CKD and CD, compared with controls, and was independently associated with Zp. Future studies are needed to determine if abnormal muscle strength contributes to progressive bone deficits in chronic disease, independent of muscle area.
Keywords: Peripheral quantitative computed tomography, muscle, bone, kidney disease, glucocorticoids, Crohn’s disease, children
INTRODUCTION
Muscle size and strength increase during growth, as do the mechanical loads on bones. Bones adapt by increasing dimensions and strength. This capacity of bone to respond to mechanical loading with increased bone strength is greatest during childhood.(1) Given the strong associations between muscle mass and bone strength, investigators have advocated a two-staged algorithm to assess the functional muscle-bone unit in chronic disease.(2) First, muscle mass is assessed relative to body size, and second, bone outcomes are assessed relative to muscle mass. This approach is intended to distinguish between primary bone disorders (muscle mass is normal and bone mass is low relative to muscle), as opposed to bone disorders that are secondary to muscle deficits (muscle mass is reduced but bone mass is ‘adequate’ for the reduced muscle mass).
Lean mass, measured by dual-energy x-ray absorptiometry (DXA), or muscle cross-sectional area (CSA), measured by peripheral quantitative computed tomography (pQCT), are often used as surrogates for mechanical loading in the interpretation of bone outcomes. For example, adjusting DXA bone outcomes for lean mass attenuated bone deficits in children with chronic disease, suggesting that bone deficits represented an adaptation to lower muscle mass.(3,4) However, it is not known if imaging-based estimates of lean mass or muscle CSA are adequate surrogates of mechanical loading in children with chronic disease. To assess the adequacy of these lean mass estimates as surrogates of mechanical loading, we examined muscle torque relative to muscle size as a measure of muscle quality. Muscle torque is a measure of muscle strength and is the gold standard for measuring muscle contractile force. Chronic diseases may be associated with lower muscle strength relative to muscle mass that is not captured by DXA or pQCT. Accordingly, direct measures of muscle torque may more fully explain bone deficits in chronic disease.
We have examined bone and body composition outcomes using DXA and pQCT in children treated with chronic glucocorticoids for steroid sensitive nephrotic syndrome (SSNS),(5–7) in children with elevated inflammatory cytokines due to incident Crohn’s disease,(8–10) and in children with chronic kidney disease (CKD).(11–13) Each of these disorders may be associated with abnormalities in muscle metabolism. Glucocorticoids and inflammatory cytokines increase protein catabolism and increase myostatin, resulting in muscle atrophy and weakness.(14,15) In addition, glucocorticoids may increase intramuscular adipose tissue which will result in an overestimate of muscle CSA on pQCT.(16) Similarly, fluid retention and edema in CKD may result in an overestimation of muscle CSA and mass since the density of water is similar to muscle. For example, the removal of fluid during maintenance dialysis resulted in acute reductions in lean mass measured by DXA.(17) CKD is associated with impaired muscle protein metabolism,(18) as well impaired mitochondrial function and swelling of individual muscle fibers.(19)
The relationships between muscle torque and muscle CSA have not been addressed in these diseases. If glucocorticoids, inflammation, and CKD are associated with lower muscle torque relative to muscle CSA, imaging-based measures of lean mass or muscle CSA that are integral to the functional muscle-bone unit algorithm to assess bone deficits may not fully capture the muscle forces acting on bone. Thus, the objectives of this pQCT study were (1) to identify deficits in muscle torque relative to muscle CSA in children and adolescents with CKD, SSNS, and Crohn’s disease, and (2) to determine whether muscle torque is associated with bone geometry in children and adolescents with these conditions, independent of muscle CSA, body weight, growth, and development. Our hypothesis was that muscle torque relative to muscle CSA would be lower in CKD, SSNS, and Crohn’s disease, and this difference would contribute to differences in bone geometry. If deficits in muscle torque contribute to bone deficits independent of muscle CSA, future studies of the muscle-bone unit should incorporate measures of both muscle torque and CSA.
METHODS
Study Participants
The study populations included children and adolescents aged 5 to 21 years that were enrolled as participants in simultaneous bone studies at the Children’s Hospital of Philadelphia.(7–9,20,21)
Participants with CKD (n = 54) were included if they had an estimated glomerular filtration rate (eGFR) less than 90 ml/min/1.73 m2 and no prior history of renal transplantation.(22) Reduced muscle torque was previously reported in this CKD cohort, but that study did not examine the association between muscle torque and bone.(12) Furthermore, the analysis here was limited to children with a primary renal diagnosis of congenital anomalies of the kidney and urinary tract in order exclude children with systemic inflammatory diseases or other renal conditions treated with glucocorticoids. Estimated GFR (ml/min/1.73 m2) was calculated from height and serum creatinine (mg/dl) using the pediatric estimating equations recently reported by the Prospective Cohort Study of Kidney Disease in Children.(22) Participants were categorized into CKD stages according to the National Kidney Foundation definitions:(23) CKD stage 2 = eGFR 60 to 89; CKD stage 3 = eGFR 30 to 59; CKD stage 4 = eGFR 15 to 29; CKD stage 5 = eGFR <15; and CKD 5D = maintenance dialysis. For the purposes of these analyses, we combined CKD stages 2 and 3 (CKD 2–3, n = 30) and CKD stages 4 to 5D (CKD 4–5D, n = 24).
The participants with SSNS (n = 51), as defined by the International Society of Kidney Disease in Children,(24) were included if they had a normal eGFR (greater than 90 ml/min/1.73 m2), at least a 6 month interval since SSNS diagnosis, a history of oral glucocorticoid therapy for SSNS within the prior 12 months, and documented urinary remission at the time of the study visit as previously described.(7) Patients in relapse were excluded to avoid the confounding effects of edema.
Participants with incident Crohn’s disease (n = 64) were evaluated within 2 weeks of diagnosis, confirmed by endoscopic, histologic, clinical, and radiographic parameters.(8) Crohn’s disease activity was assessed using the Pediatric Crohn’s Disease Activity Index (PCDAI) based on symptoms (30%), physical examination (30%), laboratory parameters (20%), and growth data (20%).(25) PCDAI scores range from 0 to 100, and disease activity was categorized as none (0 to 10), mild (11 to 30), and moderate-to-severe (> 30). For the purposes of this analysis, we categorized participants by disease activity: none to mild (PCDAI ≤ 30, n = 26) or moderate–to-severe (PCDAI > 30, n = 38). Prior publications in these participants reported muscle and bone deficits;(8–10) however, measures of muscle torque were not addressed.
The control participants (n = 264, aged 5 to 21 years) were recruited from general pediatrics practices and newspaper advertisements in the greater Philadelphia area, as previously described.(21,26) Participants were excluded for other illnesses or medications that may impact growth, nutritional status, pubertal development, or bone accrual. The control participants were included in a recent report of the functional muscle bone unit in children and young adults.(26) This study identified significant sex and race differences in muscle torque relative to muscle CSA.
The study protocol was approved by the Institutional Review Board at the Children’s Hospital of Philadelphia. Informed consent was obtained directly from study participants older than 18 years, and assent along with parental consent from participants less than 18 years of age.
Anthropometry, Physical Maturity, and Race
Height (0.1 cm) was measured with a stadiometer (Holtain, Crymych, UK) and weight (0.1 kg) with a digital scale (Scaletronix, White Plains, New York). Age- and sex-specific Z-scores for height and body mass index (BMI, kg/m2) were calculated using National Center for Health Statistics 2000 Center for Disease Control growth data.(27) The stage of pubertal development was determined using a validated self-assessment questionnaire and classified according to the method of Tanner.(28,29) Study participants, and their parent(s), if the participant was under age 18, were asked to categorize the participant’s race according to the National Institute of Health categories.
Bone and Muscle Assessment by Peripheral Quantitative Computed Tomography
Bone measures in the left tibia were obtained by pQCT using a Stratec XCT2000 device (Orthometrix, White Plains, NY) with a 12-detector unit, voxel size of 0.4 mm, slice thickness of 2.3 mm, and scan speed of 25 mm/sec. Scans were analyzed with Stratec software version 5.50. Polar section modulus (Zp, mm3) was assessed at 38% of tibia length proximal to the distal physis using Cortmode 2 (threshold, 711 mg/cm3). Section modulus was the primary outcome in our manuscript describing sex, maturation and race differences in cortical geometry and the muscle bone unit, and in our manuscript describing the relations between cortical geometry, muscle area, muscle torque and body weight in healthy controls that was the foundation for this manuscript.(21,26) The insertion region for the tibialis anterior muscle (which is primarily responsible for ankle dorsiflexion) extends to this cortical site. A prior study in athletes demonstrated that differences in cortical structure between the dominant and non-dominant leg were evident at the 38% site but not the 66% site, and a related study demonstrated that the bone-muscle relationship was similar at the 38% and 66% sites.(30,31) Zp is a summary measure of cortical dimensions calculated as {Σ (d2 × A)}/dmax, where A is the cross-sectional area of a voxel, d is the distance of the voxel from the center of gravity, dmax is the maximum distance of any of the voxels of the cortical cross-section from the center of gravity.(32) Zp explains approximately 80% of the variance in bone failure load.(33,34) The correlations of Zp with total area, cortical area and cortical thickness were 0.99, 0.98, and 0.84, respectively, consistent with our prior studies of the muscle bone relations in healthy children. Therefore, Zp was used as the primary bone outcome for this study. Muscle CSA (mm2) was assessed at the 66% site, as previously described in each of these disease cohorts. The manufacturer’s hydroxyapatite phantom was scanned daily for quality assurance. In our laboratory, the coefficient of variation (CV) for short-term precision ranged from 0.5 to 1.6% for pQCT outcomes in children and adolescents.
Stress-strain index (SSI) is a measure of Zp weighted for the density of each voxel and has been proposed as an alternative measure of bone strength. However, in a study examining structural failure in cadaveric bones specimens under loading stress, models including cortical dimensions resulted in R2 values of 0.75 or greater, while a model with SSI resulted in an R2 of 0.47.(35) Furthermore, a prior pQCT study from our group showed that muscle CSA was highly and significantly associated with cortical dimensions but was not associated with cortical bone mineral density (BMD) with the healthy controls.(21) We did not assess cortical BMD in this analysis, except to confirm that cortical BMD was not associated with muscle CSA within any of the disease groups (p > 0.31 within each disease group).
Measurement of Muscle Torque
Muscle torque was assessed using Biodex Multi-Joint System 3 Pro (Biodex Medical Systems, Inc, Shirley, NY). High intrarater (0.97 to 0.99) and interrater (0.93 to 0.96) intraclass correlation coefficients have been reported.(36) Peak isometric torque (ft-lbs) was measured in triplicate at −20, −10, 0, 10, and 20 degrees and the highest value recorded for both dorsiflexion and plantarflexion of the ankle. We report peak isometric torque (ft-lbs) in dorsiflexion (with the foot placed in 20 degrees of plantarflexion), since this measurement had the best test re-test reproducibility in our lab (CV 4.3%) and had the best fit (R2) in the Zp regression models, previously described.(26) Further, the tibialis anterior attaches directly to the tibia (the bone of interest in this study) and causes dorsiflexion of the ankle. In the Zp models described below, we performed a sensitivity analysis using measures of plantarflexion and confirmed that muscle torque measured in dorsiflexion was independently and significantly associated with Zp, but measures obtained in plantarflexion were not.
Laboratory Studies
Serum creatinine (mg/dl) was measured by spectrophotometric enzymatic assay (Vitros, Johnson & Johnson Co., Rochester, NY) with a CV of 1–5%. Reflectance spectrophotometry was used to quantify serum albumin (g/dl), with a CV from 0.9% to 2.4%.
Statistical Analysis
Stata 12.0 (Stata Corp., College Station, TX) was used for all statistical analyses. A p-value of < 0.05 was considered statistically significant, and two-sided tests of hypotheses were used throughout. The distributions of all continuous variables were examined for normality. Tibia length, muscle CSA, muscle torque, and Zp were log transformed to achieve normal distributions. Group differences were assessed using Student’s t-test or Wilcoxon signed-rank for non-normally distributed variables.
Log linear regression was used to compare muscle CSA between each disease group and controls, adjusted for tibia length, age, age2, sex, race (black vs. non-black), Tanner stage, and a multiplicative Tanner stage by sex interaction, as previously described.(26) Differences in muscle torque relative to CSA between each disease group and controls were also evaluated using log linear regression, adjusted for each of the covariates above.(26) Similar models were used to compare Zp between each disease group and controls, adjusted for the covariates above, in addition to a Tanner stage by race interaction term, as previously described.(21) Muscle CSA was first added to the Zp model to evaluate for changes in the group differences (if any) in Zp, and then measures of muscle torque and body weight were also included in the model to evaluate for further changes in group differences, independent of muscle CSA.
Because outcome variables (torque, CSA, Zp) in the models were in log form, exponentiating the beta-coefficient for the respective disease covariates generated ratios between the disease and control groups. For example, exponentiating the beta-coefficient for CKD 2–3 in the model for muscle torque resulted in a ratio of 0.857. This indicates that muscle torque was 14.3% (1 − 0.857 = 0.143) lower in CKD 2–3, compared with controls, adjusted for age, age2, sex, race, tibia length, and muscle CSA, as shown in Table 3.
Table 3.
Percent Differences in Muscle Torque Adjusted for Muscle Cross-sectional Area in Each Disease Group, Compared with Controls
| Chronic Kidney Disease R2 = 0.86 |
Nephrotic Syndrome R2 = 0.88 |
Crohn’s Disease R2 = 0.87 |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| Percent Difference (95% CI) | p | Percent Difference (95% CI) | p | Percent Difference (95% CI) | p | |
| Disease Group (vs. controls) | CKD 2–3: −14.3 (−21.9, −5.8) | 0.001 | −2.4 (−9.2, 5.0) | 0.52 | PCDAI ≤ 30: −1.7 (−10.2, 7.7) | 0.72 |
| CKD 4–5D: −22.9 (−31.1, −13.8) | < 0.001 | PCDAI > 30: −7.6 (−14.8, 0.1) | 0.05 | |||
The results represent the percent difference in muscle torque in the disease group, compared with controls. All models are adjusted for age, age2, sex, race, tibia length and muscle cross-sectional area. Each of these covariates was significant at p < 0.01.
RESULTS
Participant Characteristics
The participant characteristics are summarized in Table 1 according to disease group. CKD, SSNS, and Crohn’s disease participants had lower height Z-scores compared to the controls. Participants with CKD 4–5D had lower height Z-scores (−1.43 ± 1.26 vs. −0.13 ± 1.06 years, p < 0.01) compared to those with CKD 2–3. The Crohn’s disease participants with PCDAI scores > 30 had lower height Z-scores, compared to those with scores ≤ 30 (−0.45 ± 1.09 vs. −0.04 ± 1.10, p=0.15); however, the difference was not statistically significant. None of the other variables provided in Table 1 differed significantly between CKD 2–3 and 4–5D or between Crohn’s disease participants with PCDAI scores ≤ vs > 30. The greater BMI Z-scores in the children and adolescents with SSNS was consistent with glucocorticoid-induced obesity.(37) The proportion of participants within CKD, SSNS, and Crohn’s disease with serum albumin levels less than 3.5 g/dl were 7%, 31%, and 45%, respectively.
Table 1.
Participant Characteristics
| Variable | Chronic Kidney Disease | Nephrotic Syndrome | Crohn’s Disease | Controls |
|---|---|---|---|---|
| N | 54 | 51 | 64 | 264 |
| Age (years) | 12.7 ± 4.0 | 10.4 ± 4.1 | 12.8 ± 2.7 | 13.0 ± 4.4 |
| Sex, n (%) male | 36 (67) | 30 (59) | 38 (59) | 121 (46) |
| Race, n (%) Black | 14 (26) | 12 (24) | 4 (6) | 83 (31) |
| Tanner Stage, n (%) | ||||
| Stage 1 | 16 (31) | 26 (51) | 17 (26) | 70 (27) |
| Stage 2 | 6 (12) | 7 (14) | 16 (25) | 28 (11) |
| Stage 3 | 11 (21) | 10 (19) | 12 (19) | 34 (13) |
| Stage 4 | 10 (19) | 4 (8) | 16 (25) | 59 (22) |
| Stage 5 | 9 (17) | 4 (8) | 3 (5) | 70 (27) |
| Height | 146.2 ± 20.7 | 138.2 ± 22.0 | 151.5 ± 15.6 | 153.2 ± 18.9 |
| Height Z-score | −0.70 ± 1.31* | −0.19 ± 0.86* | −0.28 ± 1.10* | 0.37 ± 0.91 |
| Weight | 45.2 ± 20.9 | 41.1 ± 21.2 | 42.0 ± 13.6 | 50.3 ± 19.6 |
| Weight Z-score | −0.25 ± 1.40 | 0.55 ± 0.99 | −0.55 ± 1.15 | 0.49 ± 1.02 |
| BMI | 20.0 ± 5.0 | 20.2 ± 4.9 | 17.8 ± 2.8 | 20.6 ± 4.7 |
| BMI Z-score | 0.20 ± 1.12 | 0.81 ± 0.96* | −0.51 ± 1.04* | 0.37 ± 1.01 |
| Muscle CSA (mm2) | 4551 | 3876 | 4202 | 5312 |
| IQR | (3456, 5894) | (3258, 5598) | (3589, 5367) | (3999, 6609) |
| Muscle Torque (ft-lbs) | 13.5 | 9.5 | 14.7 | 17.9 |
| IQR | (7.0, 19.3) | (6.3, 21.9) | (10.1, 18.8) | (11.2, 24.8) |
| Section Modulus (mm3) | 1060 | 800 | 1189 | 1311 |
| IQR | (733, 1399) | (569, 1389) | (818, 1552) | (923, 1700) |
| Serum Albumin (g/dl) | 4.1 | 3.8 | 3.5 | |
| IQR | (3.9 to 4.2) | (3.2 to 4.1) | (3.1 to 3.9) | ----- |
| Range | (3.0 to 4.7) | (1.9 to 4.7) | (1.8 to 4.7) | |
Values are means ± SD or median [interquartile range (IQR) and/or range].
Anthropometric Z-scores were significantly different from control group (p < 0.05).
Muscle Cross-sectional Area
The multivariate regression models for muscle CSA are shown in Table 2 according to disease group and adjusted for age, age2, Tanner stage, sex, race, Tanner by sex interaction, and tibia length. Table 2 summarizes the differences in muscle CSA between each disease group compared with reference participants. For example, participants with CKD4–5 have muscle CSA that is 8.7% lower than the reference participants, adjusted for age, sex, race and maturation. Compared to controls, muscle CSA was 8.7% lower (p = 0.01) in CKD 4–5D but not significantly lower in CKD 2–3. Muscle CSA was 3.1% greater in SSNS compared to controls, but the difference was not significant. Crohn’s disease was associated with a trend toward more pronounced muscle deficits in children with greater disease activity when compared to controls (9.5% lower in PCDAI ≤ 30 vs. 14.1% lower in PCDAI > 30). These models explained 81–83% of the variability in muscle CSA. Serum albumin was not associated with muscle CSA in CKD, SSNS or Crohn’s disease.
Table 2.
Percent Differences in Muscle Cross-Sectional Area in Each Disease Group, Compared with Controls
| Chronic Kidney Disease R2 = 0.81 |
Nephrotic Syndrome R2 = 0.83 |
Crohn’s Disease R2 = 0.81 |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| Percent Difference (95% CI) | p | Percent Difference (95% CI) | p | Percent Difference (95% CI) | p | |
| Disease Group (vs. controls) | CKD 2–3: −2.9 (−8.6, 3.2) | 0.35 | 3.1 (−1.7, 8.1) | 0.20 | PCDAI ≤ 30: −9.5 (−15.1, −3.7) | 0.002 |
| CKD 4–5D: −8.7 (−15.0, −1.9) | 0.01 | PCDAI > 30: −14.1 (−18.7, −9.3) | < 0.001 | |||
The result represents the percent difference in muscle cross-sectional area in the disease group, compared with the controls. All models are adjusted for age, age2, Tanner stage, sex, race, Tanner stage x sex interaction, and tibia length.
Muscle Torque Relative to Muscle Area
The multivariate regression models for muscle torque relative to muscle CSA are shown in Table 3 according to disease group and adjusted for age, age2, sex, race, and tibia length. These models explained 86–88% of the variability in muscle torque. In CKD, muscle torque relative to muscle CSA trended towards being progressively lower with more severe CKD as compared with controls, as is seen in Figure 1 (14.3% lower in CKD 2–3 vs. 22.9% lower in CKD 4–5D). Among SSNS participants, there was no difference compared with controls. Analyses were repeated limited to the 33 SSNS participants treated with prednisone therapy at the time of the study visit; the results were unchanged. There was no significant difference in muscle torque relative to muscle CSA in Crohn’s participants with mild disease activity (PCDAI ≤ 30) compared with controls, but muscle torque was 7.6% lower (p = 0.05) in the participants with moderate-to-severe disease activity (PCDAI > 30) compared with controls.
Figure 1.
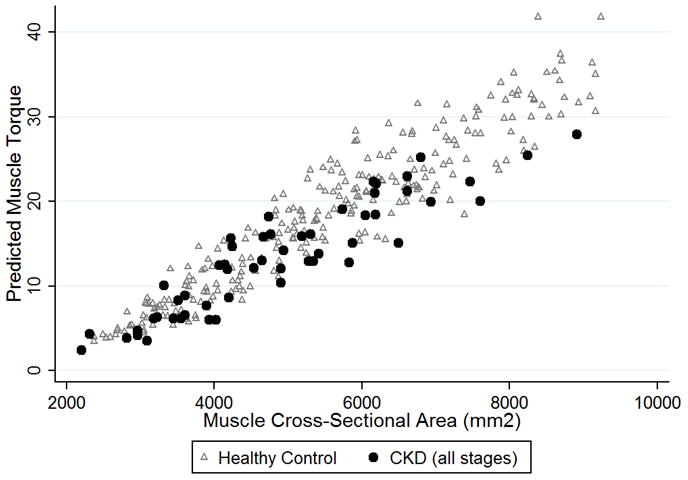
CKD participants (all stages combined) demonstrated significantly lower predicted muscle torque relative to muscle cross-sectional area, combined with healthy controls.
The Muscle-Bone Unit in Pediatric Chronic Disease
Table 4 summarizes differences in Zp between each disease group and the reference participants in a series of sequential models. For each disease, four models are presented. The first column in Table 4 shows the results of the multivariate regression for the base Zp model adjusted for age, age2, tibia length, sex, Tanner stage by sex interaction, race, and Tanner stage by race interaction. The second column illustrates that muscle CSA was significantly and positively associated with Zp in each disease group. The third column indicates that muscle torque was significantly and positively associated with Zp in each disease group, independent of muscle CSA. The fourth column demonstrates that body weight was also significantly and positively associated with Zp in each disease group, independent of muscle CSA and torque. Adjusting the models for BMI in place of weight did not impact associations between disease and control participants.
Table 4.
Percent Differences in Cortical Section Modulus in Each Disease Group, Compared with Controls
| Base Model | Base Adjusted for Muscle CSA | Base Adjusted for Muscle CSA and Muscle Torque | Base Adjusted for Muscle CSA, Muscle Torque, and Weight | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Percent Difference (95% CI) | p | Percent Difference (95% CI) | p | Percent Difference (95% CI) | p | Percent Difference (95% CI) | p | |
|
| ||||||||
| R2 = 0.88 95%CI: (0.86, 0.91) |
R2 = 0.92 95% CI: (0.90, 0.94) |
R2 = 0.92 95% CI: (0.91, 0.94) |
R2 = 0.92 95% CI: (0.91, 0.94) |
|||||
| CKD 2–3 | −7.0 (−13,4, −0.1) | 0.048 | −5.2 (−10.5, 0.5) | 0.08 | −3.6 (−9.1, 2.3) | 0.22 | −4.6 (−9.9, 1.1) | 0.11 |
| CKD 4–5D | −11.5 (−18.6, −3.7) | 0.005 | −6.7 (−12.9, 0.0) | 0.05 | −4.5 (−11.0, 2.4) | 0.19 | −5.9 (−12.1, 0.1) | 0.09 |
| Ln(muscle CSA) | + | < 0.001 | + | < 0.001 | + | < 0.001 | ||
| Ln(muscle torque) | + | 0.007 | + | 0.03 | ||||
| Ln(weight) | + | < 0.001 | ||||||
|
| ||||||||
|
R2 = 0.89 95% CI: (0.86, 0.91) |
R2 = 0.93 95% CI: (0.91, 0.94) |
R2 = 0.93 95% CI: (0.92, 0.95) |
R2 = 0.93 95% CI: (0.92, 0.95) |
|||||
|
| ||||||||
| Nephrotic Syndrome | 3.2 (−2.3, 9.1) | 0.26 | 1.3 (−3.2, 5.9) | 0.59 | 1.5 (−2.9, 6.1) | 0.50 | 0.1 (−4.2, 4.6) | 0.95 |
| Ln(muscle CSA) | + | < 0.001 | + | < 0.001 | + | < 0.001 | ||
| Ln(muscle torque) | + | < 0.001 | + | 0.004 | ||||
| Ln(weight) | + | < 0.001 | ||||||
|
| ||||||||
|
R2 = 0.87 95% CI: (0.84, 0.90) |
R2 = 0.92 95% CI: (0.90, 0.93) |
R2 = 0.92 95% CI: (0.90, 0.93) |
R2 = 0.92 95% CI: (0.91, 0.94) |
|||||
|
| ||||||||
| Crohn Disease PCDAI ≤ 30 | −2.7 (−9.8, 4.9) | 0.47 | 4.7 (−1.5, 11.2) | 0.14 | 4.8 (−1.3, 11.3) | 0.12 | 4.5 (−1.4, 10.8) | 0.14 |
| PCDAI > 30 | −4.7 (−10.8, 1.7) | 0.15 | 6.3 (0.6, 12.2) | 0.03 | 7.4 (1.7, 13.3) | 0.01 | 6.8 (1.3, 12.6) | 0.01 |
| Ln(muscle CSA) | + | < 0.001 | + | < 0.001 | + | < 0.001 | ||
| Ln(muscle torque) | + | 0.001 | + | 0.004 | ||||
| Ln(weight) | + | < 0.001 | ||||||
The results represent the percent difference in cortical section modulus in the disease group, compared with the controls. All models are adjusted for age, age2, tibia length, sex, Tanner stage by sex interaction, race, and Tanner stage by race interaction.
The β coefficients for muscle CSA and muscle torque are not provided as these are continuous variables and not expressed as percent differences in Zp between participant groups. The plus symbol represents the direction of the association.
Comparison of the percent differences in Zp across the four models within each disease group compared with controls illustrates the effects of the progressive adjustments for muscle CSA, muscle torque, and weight on the disease group effects. In the base model, Zp was significantly lower for both CKD 2–3 (7.0%) and CKD 4–5D (11.5%), compared to controls. Adjustment for the lower muscle CSA in CKD attenuated the deficits in Zp (CKD 2–3 5.2%, p = 0.08; CKD 4–5D 6.7%, p = 0.05) and improved the fit of the model with an increase in the R2 from 0.88 to 0.92. Subsequent adjustment for the muscle torque and body weight further attenuated the differences in Zp between CKD and controls, and the differences were no longer significant. With the addition of muscle torque and body weight, the model R2 was unchanged at 0.92. Last, when the base model was adjusted for muscle torque without adjustment for muscle CSA, the β-coefficient for CKD 4–5D was −5.8 (95% CI −13.3, 2.3) demonstrating that adjustment for either muscle CSA alone or muscle torque alone resulted in similar attenuation of the CKD 4–5D effect.
Among the SSNS participants, Zp was not different from controls, unadjusted or adjusted for muscle CSA and muscle torque. Model fit improved with the addition of muscle CSA, with the R2 increasing from 0.89 to 0.93, but did not further improve by adding muscle torque and body weight into the model.
In the base model for Crohn’s disease, Zp was not significantly lower in either disease category compared with controls. However, adjusting for the lower muscle CSA in Crohn’s disease resulted in a 6.3% greater Zp (p = 0.03) in the PCDAI > 30 group compared to controls with R2 increasing from 0.87 to 0.92. The magnitude of the greater Zp in this group was comparable with additional adjustment for muscle torque and body weight, while the R2 remained 0.92.
DISCUSSION
This study is the first to examine the relations among muscle torque, muscle CSA, body weight and cortical bone structure in children and adolescents with CKD, SSNS or Crohn’s disease. Given the disease-specific risk factors for impaired muscle function and the interest in the muscle-bone unit in these diseases, it is important to characterize deficits in muscle strength relative to muscle CSA, and to address the independent contributions of muscle CSA, muscle torque, and body weight to bone deficits. This study is the first to demonstrate that advanced CKD and Crohn’s disease were associated with significant deficits in muscle torque relative to muscle area. The final models demonstrated that three distinct components of biomechanical loading - muscle CSA, muscle torque, and body weight, were consistently and independently associated with Zp. These findings, combined with the fact that advanced CKD was associated with a 22.9% lower muscle torque relative to muscle area than controls, suggest that muscle CSA may be an incomplete surrogate for mechanical loading in CKD. This is consistent with our recent report that the normal positive association between changes in muscle CSA and cortical dimensions in healthy controls was absent in CKD.(12) Future longitudinal studies are needed to determine if abnormal muscle function contributes to skeletal deficits in CKD, independent of muscle area or mass.
The sample of CKD participants in this analysis was limited to those with congenital anomalies of the kidney and urinary tract in order to avoid the effects of inflammation and glucocorticoid therapy seen in other forms of CKD (e.g. glomerulonephritis). We observed 8.7% lower muscle CSA in CKD 4–5D, compared to controls. This may be due to the impaired protein synthesis and/or accelerated protein degradation. Identified mediators of muscle protein breakdown include metabolic acidosis, angiotensin II, and neural and hormonal factors that cause defects in insulin/insulin-like growth factor I intracellular signaling.(18) In addition to the deficits in muscle CSA observed here, muscle torque relative to CSA was significantly lower in CKD, compared to controls and the 22.9% reductions were striking in advanced CKD. There are multiple possible explanations for impaired muscle function. First, the reduced muscle torque relative to CSA may have been due to systemic volume overload(17) and/or edema of individual muscle fibers(19) resulting in an overestimation of muscle CSA. Second, muscle fibers exhibit abnormal biochemical properties in CKD: a recent muscle biopsy study in adult dialysis patients demonstrated a 29 to 47% reduction in oxidative capacity as measured by succinate dehydrogenase activity, compared with controls.(19)
The present analysis did not show any difference in muscle torque relative to muscle CSA between the participants with SSNS and controls. The results were unchanged when limited to the participants treated with glucocorticoids at the time of the study visit. Zp was not different from controls, adjusted or not adjusted for muscle CSA or muscle torque. Given that children and adolescents with SSNS have minimal systemic inflammation, they serve as a model of the independent effect of glucocorticoids on muscle CSA and muscle torque relative to CSA. Therefore, our findings suggest that glucocorticoids do not have long-lasting effects on muscle CSA or muscle function in children and adolescents with SSNS, even though prior studies suggest a negative role of glucocorticoids in skeletal muscle physiology.(14,16,38)
Glucocorticoids, inflammatory cytokines, and malnutrition may affect skeletal muscle CSA and function in Crohn’s disease. This study was limited to newly-diagnosed patients prior to treatment with glucocorticoids; therefore our findings highlight the effects of inflammation and malnutrition on the bone-muscle unit. Inflammatory cytokines (i.e. tumor necrosis factor-alpha, IL-1, and IL-6) cause protein degradation, myoblast apoptosis, and inhibition of myogenic differentiation.(15) We observed lower muscle CSA in Crohn’s disease, with a trend for greater deficits in participants with more advanced disease. While muscle torque relative to CSA was not significantly lower in participants with mild Crohn’s disease activity (PCDAI ≤ 30), it was significantly lower in those with moderate-to-severe disease activity (PCDAI > 30). These data suggest that inflammation and malnutrition negatively affect muscle CSA and muscle torque relative to CSA. Interestingly, Zp was lower in Crohn’s disease, compared with controls in models unadjusted for muscle CSA, muscle torque, and body weight, but was significantly greater than controls in moderate-to-severe Crohn’s disease when adjusted for these three covariates. It is possible that bone loss from decreased mechanical loading lags behind an acute loss of muscle mass and function over the interval immediately preceding diagnosis.
As detailed in the Introduction, investigators have advocated a two-staged algorithm to assess the functional muscle-bone unit in chronic disease, and to identify primary and secondary bone disorders.(2) In the CKD group, muscle CSA was lower in CKD 4–5D compared with controls, but Zp was not significantly lower compared with controls when adjusted for the low muscle CSA, muscle torque, and weight – suggesting a bone disorder potentially secondary to muscle deficits. However, the associations among lower Zp, muscle CSA and muscle torque do not prove a causal relation between muscle and bone deficits. The metabolic derangements in CKD may be an adjunctive or independent primary factor affecting bone and/or muscle directly. In the Crohn’s disease group, muscle CSA was low, but Zp was greater than expected relative to the muscle CSA, suggesting a temporal disconnect between the muscle-bone unit in newly diagnosed Crohn’s disease.
The cross-sectional design of this study is a limitation. Longitudinal data would provide greater insight into the effects of acute vs. chronic changes in muscle CSA and muscle torque on bone. Second, the study may be limited by the imprecise measures of disease activity in Crohn’s disease, the variable glucocorticoid exposure in SSNS, and the heterogeneity in CKD duration. Third, total physical activity was associated with Zp in our prior study of healthy children and young adults; unfortunately, physical activity was not assessed in these three disease groups.(26) Fourth, the measures of muscle torque were assessed relative to total calf muscle CSA, rather than the specific muscles involved in dorsiflexion (tibialis anterior, peroneus tertius, extensor digitorum longus, and extensor hallucis proprius). We were also unable to assess muscle fiber type, pennation angle, or oxidative capacity in this non-invasive study. However, the fact that our models explained greater than 85% of the variability in muscle torque (an effort-dependent outcome) suggests that we captured important determinants. We assessed tibial loading with dorsiflexion of the ankle despite the fact that additional tibial loading modalities include plantar flexion of the ankle and flexion/extension of the knee. In our sensitivity analyses, the plantar flexion covariate was not significantly associated with Zp for any of the three diseases, suggesting it does not capture biomechanical loading (data not shown). An additional limitation is the lack of measures of muscle density as an index of intramuscular adipose tissue. A recent study in older dialysis patients demonstrated that greater intramuscular adipose tissue was associated with high levels of inflammatory cytokines and reduced muscle strength.(39) Therefore, alterations in intramuscular adipose tissue may contribute to the strength deficits observed in our CKD and Crohn’s disease participants. Future studies employing measures of intra- and extra-myocellular lipids are necessary to examine associations with CKD and other diseases associated with potential risk factors for impaired muscle quality.
Other investigators have proposed that measurements of ground reaction forces, such as mechanography, better capture biomechanical loading of bone.(31,40) Anliker, et al. reported that pQCT measures of bone mineral content at the 14% site were better correlated with ground reaction forces than with calf muscle CSA (R2 = 0.84 vs. 0.72 in males, and 0.77 vs. 0.60 in females). A recent study in children and adults with X-linked hypophosphatemic rickets (XLH) included functional measures of vertical ground reaction forces and pQCT measures of muscle CSA and cortical bone total CSA.(41) Assessment of the muscle-bone unit revealed that the model incorporating ground reaction force explained 58% of the variability in bone CSA, while the model incorporating muscle CSA explained 69% of the variability in bone CSA. The authors concluded that the muscle-bone interaction can be assessed using pQCT muscle CSA. They did not evaluate the combination of muscle CSA and force in a single model; therefore we do not know if the combination of anatomic and functional measures would improve the prediction of cortical dimensions in XLH.
In summary, this is the first study to examine muscle strength, muscle size, and cortical bone structure in children and adolescents at risk for reduced muscle function due to glucocorticoid therapy, inflammation, malnutrition and CKD. This study is further strengthened by the inclusion of a robust reference group that facilitated the adjustment of the models for group differences in body size and maturation. Muscle torque relative to muscle CSA was significantly lower in CKD and moderate-to-severe CD, and muscle torque was associated with Zp, independent of muscle CSA in all three disorders. Future studies are needed to assess additional measures of biomechanical loading, such as ground reaction forces, and non-invasive measures of muscle metabolic function, such as 31P-magnetic resonance spectroscopy,(42) in chronic diseases. Longitudinal intervention studies are needed to determine if strategies to improve muscle mass and quality result in improved bone outcomes.
Acknowledgments
Authors’ roles: Study design: DL, RW, JS, ML. Study conduct: RW, BZ, BF, RH, DF. Data collection: RH, DF. Data analysis: DL, RW, JS, ML. Data interpretation: DL, RW, BZ, ML. Drafting manuscript: DL, RW, ML. Revising manuscript content: DL, RW, JO, ML. Approving final version of manuscript: DL, RW, BZ, JS, BF, RH, DF, JO, ML. DL takes responsibility for the integrity of the data analysis.
We greatly appreciate the dedication and enthusiasm of the children and their families who participated in this study. Special thanks to the nurses and physicians in the Division of Nephrology and the Division of Gastroenterology, Hepatology and Nutrition at CHOP. Additional thanks to Daniel Schiferl at Bone Diagnostic, Inc. for technical assistance with the pQCT scans.
Funding Sources: NIH R01-DK060030, R01-HD040714, K24-DK076808, T32-DK007740, and UL1-RR-024134 from the National Center for Research Resources.
Footnotes
Disclosures: The authors state that they have no conflicts of interest.
Contributor Information
Dale Y. Lee, Email: leed1@email.chop.edu.
Rachel J. Wetzsteon, Email: wetzsteonr@email.chop.edu.
Babette S. Zemel, Email: zemel@email.chop.edu.
Justine Shults, Email: jshults@mail.med.upenn.edu.
Jason M. Organ, Email: jorgan@iupui.edu.
Bethany J. Foster, Email: beth.foster@muhc.mcgill.ca.
Rita M. Herskovitz, Email: herskovitz@email.chop.edu.
Debbie L. Foerster, Email: foerster@email.chop.edu.
Mary B. Leonard, Email: leonard5@stanford.edu.
References
- 1.Daly RM. The effect of exercise on bone mass and structural geometry during growth. Med Sport Sci. 2007;51:33–49. doi: 10.1159/000103003. [DOI] [PubMed] [Google Scholar]
- 2.Schoenau E, Neu CM, Beck B, Manz F, Rauch F. Bone mineral content per muscle cross-sectional area as an index of the functional muscle-bone unit. J Bone Miner Res. 2002;17(6):1095–101. doi: 10.1359/jbmr.2002.17.6.1095. [DOI] [PubMed] [Google Scholar]
- 3.Burnham JM, Shults J, Petit MA, Semeao E, Beck TJ, Zemel BS, Leonard MB. Alterations in proximal femur geometry in children treated with glucocorticoids for Crohn disease or nephrotic syndrome: impact of the underlying disease. J Bone Miner Res. 2007;22(4):551–9. doi: 10.1359/jbmr.070110. [DOI] [PubMed] [Google Scholar]
- 4.Petit MA, Beck TJ, Shults J, Zemel BS, Foster BJ, Leonard MB. Proximal femur bone geometry is appropriately adapted to lean mass in overweight children and adolescents. Bone. 2005;36(3):568–76. doi: 10.1016/j.bone.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Foster BJ, Shults J, Zemel BS, Leonard MB. Interactions between growth and body composition in children treated with high-dose chronic glucocorticoids. Am J Clin Nutr. 2004;80(5):1334–41. doi: 10.1093/ajcn/80.5.1334. [DOI] [PubMed] [Google Scholar]
- 6.Tsampalieros A, Gupta P, Denburg MR, Shults J, Zemel BS, Mostoufi-Moab S, Wetzsteon RJ, Herskovitz RM, Whitehead KM, Leonard MB. Glucocorticoid effects on changes in bone mineral density and cortical structure in childhood nephrotic syndrome. J Bone Miner Res. 2013;28(3):480–8. doi: 10.1002/jbmr.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wetzsteon RJ, Shults J, Zemel BS, Gupta PU, Burnham JM, Herskovitz RM, Howard KM, Leonard MB. Divergent effects of glucocorticoids on cortical and trabecular compartment BMD in childhood nephrotic syndrome. J Bone Miner Res. 2009;24(3):503–13. doi: 10.1359/JBMR.081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubner SE, Shults J, Baldassano RN, Zemel BS, Thayu M, Burnham JM, Herskovitz RM, Howard KM, Leonard MB. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn’s disease. Gastroenterology. 2009;136(1):123–30. doi: 10.1053/j.gastro.2008.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thayu M, Denson LA, Shults J, Zemel BS, Burnham JM, Baldassano RN, Howard KM, Ryan A, Leonard MB. Determinants of changes in linear growth and body composition in incident pediatric Crohn’s disease. Gastroenterology. 2010;139(2):430–8. doi: 10.1053/j.gastro.2010.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsampalieros A, Lam CK, Spencer JC, Thayu M, Shults J, Zemel BS, Herskovitz RM, Baldassano RN, Leonard MB. Long-term inflammation and glucocorticoid therapy impair skeletal modeling during growth in childhood Crohn disease. J Clin Endocrinol Metab. 2013;98(8):3438–45. doi: 10.1210/jc.2013-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster BJ, Kalkwarf HJ, Shults J, Zemel BS, Wetzsteon RJ, Thayu M, Foerster DL, Leonard MB. Association of chronic kidney disease with muscle deficits in children. J Am Soc Nephrol. 2011;22(2):377–86. doi: 10.1681/ASN.2010060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsampalieros A, Kalkwarf HJ, Wetzsteon RJ, Shults J, Zemel BS, Foster BJ, Foerster DL, Leonard MB. Changes in bone structure and the muscle-bone unit in children with chronic kidney disease. Kidney Int. 2013;83(3):495–502. doi: 10.1038/ki.2012.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wetzsteon RJ, Kalkwarf HJ, Shults J, Zemel BS, Foster BJ, Griffin L, Strife CF, Foerster DL, Jean-Pierre DK, Leonard MB. Volumetric bone mineral density and bone structure in childhood chronic kidney disease. J Bone Miner Res. 2011;26(9):2235–44. doi: 10.1002/jbmr.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma K, Mallidis C, Bhasin S, Mahabadi V, Artaza J, Gonzalez-Cadavid N, Arias J, Salehian B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am J Physiol Endocrinol Metab. 2003;285(2):E363–71. doi: 10.1152/ajpendo.00487.2002. [DOI] [PubMed] [Google Scholar]
- 15.Spate U, Schulze PC. Proinflammatory cytokines and skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7(3):265–9. doi: 10.1097/00075197-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Brethour JR. Effects of acute injections of dexamethasone on selective deposition of bovine intramuscular fat. J Anim Sci. 1972;35(2):351–6. doi: 10.2527/jas1972.352351x. [DOI] [PubMed] [Google Scholar]
- 17.Georgiou E, Virvidakis K, Douskas G, Lambrinoudaki I, Voudiklari S, Katsoudas S, Mountokalakis T, Proukakis C. Body composition changes in chronic hemodialysis patients before and after hemodialysis as assessed by dual-energy x-ray absorptiometry. Metabolism. 1997;46(9):1059–62. doi: 10.1016/s0026-0495(97)90278-x. [DOI] [PubMed] [Google Scholar]
- 18.Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr. 2010;91(4):1128S–1132S. doi: 10.3945/ajcn.2010.28608B. [DOI] [PubMed] [Google Scholar]
- 19.Lewis MI, Fournier M, Wang H, Storer TW, Casaburi R, Cohen AH, Kopple JD. Metabolic and morphometric profile of muscle fibers in chronic hemodialysis patients. J Appl Physiol (1985) 2012;112(1):72–8. doi: 10.1152/japplphysiol.00556.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thayu M, Shults J, Burnham JM, Zemel BS, Baldassano RN, Leonard MB. Gender differences in body composition deficits at diagnosis in children and adolescents with Crohn’s disease. Inflamm Bowel Dis. 2007;13(9):1121–8. doi: 10.1002/ibd.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonard MB, Elmi A, Mostoufi-Moab S, Shults J, Burnham JM, Thayu M, Kibe L, Wetzsteon RJ, Zemel BS. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95(4):1681–9. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–37. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 24.The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr. 1981;98(4):561–4. doi: 10.1016/s0022-3476(81)80760-3. [DOI] [PubMed] [Google Scholar]
- 25.Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, Griffiths AM, Katz AJ, Grand RJ, Boyle JT, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12(4):439–47. [PubMed] [Google Scholar]
- 26.Wetzsteon RJ, Zemel BS, Shults J, Howard KM, Kibe LW, Leonard MB. Mechanical loads and cortical bone geometry in healthy children and young adults. Bone. 2011;48(5):1103–8. doi: 10.1016/j.bone.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogden CL, Carroll ME, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. National Center for Health Statistics Data Brief. 2012;82:1–8. [PubMed] [Google Scholar]
- 28.Morris NM, Udry JR. Validation of a Self-Administered Instrument to Assess Stage of Adolescent Development. Journal of Youth and Adolescence. 1980;9(3):271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 29.Tanner JM. Growth at Adolescence. 2. Blackwell Scientific Publication; Oxford, United Kingdom: 1962. [Google Scholar]
- 30.Anliker E, Sonderegger A, Toigo M. Side-to-side differences in the lower leg muscle-bone unit in male soccer players. Med Sci Sports Exerc. 2013;45(8):1545–52. doi: 10.1249/MSS.0b013e31828cb712. [DOI] [PubMed] [Google Scholar]
- 31.Anliker E, Rawer R, Boutellier U, Toigo M. Maximum ground reaction force in relation to tibial bone mass in children and adults. Med Sci Sports Exerc. 2011;43(11):2102–9. doi: 10.1249/MSS.0b013e31821c4661. [DOI] [PubMed] [Google Scholar]
- 32.Schoenau E, Neu CM, Rauch F, Manz F. The development of bone strength at the proximal radius during childhood and adolescence. J Clin Endocrinol Metab. 2001;86(2):613–8. doi: 10.1210/jcem.86.2.7186. [DOI] [PubMed] [Google Scholar]
- 33.Kontulainen SA, Johnston JD, Liu D, Leung C, Oxland TR, McKay HA. Strength indices from pQCT imaging predict up to 85% of variance in bone failure properties at tibial epiphysis and diaphysis. J Musculoskelet Neuronal Interact. 2008;8(4):401–9. [PubMed] [Google Scholar]
- 34.Liu D, Manske SL, Kontulainen SA, Tang C, Guy P, Oxland TR, McKay HA. Tibial geometry is associated with failure load ex vivo: a MRI, pQCT and DXA study. Osteoporos Int. 2007;18(7):991–7. doi: 10.1007/s00198-007-0325-0. [DOI] [PubMed] [Google Scholar]
- 35.Ashe MC, Khan KM, Kontulainen SA, Guy P, Liu D, Beck TJ, McKay HA. Accuracy of pQCT for evaluating the aged human radius: an ashing, histomorphometry and failure load investigation. Osteoporos Int. 2006;17(8):1241–51. doi: 10.1007/s00198-006-0110-5. [DOI] [PubMed] [Google Scholar]
- 36.Leggin BG, Neuman RM, Iannotti JP, Williams GR, Thompson EC. Intrarater and interrater reliability of three isometric dynamometers in assessing shoulder strength. J Shoulder Elbow Surg. 1996;5(1):18–24. doi: 10.1016/s1058-2746(96)80026-7. [DOI] [PubMed] [Google Scholar]
- 37.Leonard MB, Feldman HI, Shults J, Zemel BS, Foster BJ, Stallings VA. Long-term, high-dose glucocorticoids and bone mineral content in childhood glucocorticoid-sensitive nephrotic syndrome. N Engl J Med. 2004;351(9):868–75. doi: 10.1056/NEJMoa040367. [DOI] [PubMed] [Google Scholar]
- 38.Seene T. Turnover of skeletal muscle contractile proteins in glucocorticoid myopathy. J Steroid Biochem Mol Biol. 1994;50(1–2):1–4. doi: 10.1016/0960-0760(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 39.Cheema B, Abas H, Smith B, O’Sullivan AJ, Chan M, Patwardhan A, Kelly J, Gillin A, Pang G, Lloyd B, Berger K, Baune BT, Singh MF. Investigation of skeletal muscle quantity and quality in end-stage renal disease. Nephrology (Carlton) 2010;15(4):454–63. doi: 10.1111/j.1440-1797.2009.01261.x. [DOI] [PubMed] [Google Scholar]
- 40.Anliker E, Toigo M. Functional assessment of the muscle-bone unit in the lower leg. J Musculoskelet Neuronal Interact. 2012;12(2):46–55. [PubMed] [Google Scholar]
- 41.Veilleux LN, Cheung MS, Glorieux FH, Rauch F. The muscle-bone relationship in X-linked hypophosphatemic rickets. J Clin Endocrinol Metab. 2013;98(5):E990–5. doi: 10.1210/jc.2012-4146. [DOI] [PubMed] [Google Scholar]
- 42.Edwards LM, Tyler DJ, Kemp GJ, Dwyer RM, Johnson A, Holloway CJ, Nevill AM, Clarke K. The reproducibility of 31-phosphorus MRS measures of muscle energetics at 3 Tesla in trained men. PLoS One. 2012;7(6):e37237. doi: 10.1371/journal.pone.0037237. [DOI] [PMC free article] [PubMed] [Google Scholar]


