Abstract
Leptin was initially best known for its role in energy homeostasis and regulation of energy expenditure. In the past few years we have realized that leptin also plays a major role in neuroendocrine regulation and bone metabolism. Here, we review the literature on indirect and direct pathways through which leptin acts to influence bone metabolism and discuss bone abnormalities related to leptin deficiency in both animal and human studies. The clinical utility of leptin in leptin deficient individuals and its potential to improve metabolic bone disease are also discussed. We are beginning to understand the critical role leptin plays in bone metabolism; future randomized studies are needed to fully assess the potential and risk – benefit of leptin's use in metabolic bone disease particularly in leptin deficient individuals.
Keywords: leptin, bone, osteoporosis
1. Introduction
Leptin is an adipokine composed of 167 amino acids which is secreted in a pulsatile fashion to maintain energy homeostasis [1]. Leptin is primarily secreted from adipocytes at levels determined mainly by the number of adipocytes, and thus amount of body fat, and secondarily by acute changes in food intake [2]. Although circulating leptin levels mainly signify the amount of energy stored in adipose tissue, and thus reflect obesity, insulin levels and alcohol intake have also been associated with increased circulating leptin levels [3]. In epidemiology studies, a wide variability in leptin levels has been reported, even among individuals with the same body mass index (BMI) implying the influence of both genetic and environmental factors. For instance, a comparison of heterozygous relatives of congenitally leptin deficient individuals with control subjects of the same ethnicity and BMI reveals an increased percentage of body fat and reduced leptin levels [4]. In addition to genetic determinants, the circulation of leptin also responds to acute caloric changes, decreasing with acute energy deprivation [5]. Sleep and fasting as well as circulating hormone and cytokine levels have been shown to regulate leptin levels in healthy individuals. Finally, circadian sleep/wake cycle is intimately linked in the regulation of leptin levels and disturbance of which could potentially cause an increase in circulating leptin [6].
The primary actions of leptin have been thought to occur in the arcuate nucleus of the hypothalamus, where leptin inhibits the actions of neuropeptide Y (NPY) and agouti-related peptide (AgRP) and enhances the actions of pro-opiomelanocortin (POMC) and cocaine- and amphetamine-related transcript (CART) to decrease food intake (Figure 1) [7-9]. Leptin also affects hypothalamic pathways to regulate reproduction and development [10-13] but importantly it also acts in several peripheral metabolically important organs. These actions of leptin are mediated through the leptin receptor (LepRb) which is found throughout the brain and brain stem as well as in peripheral organs [14, 15]. Once leptin binds to LepRb, the receptor dimerizes and initiates a downstream cascade (including janus kinase 2(JAK2)/signal transducer and activator of transcription 3 (STAT3), src homology-2-containing protein tyrosine phosphatase 2 (SHP2)/mitogen-activated protein kinase (MAPK)/forkhead box protein O1 (FoxO1)/phosphatidylinositol 3 kinase (PI3K)/Protein Kinase B (Akt)/mammalian target of rapamycin (mTOR)/adenosine monophosphate-activated protein kinase (AMPK), Suppressor of cytokine signaling 3 (SOCS3), Src homology-2 protein tyrosine phosphatase (SHP2), Protein-tyrosine phosphatase 1B (PTP1B), regulating several physiological functions including energy homeostasis, neuroendocrine action and insulin resistance [16]. The range of signaling pathways activated by leptin, as well as the number of peripheral tissues that leptin targets, have recently been expanded. Several of these novel pathways, including inflammatory activation through nuclear factor kappa-light-chain-enhancer of activated B cells (NFkB)/IKK need to be further delineated in the future [17]. Research continues to illuminate and define these pathways, which have widespread impacts throughout the brain as well as in the periphery.
Figure 1.
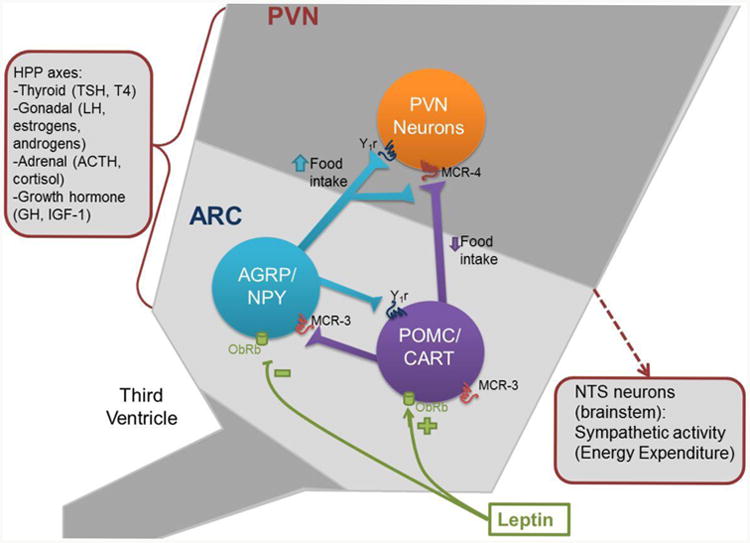
Actions of leptin to alter food intake and energy expenditure. Leptin inhibits AGRP/NPY and activates POMC/CART neurons in the arcuate nucleus of the hypothalamus. These neurons in turn act on paraventricular (PVN) neurons to increase or decrease food intake as well as to modulate sympathetic activity and energy expenditure through the Nucleus of the Solitary Tract. Leptin signaling in the hypothalamus also activates other hypothalamic-pituitary-peripheral (HPP) axes, which also have consequences for bone metabolism.
2. Leptin and Neuroendocrine Regulation
In addition to regulating energy homeostasis, leptin also regulates several hypothalamic pituitary peripheral neuroendocrine axes, including the thyroid, gonadal, cortisol and growth hormone axes [10-13]. It is important to understand how all these HPP axes are influenced by leptin and/or leptin deficiency, as they may all mediate the connection between leptin and bone which is further discussed below.
Farooqi et al. [4, 11, 18] and Ozata et al. [19] identified leptin deficient homozygous individuals and described neuroendocrine responses in this phenotype. A missense leptin gene mutation was identified in his family and homozygous individuals were found to have extreme obesity [10]. Thus, congenital, complete leptin deficiency is associated with extreme obesity, and leptin replacement in such individuals has led to improvement in obese state by increasing energy expenditure and reducing caloric intake [11]. Additionally, leptin therapy for patients who have disturbed neuroendocrine axes has been shown to restore functioning of other hypothalamic axes, including the thyroid, gonadal, cortisol, and growth hormone [11, 20-23], which are all linked to bone metabolism.
All heterozygous members of the extended family with leptin deficiency studied by Ozata et al. [19] had normal weight while homozygous members had morbid obesity. Out of the four heterozygous individuals, the adult patients (2 females and 1 male) had normal thyroid function while the child had elevated thyroid-stimulating hormone (TSH), negative antibodies and exaggerated response to TSH stimulation [19]. Significant elevations of TSH levels have been seen in patients with leptin deficiency which has normalized on leptin replacement therapy and has subsequently led to treatment discontinuation of levothyroxine [24]. Studies in healthy, lean men were done to see the changes in neuroendocrine hormones in well fed state as compared to a 72 hour fasting state with placebo or replacement doses of metreleptin [5]. Changes in hypothalamic-pituitary-gonadal axis, in part changes of hypothalamic-pituitary-thyroid axis and binding capacity of insulin-like growth factor 1 (IGF1) in serum were rescued in patients who were starving but received replacement doses of r-met Huleptin as opposed to the patients who received placebo [5].
Leptin also plays a significant role in the maintenance of hypothalamic-gonadal-pituitary axis. Delayed puberty is often seen in leptin deficient states. Indeed, both congenital and acquired leptin deficiencies have been associated with hypothalamic amenorrhea or the cessation of the menstrual cycle and infertility [25]. Decrease leptin levels and increase soluble leptin receptor protein (sLep-R) were also seen in healthy volunteers after a four-week reduced calorie diet of 1000-1200 kcal/day intake [26]. Ozata et al. [19] described hypogonadism in all three adult homozygous patients in his study. Normal gonadotropin responses were demonstrated in all these patients in response to gonadotropin releasing hormone (GnRH) stimulation indicating a hypothalamic defect in these individuals. A leptin rise of approximately 50% was described just before the onset of puberty in prepubertal boys, which decreased to baseline levels after the initiation of puberty [27]. Normal pituitary gonadal axis was noted in healthy men after 72 hour fast with replacement of recombinant leptin as opposed to placebo, indicating important physiological role of leptin in regulation of neuroendocrine axes in healthy individuals [5]. Leptin replacement for 2-3 months was also shown to result in resumption in ovulation, increase in LH and estradiol levels in blood and increase in follicular diameter and number in women with hypothalamic amenorrhea as compared to control subjects [28]. Moreover, replacement of leptin in deficient individuals has led to the successful treatment of hypogonadism by gonadotropin secretion and the restoration of puberty and fertility [29].
An inverse relationship has been described between leptin levels and serum cortisol and adrenocorticotropic hormone (ACTH) levels [30]. The homozygous leptin deficient patients were also found to have high cortisol, high ACTH levels, a disturbed diurnal variation, but a normal dexamethasone suppression response [19]. Higher body fat has been shown to be associated with decreased cortisol inhibitory feedback signaling [31]. In a study of leptin therapy in lean humans, no significant change was noted in cortisol or corticotropin levels from their baseline during leptin treatment [28]. Also, no significant change in corticotropin pulsatility was noted after two week treatment with metreleptin [28]. Similarly no significant change was noted in the baseline cortisol or 24-hour urine free cortisol with fasting and/or metreleptin replacement healthy volunteers after a 72 hour fast [5]. Longer, randomized controlled trials of leptin administration demonstrated an effect of leptin to normalize the ACTH-cortisol axis in women with exercise induced hypothalamic amenorrhea [5].
Studies on growth hormone deficient individuals as compared to healthy adults showed a negative correlation of leptin to IGF1 [32]. Blunt growth hormone response is seen in obese individuals in response to insulin induced hypoglycemia as compared to healthy people [33]. In normal healthy men, after a 72-hour fasting, a rise in serum growth hormone (GH) levels, pulsatile frequency of GH and 24-hour integrated GH concentrations, but a decrease in IGF1, were noted, which was not reversed with leptin recombinant therapy [5]. Although no change was noticed in free IGF-1 with leptin replacement, total IGF-1 levels were increased, reflecting increase in binding capacity in the serum [5]. In women with hypothalamic amenorrhea increase in IGF1 was seen in month 1 and an increase in IGF binding protein 3 (IGF-BP3) was seen in months 2 and 3. IGF1 levels declined to baseline on follow-up at 2 and 3 months [28].
Several animal studies have been done to establish relationship between leptin and its effect on sympathetic system, but similar studies have failed to demonstrate a similar role in response to at least short term leptin changes in humans [34]. Since the main role of leptin as an adipokine is to maintain energy homeostasis, it can be considered a messenger that relays information about energy stores in the body to the brain. Its role in bone formation was thought to be regulated by sympathetic nervous system. Offspring whose mothers were on a high fat diet have altered sensitivity to leptin and ghrelin in the hypothalamus that results in adverse cardiovascular outcomes [35]. Central leptin infusion increased insulin sensitivity via sympathetic regulation of insulin-like growth factor binding protein 2 (IGFBP-2) levels in animal models. An intracerberoventricular leptin infusion in sheep was shown to increase skeletal muscle IGFBP-2 resulting in improved glucose tolerance and increased insulin levels in response to a glucose challenge, which was blocked by a beta-adrenergic blocker, indicating sympathetic regulation of leptin [36]. High bone mass is shown to result after ablation of adrenergic signaling, which is even resistant to correction by intracerebroventricular leptin [37]. Decreased leptin states show a decline in sympathetic nervous system tone [38]. This may only be the case in animals, as these findings have not been reliably replicated in humans. For instance, it was found that changes in heart rate, catecholamines, and other sympathetic nervous system parameters during fasting were independent of leptin levels in healthy humans [34].
3. Leptin's Impacts on Bone Metabolism
3.1. Direct Mechanisms
The leptin receptor can be found in adult primary osteoblasts and chondrocytes, suggesting that the effects of leptin on bone growth and metabolism may be direct [39]. Other studies have shown that leptin may impact bone growth through the activation of fibroblast growth factor 23 (FGF-23) [40]. Leptin also impacts and regulates osteocalcin, which in turn regulates not only bone metabolism, but also insulin sensitivity and energy expenditure [41]. Locally, bone marrow adipocytes have been found to secrete leptin, and this may mediate leptin's local effects on bone [42]. Indeed, replacement of bone tissue in mice that lack a functional leptin receptor (db/db) increases bone mass without affecting energy homeostasis, suggesting that some of the effects of leptin on bone metabolism may be peripheral rather than central [43].
3.2. Indirect Mechanisms
Although leptin may act peripherally on bone, central leptin administration in ob/ob mice has been found to restore bone mass to control levels, suggesting that leptin may indirectly impact bone mass [44]. The ventromedial hypothalamus (VMH) may activate local noradrenergic signaling at the osteoblasts in response to leptin, mediating this effect [37]. Indeed, lesions of the VMH have been found to prevent the restoration of bone mass with leptin administration for ob/ob mice, suggesting that the VMH is key to leptin's control of bone mass [37].
Leptin may also act indirectly through the brainstem and serotonergic signaling, though these effects shown in animal models have not been shown in humans yet. Leptin and serotonin have opposite effects on bone mass [45]. Leptin appears to decrease serotonin synthesis and inhibit serotonergic receptors [45]. Serotonin appears to bind to the serotonin 2c receptors in the VMH and serotonin 1b receptor on osteoblasts to inhibit bone growth [45, 46]. In cases of leptin inhibiting serotonin, these effects would be reversed, inducing bone growth.
In the most human studies, it is difficult to parcel apart the effects of leptin per se vs. its hypothalamic effectors, such as estrogen, cortisol, IGF-1and parathyroid hormone on bone mass [47]. Leptin therapy increases all of these hormones along with improving bone mass, and thus whether the effects on bone mass occur directly or indirectly through other hormones remains to be fully clarified [12, 48]. Estrogen, activated through the hypothalamic-pituitary-gonadal axis by leptin [49], itself induces growth of human osteoblasts [50, 51]. The effect of hormonal replacement therapy in women with postmenopausal osteoporosis on the increase in bone density and reduction of osteoporotic fracture is established [52, 53] although a few studies have not linked improvement in estrogen levels with improvements in bone density [54-56]. Although the potential role of estrogen indirectly modulating this connection cannot be discounted, the combination of low bone density or mass with low estrogen levels may be more of an impact of leptin on both estrogen and bone mass than of estrogen on bone mass.
Cortisol is another potential indirect pathway for leptin to act on bone, as it is inhibited through the hypothalamic-pituitary-adrenal axis by leptin [57]. Cortisol has been found to inhibit the growth of osteoblasts and osteoclasts, as well as inhibiting growth hormone, which also have an anabolic effect on bone [58-60]. Indeed, strong correlations have been seen between cortisol and markers of bone growth, where higher cortisol levels correlate with decreased bone mass and growth markers like osteocalcin [58, 61]. The effect of cortisol and other glucocorticoids on bone may be mediated through pathways such as the hepatocyte growth factor signaling pathways (e.g. IGF-1) [59]. In the case of high adiposity, which can increase leptin and cortisol, central leptin resistance may mediate the unexpected negative effects of obesity on bone metabolism [62, 63]. Thus, leptin's inhibition of cortisol and glucocorticoids may help to improve bone growth.
Thyroid and parathyroid hormones may also mediate relationships between leptin and bone metabolism. Leptin activates thyroid hormones through the hypothalamic-pituitary-thyroid axis [64]. Leptin is known to regulate thyroid-stimulating hormone (TSH) levels and thus influence this axis [65]. Parathyroid hormone activates osteoblasts and bone growth when administered intermittently, whereas it has catabolic action in bone when it is stably increased (e.g., in hyperparathyroidism or hypothalamic amenorrhea) [66]. Parathyroid hormone also increases calcium absorption in the intestines and reabsorption in the kidneys [67]. Metreleptin decreased parathyroid hormone and RANKL and increased osteoprotegerin (OPG) in women with hypothalamic amenorrhea together with an increase in bone mass [68].
Growth hormone and IGF-1 are other potential mediators, activated through the hypothalamic-pituitary-growth hormone axis by leptin [69]. Growth hormone causes IGF-1 secretion from the liver and bone [70]. Importantly, growth hormone is not the only activator of IGF-1, but parathyroid hormone, estrogen and cortisol have also been shown to affect IGF-1 levels at bone [71-76]. Given these complex relationships, it is not hard to believe that leptin may act indirectly to affect bone metabolism.
4. Impacts of leptin deficiency on bone mass: Evidence from animal studies
Leptin has been linked to decreased bone mass in both cases of obesity with hyperleptinemia but leptin resistance, and in cases of extreme leanness with hypoleptinemia. Mice who cannot produce leptin (ob/ob) are obese and have reduced bone mass [77]. Hamrick et al. [78] first studied the bone microarchitecture in leptin deficient obese mice as compared to lean controls. They had reported a differential leptin action on bone density and mineralization in axial and appendicular skeleton. In the peripheral skeleton, namely femur, leptin-deficient mice had shorter length, decreased mineralization and low bone mineral density. Cortical thickness, and trabecular bone volume of femur was also low as compared to the controls. On the other hand, in the axial skeleton (lumbar vertebrae) of leptin deficient mouse increase in trabecular volume, cortical thickness, mineralization and density were observed. Increased number of adipocytes were noted in femoral bone marrow and decreased in vertebrae bone marrow. Muscle mass likely may contribute to this difference, as low muscle mass (sarcopenia) in obese mice were associated with low mineral density [78]. Intracerebrovesicular infusion of leptin in leptin deficient mice was initially shown to result in bone loss indicating that leptin, through central nervous system, inhibits bone formation [44]. However, more recently, intracerebroventricular injection of leptin was shown to promote the expression of pro-osteogenic factors in bone marrow, leading to enhanced bone formation in ob/ob mice [79]. Similarly, peripheral effect of leptin on bone was found to be anabolic. Leptin increased proliferation of isolated fetal rat osteoblasts in bone and inhibited osteoclastogenesis in bone marrow, leading to new bone formation, higher bone density and reduction in fracture risk [80]. Similarly, other authors recently observed decreases in bone growth, osteoblast-lined bone perimeter and bone formation rate were observed in ob/ob mice, which was greatly increased following subcutaneous administration of leptin [81]. Similarly, hypothalamic leptin gene therapy increased osteoblast-lined bone perimeter in ob/ob mice. In spite of normal osteoclast-lined bone perimeter, db/db mice exhibited a mild but generalized osteopetrotic-like (calcified cartilage encased by bone) skeletal phenotype and greatly reduced serum markers of bone turnover [81]. The authors of this study supported that leptin, acting primarily through peripheral pathways, increases osteoblast number and activity [81]. Therefore, it seems that, regardless of intracerebroventricular or subcutaneous leptin administration, leptin increased muscle mass, bone mineral density, bone mineral content, bone area, marrow adipocyte number and mineral apposition rate in both the appendicular and axial skeleton [43, 79].
Furthermore, it was reported that leptin is expressed in a unique time course during fracture healing. Delay in callus maturation was demonstrated radiographically and histologically in the ob/ob mice, which was reversed by local leptin administration, thereby indicating that leptin deficiency (ob/ob mice) leads to impaired fracture healing, which is reversed by its administration [82].
5. Bone abnormalities in hypoleptinemia and leptin resistance: Evidence from human studies
Individuals with anorexia nervosa have low leptin levels that correlate directly to low BMI and percent body fat [83]. Additionally, low BMI in constitutionally thin women is also associated with lower bone mass and poor bone mineralization [84]. Higher bone mass density (BMD) in obese patients was believed to be protective effect of obesity on bone health and mineralization [85], which may be partially true, since obese patients with sarcopenia may have low bone density and increased fragility [86]. Poor bone quality and increased fracture risk is found in patients with anorexia nervosa and hypoleptinemia. Low bone mineral density was seen in women with anorexia nervosa at lateral spine, AP spine and total hip [87]. Although bony abnormalities are multifactorial in anorexia nervosa, leptin has been shown to play a major role in bone health. Leptin levels are positive associated with bone microarchitecture and structural integrity [88]. Abnormal microarchitecture, even in the presence of normal BMD, results in increased fracture risk, thus placing low leptin state conditions with abnormal microarchitecture at a higher fracture risk category.
In cases of obesity, a state of leptin resistance, there have also been observed abnormalities. Obesity caused decreased bone mass density in a controlled study of rats [62]. Obesity may also cause increased fracture risk in humans [63]. Although in the past, it was thought that obesity was protective against osteoporosis and bone fracture risk, new evidence may suggest that obesity, implicated with low-grade inflammation and sarcopenia, may not confer benefits on bone mass [63, 89]. This relationship may be altered through the states of leptin or insulin resistance found in obesity and which in turn seem to relate to poorer bone health outcomes [90, 91]. In a large study of lean, healthy adolescents, bone mass was found to be inversely related with percent fat mass, when body weight was controlled [92]. Several other studies have found similar results of increasing adiposity leading to decreased bone mass with obese and/or lean participants, an effect that is most pronounced in obesity [93-96]. Certain bone regions may be more sensitive to these effects. For instance, cortical bone may be more sensitive to adiposity than trabecular bone [92, 94]. At higher levels, leptin, acting as a proinflammatory adipokine, may activate inflammatory pathways in osteoblasts that may cause poorer bone and cartilage health [97].
6. Interventional studies in humans
Several interventional studies have been done with leptin to look at its effect on body mass, body fat content, bone composition and bone mass, particularly in individuals with hypothalamic amenorrhea and lipodystrophy. Much of the evidence comes from women with hypothalamic amenorrhea, a state of infertility which can be caused by energy deficiency-through excess exercise and/or inadequate food intake [25]. Women with hypothalamic amenorrhea have markedly low leptin levels in addition to decreased estrogen and other hypothalamic output hormones, including thyroid hormones and growth hormones [12, 25]. They also have poor bone mass density, which can lead to low-energy bone fractures despite young age [25]. Remarkably, all hormonal abnormalities and inappropriate bone density can be reversed by metreleptin therapy [12, 25, 28, 98]. Welt et al. [28] examined eight women with more than six month long hypothalamic amenorrhea due to strenuous exercise, i.e. by definition women with decreased bone mass. All the study patients were treated with metreleptin subcutaneously for two to three months, with forty percent of the daily dose of leptin given in the morning and the remaining sixty percent at night to mimic natural diurnal variation [28]. Women were studied on and off treatment, serving as their own controls, in addition to a separate untreated control group [28]. Leptin treatment resulted in increased mean luteinizing hormone (LH) levels and LH pulse frequency, as well as in increased levels of estrogen, IGF1, IGF-BP3, and thyroxine, all of which have positive impacts on bone health [28]. It also increased levels of bone turn over markers, including bone alkaline phosphatase and osteocalcin (markers of bone formation), thereby indicating an osteoanabolic action [28]. Bone mineral density remained stable at a 3 months follow-up visit of the study [28], but the duration of this pilot study may not be long enough to detect changes in bone density. A longer study in young women with hypothalamic amenorrhea who underwent metreleptin treatment for two years showed significant improvements in bone mineral density and content at the lumbar spine [98]. The bone mineral density of hip and radius showed a trend towards improvement as well [98]. This may be related to the influences of estrogen, a hormone which also improves with leptin therapy in hypothalamic amenorrhea [98] in addition to other hormonal axes that were improved in response to exogenously administered leptin. Indeed, leptin's effect on bone is very similar to that of estrogen. Like estrogen, it also increases osteoprotegerin (OPG) levels, which leads to binding receptor activator of nuclear factor kappa-b ligand (RANKL), and in turn, results in reducing osteoclast activity [99]. Therefore, in clinical trials, it is difficult to parcel apart which hormone may causing the end effects of improving bone density and further studies may need to determine whether this is a direct or indirect effect of leptin. Regardless, these findings do show that leptin does, whether directly or indirectly, restore normal hypothalamic and bone metabolism/functioning. While leptin levels have shown strong positive correlation with BMD in women, it seems to have a weaker effect in men [100]. It seems to be rational, since testosterone, rather than estrogen, is a stronger determinant of bone mass in men; in this regard, it would be of interest to investigate the effect of metreleptin treatment in men with hypogonadotrophic hypogonadism and hypoleptinemia.
Leptin therapies, in the context of non randomized uncontrolled studies, have also be proven useful for lipodystrophic patients [101]. Lipodystrophy is characterized by a complete or partial loss of adipose tissue [101]. Moran et al. [102] studied 14 patients (3 men and 11 women) with congenital hypoleptinemia due to congenital or acquired lipodystrophy. At baseline, they had decreased fat mass, BMI and very low leptin levels, whereas their baseline BMD was normal. By four months of therapy, leptin levels were restored [102]. Leptin administration decreased lean body mass and fat content, decreased energy expenditure and caloric intake, but had no impact on bone mineralization/BMD, bone resorption, or bone metabolism biomarkers in these patients with lipodystrophy [102]. However, high baseline BMD may partly account for this paradox. Unlike patients with hypothalamic amenorrhea, patients with lipodystrophy often have comorbid insulin resistance which may increase their bone density due to the high insulin and IGF1 levels present [103].
Summarizing the aforementioned data, leptin may normalize bone density in hypoleptinemic individuals, when it is impaired, whereas it may have no or minimal action when it is not. However, further larger studies are needed to elucidate the effect of metreleptin treatment on bone metabolism.
7. Clinical Utility of Leptin
Leptin plays a crucial role in regulation of neuroendocrine axes, fat and glucose metabolism and hence has been studied in detail in several “proof of concept” clinical trials as a potential therapeutic agent in leptin deficient states. Treatment with leptin in deficient individuals not only decreases appetite and weight but also has significant effect on neuroendocrine axes leading to normalization of several hormone levels [103, 104]. In addition to congential lipodystrophy mentioned above, metreleptin replacement in leptin deficient HIV patients with highly active antiretroviral therapy (HAART)- induced lipoatrophy has shown improvement in fasting insulin levels, insulin resistance, body fat-mass (especially truncal) and high-density lipoprotein levels [105]. Leptin replacement has also shown benefit in balancing immune function in deficient individuals. Interventional studies have shown improvement in circulating cytokines and CD4 (+) T cells with leptin replacement in congenitally deficient individuals [18]. In lean women with chronic energy deprivation and relative leptin deficiency, replacement with recombinant leptin for 8 weeks showed increase in TNFα receptor levels, indicating correction of immunological function [106]. Myalept (metreleptin) is now approved for use in individuals with lipodystrophy but not those with HIV lipodystrophy. The potential side effects and contraindications include headache, weight loss, abdominal pain, arthralgia, dizziness, ear infection, fatigue, nausea, anemia, back pain, and diarrhea. It also bears the risks of neutralizing antibodies, lymphoma, hypoglycemia (when used with insulin), autoimmune disease, and allergic reactions to the compound.
Leptin replacement has shown significant improvement in bone mineral density of lumbar spine giving hope of leptin use in metabolic bone disease, including osteoporosis, particularly in leptin deficient individuals [98]. Osteoporosis is characterized by low bone mass and density that makes bone fragile and increases fracture risk. Over 10 million people worldwide have proven to have osteoporosis and 34 million have osteopenia. The disease carries a huge burden and osteoporosis related fractures cost the U.S. healthcare system nearly $17 billion annually with an increasing curve every year [107]. Several pharmacological drugs have been FDA approved for treatment of osteoporosis. Most of them are antiresorptive agents that directly or indirectly inhibit osteoclasts (e.g. bisphosphonates and calcitonin).
Given the heavy socio-economic burden of osteoporosis, newer molecules, ideally in the context of a more personalized treatment, are needed. Leptin provides a potentially promising future anabolic therapy for leptin deficient individuals, due to its effect on bone formation markers. Osteocalcin and bone alkaline phosphatase have shown significant increases after treatment with leptin in hypothalamic amenorrheic women. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women [12, 98]. There is currently no approved therapy for women with hypothalamic amenorrhea, and this unmet clinical need should be addressed; in this regard, current data warrant the design of larger controlled phase III clinical trials involving metreleptin administration [12].
Long-term trials with recombinant leptin therapy and its effect on bone mineral density and turnover markers in leptin deficient osteoporotic women should also be studied. Studies have definitively shown positive correlations of leptin with BMD, especially in postmenopausal women. The effect of leptin treatment on bone density, bone turnover markers, and mainly on low-energy fracture of hypoleptinemic postmenopausal women needs further clarification. Although the association between leptin treatment and lymphoma remains to be elucidated, leptin treatment seems to be safe and well tolerated [108]. However, it remains unknown whether the side effects and potential complications mentioned in trials on lipodystrophic individuals would also be seen in the context of randomized, placebo controlled studies in women with osteoporosis or osteopenia.
This adipokine can possibly provide therapeutic option in osteoporosis and other metabolic bone diseases in leptin deficient individuals, and future research should test and expand on this possibility.
Highlights.
Leptin has both direct and indirect effects on bone metabolism.
Leptin therapy has a normalizing effect on bone density of hypoleptinemic subjects.
Future studies need to assess benefits and risk of leptin's usefulness in bone disease.
Acknowledgments
Jagriti Upadhyay is supported by Department of Veterans Affairs Fellowship sponsored by the Office of Academic Affiliations. Olivia M. Farr is supported by training grant NICHD 5T32HD052961.
Funding: NIH 5T32HD052961
Abbreviations
- BMI
body mass index
- ARC
arcuate nucleus of hypothalamus
- NPY
neuropeptide Y
- AgRP
agouti-related peptide
- POMC
pro-opiomelanocortin
- CART
cocaine-and amphetamine-related transcript
- LepRb
leptin receptor
- JAK2
janus kinase 2
- STAT3
signal transducer and activator of transcription 3
- SHP2
src homology-2-containing protein tyrosine phosphatase 2
- MAPK
mitogen-activated protein kinase
- PI3K
phosphatidylinositol 3 kinase
- AMPK
adenosine monophosphate-activated protein kinase
- Mtor
mammalian target of rapamycin
- FoxO1
forkhead box protein O1
- TSH
thyroid-stimulating hormone
- FGF23
fibroblast growth factor 23
- VMH
ventromedial hypothalamus
- IGF1
insulin-like growth factor 1
- IGF-BP2/3
insulin-like growth factor binding protein 2/3
- GH
growth hormone
- ACTH
adrenocorticotropic hormone
- HAART
highly active antiretroviral therapy
- Akt
Protein Kinase B
- SOCS-3
Suppressor of cytokine signaling 3
Footnotes
Disclosure Statement: Dr. Mantzoros has served as consultant for Astra Zeneca.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sinha MK, Sturis J, Ohannesian J, et al. Ultradian oscillations of leptin secretion in humans. Biochem Biophys Res Commun. 1996;228(3):733–8. doi: 10.1006/bbrc.1996.1724. [DOI] [PubMed] [Google Scholar]
- 2.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: Measurement of plasma leptin and ob rna in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–61. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 3.Mantzoros CS, Liolios AD, Tritos NA, et al. Circulating insulin concentrations, smoking, and alcohol intake are important independent predictors of leptin in young healthy men. Obes Res. 1998;6(3):179–86. doi: 10.1002/j.1550-8528.1998.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 4.Farooqi IS, Keogh JM, Kamath S, et al. Partial leptin deficiency and human adiposity. Nature. 2001;414(6859):34–5. doi: 10.1038/35102112. [DOI] [PubMed] [Google Scholar]
- 5.Chan JL, Heist K, DePaoli AM, et al. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. Journal of Clinical Investigation. 2003;111(9):1409–21. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shea SA, Hilton MF, Orlova C, et al. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90(5):2537–44. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broberger C, Johansen J, Johansson C, et al. The neuropeptide y/agouti gene-related protein (agrp) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(25):15043–8. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huszar D, Lynch CA, Fairchild-Huntress V, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–41. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 9.Cone RD. Anatomy and regulation of the central melanocortin system. Nature Neuroscience. 2005;8(5):571–8. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 10.Strobel A, Issad T, Camoin L, et al. A leptin missense mutation associated with hypogonadism and morbid obesity. Nature Genetics. 1998;18(3):213–5. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- 11.Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341(12):879–84. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 12.Chou SH, Chamberland JP, Liu X, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(16):6585–90. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Audi L, Mantzoros CS, Vidal-Puig A, et al. Leptin in relation to resumption of menses in women with anorexia nervosa. Molecular Psychiatry. 1998;3(6):544–7. doi: 10.1038/sj.mp.4000418. [DOI] [PubMed] [Google Scholar]
- 14.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272(10):6093–6. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 15.Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–5. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 16.Dalamaga M, Chou SH, Shields K, et al. Leptin at the intersection of neuroendocrinology and metabolism: Current evidence and therapeutic perspectives. Cell Metab. 2013;18(1):29–42. doi: 10.1016/j.cmet.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Moon HS, Huh JY, Dincer F, et al. Identification, and saturable nature, of signaling pathways induced by metreleptin in humans: Comparative evaluation of in vivo, ex vivo and in vitro administration. Diabetes. 2014 doi: 10.2337/db14-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, t cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110(8):1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: Multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84(10):3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 20.Aronis KN, Kilim H, Chamberland JP, et al. Preadipocyte factor-1 levels are higher in women with hypothalamic amenorrhea and are associated with bone mineral content and bone mineral density through a mechanism independent of leptin. Journal of Clinical Endocrinology and Metabolism. 2011;96(10):E1634–9. doi: 10.1210/jc.2011-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank S, Heni M, Moss A, et al. Long-term stabilization effects of leptin on brain functions in a leptin-deficient patient. PloS One. 2013;8(6):e65893. doi: 10.1371/journal.pone.0065893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank S, Heni M, Moss A, et al. Leptin therapy in a congenital leptin-deficient patient leads to acute and long-term changes in homeostatic, reward, and food-related brain areas. Journal of Clinical Endocrinology and Metabolism. 2011;96(8):E1283–7. doi: 10.1210/jc.2010-2713. [DOI] [PubMed] [Google Scholar]
- 23.Matarese G, La Rocca C, Moon HS, et al. Selective capacity of metreleptin administration to reconstitute cd4+ t-cell number in females with acquired hypoleptinemia. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(9):E818–27. doi: 10.1073/pnas.1214554110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson WT, Farooqi IS, Moreau M, et al. Congenital leptin deficiency due to homozygosity for the delta133g mutation: Report of another case and evaluation of response to four years of leptin therapy. J Clin Endocrinol Metab. 2004;89(10):4821–6. doi: 10.1210/jc.2004-0376. [DOI] [PubMed] [Google Scholar]
- 25.Chou SH, Mantzoros C. 20 years of leptin: Role of leptin in human reproductive disorders. J Endocrinol. 2014;223(1):T49–T62. doi: 10.1530/JOE-14-0245. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe BE, Jimerson DC, Orlova C, et al. Effect of dieting on plasma leptin, soluble leptin receptor, adiponectin and resistin levels in healthy volunteers. Clin Endocrinol (Oxf) 2004;61(3):332–8. doi: 10.1111/j.1365-2265.2004.02101.x. [DOI] [PubMed] [Google Scholar]
- 27.Mantzoros CS, Flier JS, Rogol AD. A longitudinal assessment of hormonal and physical alterations during normal puberty in boys. V. Rising leptin levels may signal the onset of puberty. J Clin Endocrinol Metab. 1997;82(4):1066–70. doi: 10.1210/jcem.82.4.3878. [DOI] [PubMed] [Google Scholar]
- 28.Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351(10):987–97. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 29.Licinio J, Caglayan S, Ozata M, et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci U S A. 2004;101(13):4531–6. doi: 10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Licinio J, Mantzoros C, Negrao AB, et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat Med. 1997;3(5):575–9. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 31.Aschbacher K, Rodriguez-Fernandez M, van Wietmarschen H, et al. The hypothalamic-pituitary-adrenal-leptin axis and metabolic health: A systems approach to resilience, robustness and control. Interface Focus. 2014;4(5):20140020. doi: 10.1098/rsfs.2014.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li ZP, Zhang M, Gao J, et al. Study of the correlation between growth hormone deficiency and serum leptin, adiponectin, and visfatin levels in adults. Genet Mol Res. 2014;13(2):4050–6. doi: 10.4238/2014.February.14.16. [DOI] [PubMed] [Google Scholar]
- 33.Bernini GP, Argenio GF, Vivaldi MS, et al. Impaired growth hormone response to insulin-induced hypoglycaemia in obese patients: Restoration blocked by ritanserin after fenfluramine administration. Clin Endocrinol (Oxf) 1990;32(4):453–9. doi: 10.1111/j.1365-2265.1990.tb00885.x. [DOI] [PubMed] [Google Scholar]
- 34.Chan JL, Mietus JE, Raciti PM, et al. Short-term fasting-induced autonomic activation and changes in catecholamine levels are not mediated by changes in leptin levels in healthy humans. Clin Endocrinol (Oxf) 2007;66(1):49–57. doi: 10.1111/j.1365-2265.2006.02684.x. [DOI] [PubMed] [Google Scholar]
- 35.Prior LJ, Davern PJ, Burke SL, et al. Exposure to a high-fat diet during development alters leptin and ghrelin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2014;63(2):338–45. doi: 10.1161/HYPERTENSIONAHA.113.02498. [DOI] [PubMed] [Google Scholar]
- 36.Yau SW, Henry BA, Russo VC, et al. Leptin enhances insulin sensitivity by direct and sympathetic nervous system regulation of muscle igfbp-2 expression: Evidence from nonrodent models. Endocrinology. 2014;155(6):2133–43. doi: 10.1210/en.2013-2099. [DOI] [PubMed] [Google Scholar]
- 37.Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–17. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 38.Rosenbaum M, Leibel RL. 20 years of leptin: Role of leptin in energy homeostasis in humans. J Endocrinol. 2014;223(1):T83–t96. doi: 10.1530/JOE-14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Driessler F, Baldock PA. Hypothalamic regulation of bone. J Mol Endocrinol. 2010;45(4):175–81. doi: 10.1677/JME-10-0015. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji K, Maeda T, Kawane T, et al. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin d3 synthesis in leptin-deficient mice. J Bone Miner Res. 2010;25(8):1711–23. doi: 10.1002/jbmr.65. [DOI] [PubMed] [Google Scholar]
- 41.Ferron M, Lacombe J. Regulation of energy metabolism by the skeleton: Osteocalcin and beyond. Arch Biochem Biophys. 2014;561c:137–46. doi: 10.1016/j.abb.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 42.Laharrague P, Larrouy D, Fontanilles AM, et al. High expression of leptin by human bone marrow adipocytes in primary culture. Faseb j. 1998;12(9):747–52. doi: 10.1096/fasebj.12.9.747. [DOI] [PubMed] [Google Scholar]
- 43.Turner RT, Kalra SP, Wong CP, et al. Peripheral leptin regulates bone formation. Journal of Bone and Mineral Research. 2013;28(1):22–34. doi: 10.1002/jbmr.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell. 2000;100(2):197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 45.Yadav VK, Oury F, Suda N, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138(5):976–89. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yadav VK, Ryu JH, Suda N, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135(5):825–37. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan SM, Hamnvik OP, Brinkoetter M, et al. Leptin as a modulator of neuroendocrine function in humans. Yonsei Med J. 53(4):671–9. doi: 10.3349/ymj.2012.53.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foo JP, Hamnvik OP, Mantzoros CS. Optimizing bone health in anorexia nervosa and hypothalamic amenorrhea: New trials and tribulations. Metabolism: Clinical and Experimental. 2012;61(7):899–905. doi: 10.1016/j.metabol.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu WH, Kimura M, Walczewska A, et al. Role of leptin in hypothalamic-pituitary function. Proc Natl Acad Sci U S A. 1997;94(3):1023–8. doi: 10.1073/pnas.94.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson JA, Waters KM, Turner RT, et al. Direct action of naturally occurring estrogen metabolites on human osteoblastic cells. Journal of Bone and Mineral Research. 2000;15(3):499–506. doi: 10.1359/jbmr.2000.15.3.499. [DOI] [PubMed] [Google Scholar]
- 51.Delaveyne-Bitbol R, Garabedian M. In vitro responses to 17beta-estradiol throughout pubertal maturation in female human bone cells. Journal of Bone and Mineral Research. 1999;14(3):376–85. doi: 10.1359/jbmr.1999.14.3.376. [DOI] [PubMed] [Google Scholar]
- 52.Cauley JA, LaCroix AZ, Robbins JA, et al. Baseline serum estradiol and fracture reduction during treatment with hormone therapy: The women's health initiative randomized trial. Osteoporos Int. 2010;21(1):167–77. doi: 10.1007/s00198-009-0953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bagger YZ, Tanko LB, Alexandersen P, et al. Two to three years of hormone replacement treatment in healthy women have long-term preventive effects on bone mass and osteoporotic fractures: The perf study. Bone. 2004;34(4):728–35. doi: 10.1016/j.bone.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 54.Bolton JG, Patel S, Lacey JH, et al. A prospective study of changes in bone turnover and bone density associated with regaining weight in women with anorexia nervosa. Osteoporosis International. 2005;16(12):1955–62. doi: 10.1007/s00198-005-1972-7. [DOI] [PubMed] [Google Scholar]
- 55.Mika C, Holtkamp K, Heer M, et al. A 2-year prospective study of bone metabolism and bone mineral density in adolescents with anorexia nervosa. Journal of Neural Transmission. 2007;114(12):1611–8. doi: 10.1007/s00702-007-0787-4. [DOI] [PubMed] [Google Scholar]
- 56.Kiriike N, Iketani T, Nakanishi S, et al. Reduced bone density and major hormones regulating calcium metabolism in anorexia nervosa. Acta Psychiatrica Scandinavica. 1992;86(5):358–63. doi: 10.1111/j.1600-0447.1992.tb03280.x. [DOI] [PubMed] [Google Scholar]
- 57.Pralong FP, Roduit R, Waeber G, et al. Leptin inhibits directly glucocorticoid secretion by normal human and rat adrenal gland. Endocrinology. 1998;139(10):4264–8. doi: 10.1210/endo.139.10.6254. [DOI] [PubMed] [Google Scholar]
- 58.Misra M, Miller KK, Almazan C, et al. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. Journal of Clinical Endocrinology and Metabolism. 2004;89(10):4972–80. doi: 10.1210/jc.2004-0723. [DOI] [PubMed] [Google Scholar]
- 59.Tsunashima Y, Kondo A, Matsuda T, et al. Hydrocortisone inhibits cellular proliferation by downregulating hepatocyte growth factor synthesis in human osteoblasts. Biological and Pharmaceutical Bulletin. 2011;34(5):700–3. doi: 10.1248/bpb.34.700. [DOI] [PubMed] [Google Scholar]
- 60.Ventura A, Brunetti G, Colucci S, et al. Glucocorticoid-induced osteoporosis in children with 21-hydroxylase deficiency. Biomed Res Int. 2013;2013:250462. doi: 10.1155/2013/250462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grinspoon S, Miller K, Coyle C, et al. Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic amenorrhea. Journal of Clinical Endocrinology and Metabolism. 1999;84(6):2049–55. doi: 10.1210/jcem.84.6.5792. [DOI] [PubMed] [Google Scholar]
- 62.Feresin RG, Johnson SA, Elam ML, et al. Effects of obesity on bone mass and quality in ovariectomized female zucker rats. 2014;2014:690123. doi: 10.1155/2014/690123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caffarelli C, Alessi C, Nuti R, et al. Divergent effects of obesity on fragility fractures. Clin Interv Aging. 2014;9:1629–36. doi: 10.2147/CIA.S64625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Kroon PH, Boldewijn H, Langeveld-Soeter N. Congenital hypothyroidism in latent obese (ob/ob) mice. Int J Obes. 1982;6(1):83–90. [PubMed] [Google Scholar]
- 65.Sanchez VC, Goldstein J, Stuart RC, et al. Regulation of hypothalamic prohormone convertases 1 and 2 and effects on processing of prothyrotropin-releasing hormone. J Clin Invest. 2004;114(3):357–69. doi: 10.1172/JCI21620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Montagnani A. Bone anabolics in osteoporosis: Actuality and perspectives. World J Orthop. 2014;5(3):247–54. doi: 10.5312/wjo.v5.i3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hodsman AB, Bauer DC, Dempster DW, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: A review of the evidence and suggested guidelines for its use. Endocrine Reviews. 2005;26(5):688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- 68.Foo JP, Polyzos SA, Anastasilakis AD, et al. The effect of leptin replacement on parathyroid hormone, rankl-osteoprotegerin axis and wnt inhibitors in young women with hypothalamic amenorrhea. J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2014-2491. jc20142491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chan JL, Williams CJ, Raciti P, et al. Leptin does not mediate short-term fasting-induced changes in growth hormone pulsatility but increases igf-i in leptin deficiency states. J Clin Endocrinol Metab. 2008;93(7):2819–27. doi: 10.1210/jc.2008-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohlsson C, Bengtsson BA, Isaksson OG, et al. Growth hormone and bone. Endocrine Reviews. 1998;19(1):55–79. doi: 10.1210/edrv.19.1.0324. [DOI] [PubMed] [Google Scholar]
- 71.Swolin D, Brantsing C, Matejka G, et al. Cortisol decreases igf-i mrna levels in human osteoblast-like cells. Journal of Endocrinology. 1996;149(3):397–403. doi: 10.1677/joe.0.1490397. [DOI] [PubMed] [Google Scholar]
- 72.McCarthy TL, Centrella M, Canalis E. Parathyroid hormone enhances the transcript and polypeptide levels of insulin-like growth factor i in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1989;124(3):1247–53. doi: 10.1210/endo-124-3-1247. [DOI] [PubMed] [Google Scholar]
- 73.McCarthy TL, Centrella M, Canalis E. Cortisol inhibits the synthesis of insulin-like growth factor-i in skeletal cells. Endocrinology. 1990;126(3):1569–75. doi: 10.1210/endo-126-3-1569. [DOI] [PubMed] [Google Scholar]
- 74.McCarthy TL, Centrella M, Raisz LG, et al. Prostaglandin e2 stimulates insulin-like growth factor i synthesis in osteoblast-enriched cultures from fetal rat bone. Endocrinology. 1991;128(6):2895–900. doi: 10.1210/endo-128-6-2895. [DOI] [PubMed] [Google Scholar]
- 75.Ernst M, Heath JK, Rodan GA. Estradiol effects on proliferation, messenger ribonucleic acid for collagen and insulin-like growth factor-i, and parathyroid hormone-stimulated adenylate cyclase activity in osteoblastic cells from calvariae and long bones. Endocrinology. 1989;125(2):825–33. doi: 10.1210/endo-125-2-825. [DOI] [PubMed] [Google Scholar]
- 76.Ernst M, Rodan GA. Estradiol regulation of insulin-like growth factor-i expression in osteoblastic cells: Evidence for transcriptional control. Molecular Endocrinology. 1991;5(8):1081–9. doi: 10.1210/mend-5-8-1081. [DOI] [PubMed] [Google Scholar]
- 77.Turner RT, Philbrick KA, Wong CP, et al. Morbid obesity attenuates the skeletal abnormalities associated with leptin deficiency in mice. J Endocrinol. 2014;223(1):M1–m15. doi: 10.1530/JOE-14-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamrick MW, Pennington C, Newton D, et al. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34(3):376–83. doi: 10.1016/j.bone.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 79.Bartell SM, Rayalam S, Ambati S, et al. Central (icv) leptin injection increases bone formation, bone mineral density, muscle mass, serum igf-1, and the expression of osteogenic genes in leptin-deficient ob/ob mice. J Bone Miner Res. 2011;26(8):1710–20. doi: 10.1002/jbmr.406. [DOI] [PubMed] [Google Scholar]
- 80.Cornish J, Callon KE, Bava U, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175(2):405–15. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- 81.Turner RT, Kalra SP, Wong CP, et al. Peripheral leptin regulates bone formation. J Bone Miner Res. 2013;28(1):22–34. doi: 10.1002/jbmr.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khan SN, DuRaine G, Virk SS, et al. The temporal role of leptin within fracture healing and the effect of local application of recombinant leptin on fracture healing. J Orthop Trauma. 2013;27(11):656–62. doi: 10.1097/BOT.0b013e3182847968. [DOI] [PubMed] [Google Scholar]
- 83.Grinspoon S, Gulick T, Askari H, et al. Serum leptin levels in women with anorexia nervosa. J Clin Endocrinol Metab. 1996;81(11):3861–3. doi: 10.1210/jcem.81.11.8923829. [DOI] [PubMed] [Google Scholar]
- 84.Galusca B, Zouch M, Germain N, et al. Constitutional thinness: Unusual human phenotype of low bone quality. J Clin Endocrinol Metab. 2008;93(1):110–7. doi: 10.1210/jc.2007-1591. [DOI] [PubMed] [Google Scholar]
- 85.Albala C, Yanez M, Devoto E, et al. Obesity as a protective factor for postmenopausal osteoporosis. Int J Obes Relat Metab Disord. 1996;20(11):1027–32. [PubMed] [Google Scholar]
- 86.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grinspoon S, Thomas E, Pitts S, et al. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med. 2000;133(10):790–4. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lawson EA, Miller KK, Bredella MA, et al. Hormone predictors of abnormal bone microarchitecture in women with anorexia nervosa. Bone. 2010;46(2):458–63. doi: 10.1016/j.bone.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De Laet C, Kanis JA, Oden A, et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos Int. 2005;16(11):1330–8. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 90.Shin D, Kim S, Kim KH, et al. Association between insulin resistance and bone mass in men. J Clin Endocrinol Metab. 2014;99(3):988–95. doi: 10.1210/jc.2013-3338. [DOI] [PubMed] [Google Scholar]
- 91.Choi YJ, Kim DJ, Lee Y, et al. Insulin is inversely associated with bone mass, especially in the insulin-resistant population: The korea and us national health and nutrition examination surveys. J Clin Endocrinol Metab. 2014;99(4):1433–41. doi: 10.1210/jc.2013-3346. [DOI] [PubMed] [Google Scholar]
- 92.Hong X, Arguelles LM, Liu X, et al. Percent fat mass is inversely associated with bone mass and hip geometry in rural chinese adolescents. J Bone Miner Res. 2010;25(7):1544–54. doi: 10.1002/jbmr.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goulding A, Taylor RW, Jones IE, et al. Overweight and obese children have low bone mass and area for their weight. Int J Obes Relat Metab Disord. 2000;24(5):627–32. doi: 10.1038/sj.ijo.0801207. [DOI] [PubMed] [Google Scholar]
- 94.Pollock NK, Laing EM, Baile CA, et al. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am J Clin Nutr. 2007;86(5):1530–8. doi: 10.1093/ajcn/86.5.1530. [DOI] [PubMed] [Google Scholar]
- 95.Janicka A, Wren TA, Sanchez MM, et al. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92(1):143–7. doi: 10.1210/jc.2006-0794. [DOI] [PubMed] [Google Scholar]
- 96.Weiler HA, Janzen L, Green K, et al. Percent body fat and bone mass in healthy canadian females 10 to 19 years of age. Bone. 2000;27(2):203–7. doi: 10.1016/s8756-3282(00)00314-8. [DOI] [PubMed] [Google Scholar]
- 97.Yang WH, Tsai CH, Fong YC, et al. Leptin induces oncostatin m production in osteoblasts by downregulating mir-93 through the akt signaling pathway. Int J Mol Sci. 2014;15(9):15778–90. doi: 10.3390/ijms150915778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sienkiewicz E, Magkos F, Aronis KN, et al. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism. 2011;60(9):1211–21. doi: 10.1016/j.metabol.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 99.Legiran S, Brandi ML. Bone mass regulation of leptin and postmenopausal osteoporosis with obesity. Clin Cases Miner Bone Metab. 2012;9(3):145–9. [PMC free article] [PubMed] [Google Scholar]
- 100.Thomas T, Burguera B, Melton LJ, 3rd, et al. Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone. 2001;29(2):114–20. doi: 10.1016/s8756-3282(01)00487-2. [DOI] [PubMed] [Google Scholar]
- 101.Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: Pathophysiology and advances in treatment. Nat Rev Endocrinol. 2011;7(3):137–50. doi: 10.1038/nrendo.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moran SA, Patten N, Young JR, et al. Changes in body composition in patients with severe lipodystrophy after leptin replacement therapy. Metabolism. 2004;53(4):513–9. doi: 10.1016/j.metabol.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 103.Bluher S, Mantzoros CS. Leptin in humans: Lessons from translational research. Am J Clin Nutr. 2009;89(3):991S–7S. doi: 10.3945/ajcn.2008.26788E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mantzoros CS, Magkos F, Brinkoetter M, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301(4):E567–84. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee JH, Chan JL, Sourlas E, et al. Recombinant methionyl human leptin therapy in replacement doses improves insulin resistance and metabolic profile in patients with lipoatrophy and metabolic syndrome induced by the highly active antiretroviral therapy. J Clin Endocrinol Metab. 2006;91(7):2605–11. doi: 10.1210/jc.2005-1545. [DOI] [PubMed] [Google Scholar]
- 106.Chan JL, Moschos SJ, Bullen J, et al. Recombinant methionyl human leptin administration activates signal transducer and activator of transcription 3 signaling in peripheral blood mononuclear cells in vivo and regulates soluble tumor necrosis factor-alpha receptor levels in humans with relative leptin deficiency. J Clin Endocrinol Metab. 2005;90(3):1625–31. doi: 10.1210/jc.2004-1823. [DOI] [PubMed] [Google Scholar]
- 107.Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the united states, 2005-2025. J Bone Miner Res. 2007;22(3):465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 108.Mantzoros CS, Flier JS. Editorial: Leptin as a therapeutic agent--trials and tribulations. J Clin Endocrinol Metab. 2000;85(11):4000–2. doi: 10.1210/jcem.85.11.7062. [DOI] [PubMed] [Google Scholar]


