Abstract
In this study, the anoxic oxidation of arsenite (As(III)) linked to chemolithotrophic denitrification was shown to be feasible in continuous bioreactors. Biological oxidation of As(III) was stable over prolonged periods of operation ranging up to 3 years in continuous denitrifying bioreactors with granular biofilms. As(III) was removed with a high conversion efficiency (> 92%) to arsenate (As(V)) in periods with high volumetric loadings (e.g. 3.5 to 5.1 mmol As Lreactor−1 d−1). The maximum specific activity of sampled granular sludge from the bioreactors was 0.98±0.04 mmol As(V) formed g−1 VSS d−1 when determined at an initial concentration of 0.5 mM As(III). The microbial population adapted to high influent concentrations of As(III) up to 5.2 mM. However, the As(III) oxidation process was severely inhibited when 7.6 to 8.1 mM As(III) was fed. Activity was restored upon lowering the As(III) concentration to 3.8 mM. Several experimental strategies were utilized to demonstrate a dependence of the nitrate removal on As(III) oxidation as well as a dependence of the As(III) removal on nitrate reduction. The molar stoichiometric ratio of As(V) formed to nitrate removed (corrected for endogenous denitrification) in the bioreactors approximated 2.5, indicating complete denitrification was occurring. As(III) oxidation was also shown to be linked to the complete denitrification of NO3− to N2 gas by demonstrating a significantly enhanced production of N2 beyond the background endogenous production in a batch bioassay spiked with 3.5 mM As(III). The N2 production also corresponded closely to the expected stoichiometry of 2.5 mol As(III) mol−1 N2-N for complete denitrification.
Keywords: Chemolithotrophic, Arsenic, Denitrification, Anaerobic, Continuous bioreactor, Arsenate, UASB
Introduction
Arsenic (As) contamination in drinking water resources is a global health problem affecting numerous countries (Smedley and Kinniburgh 2002). Industrial, agricultural and mining activities as well as the natural weathering of arsenic bearing rocks and sediments are important sources of arsenic in groundwater (Oremland and Stolz 2003; Sierra-Alvarez et al. 2004; Smedley and Kinniburgh 2002). In circumneutral aqueous environments, the predominant forms of As are inorganic arsenate (As(V)) and arsenite (As(III)). As(III) is substantially more toxic than As(V) (Abdullaev et al. 2001; Sierra-Alvarez et al. 2004). As(III) is also more weakly bound to the surface of clay and aluminum hydroxides (Lin and Wu 2001; Manning and Goldberg 1997). Therefore microbial conversions between As species have a significant impact on the toxicity and mobility of As (Oremland et al. 2005).
As(III)-oxidizing microorganisms are widespread in the environment (Inskeep et al. 2007; Rhine et al. 2007; Stolz et al. 2006). Heterotrophic and chemoautotrophic microorganisms have been reported to oxidize As(III) under aerobic (Inskeep et al. 2007; Rhine et al. 2007) and anaerobic denitrifying conditions (Oremland et al. 2002; Rhine et al. 2006; Sun et al. 2009; Sun et al. 2008). Several bioreactor technologies have been used to investigate the biological oxidation of As(III) in aerobic environments (Battaglia-Brunet et al. 2006; Lievremont et al. 2003; Simeonova et al. 2005). Although aerobic microbial oxidation of As(III) occurs readily and rapidly, this process would not be feasible in anaerobic groundwater. Nitrate can considered as an alternative oxidant with advantages over elemental oxygen due to its high solubility and lower reactivity which together enable it to be better dispersed in the saturated subsurface (Nolan et al. 1997). Chemolithotrophic denitrifying bioreactors have been proposed for groundwater treatment utilizing various inorganic electron donors such as elemental sulfur (S°) (Sierra-Alvarez et al. 2007), sulfide (H2S) (Kleerebezem and Mendez 2002), and hydrogen (H2) (Lee and Rittmann 2002).
The objective of this research was to explore whether As(III) can be efficiently oxidized in a continuous denitrifying bioreactor. The specific goals of this study were to demonstrate the dependence of the anoxic oxidation of As(III) on the presence of nitrate, determine if the reaction is due to complete denitrification, and acclimate the microbial population to high influent concentrations of As(III).
Material and Methods
Microorganisms
Chemolithotrophic sulfoxidizing denitrifying granular sludge was obtained from a laboratory-scale denitrifying bioreactor (TDE) fed with a synthetic wastewater containing nitrate (39 mM) and thiosulfate (20 mM). Methanogenic granular sludge was obtained from a full-scale upward flow anaerobic sludge blanked (UASB) treating alcohol distillery wastewater (NGS) (Nedalco, Bergemop Zoom, The Netherlands). The content of volatile suspended solids (VSS) in the denitrifying and methanogenic sludges was 5.76±0.18 and 5.96±0.28% on a wet weight basis, respectively. The granular sludge samples were washed and sieved before use to remove fines. The inocula were stored in N2 gas at 4°C.
Basal medium
The standard basal medium for the continuous columns was prepared using Milli-Q water and contained the following (mg l−1): NH4HCO3 (3.16); NaHCO3 (672); CaCl2 (10), MgSO4.7H2O (40); K2HPO4 (300); KH2PO4.2H2O (800); Yeast extract (0.3) and 0.2 ml l−1 of a trace element solution containing (mg l−1): FeC13.4H20 (2,000); CoCl2. 6 H20 (2000); MnCl2 4H20 (500); AlCl3.6H20 (90); CuCl2.2H20 (30); ZnCl2 (50); H3BO3 (50); (NH4)6Mo7O24.4H2O (50); Na2SeO3.5 H2O (100); NiCl2.6H20 (50); EDTA (1,000); resazurin (200); HCl 36% (1 ml).
Continuous columns
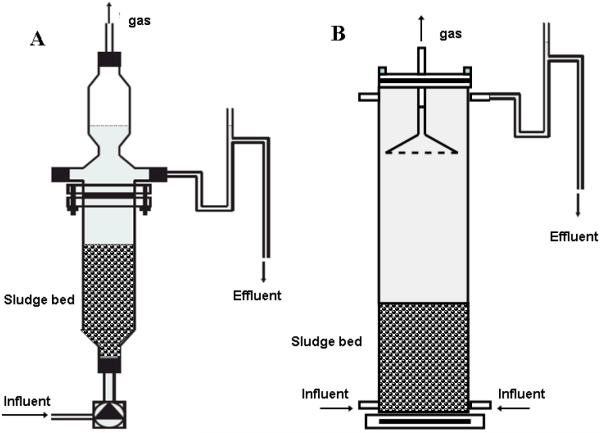
Two laboratory-scale flow-through columns (420 ml), R1 and R2, were operated in parallel (Figure 1A) under anoxic conditions. Both reactors were seeded with 12.44 g VSS l−1 TDE. R1 was continuously fed with As(III) and nitrate; R2 was a control reactor without nitrate. R1 was supplied with the basal medium containing As(III) (0.5, 2.5 and 3.8 mM, depending on period), nitrate (2.5 and 6.4 mM), and bicarbonate. The feed of R2 was prepared similarly but nitrate addition was omitted. The columns were operated for a period of 608 days and the operation was divided into five periods, which could be distinguished based on the concentration of arsenic as shown in the Table S1 (supporting information). A larger bench-scale UASB column (2 l), R3, was used in a second experiment (Figure 1B). This reactor was inoculated with 27 g VSS l−1 of NGS. The reactor was fed with the basal medium and As(III), nitrate (6.57±0.46 mM) and and bicarbonate. The concentrations of As(III) varied according to operational periods (Table S2, supporting information). The average hydraulic retention time (HRT) of the columns in both experiments was approximately 1 d. All columns were placed in a climate controlled room at 30±2°C and covered with aluminum foil to avoid light exposure. The pH of the influent was adjusted to 7.2 with NaOH or HCl, as required. The influent was maintained at all times under an N2 atmosphere to prevent dissolved oxygen from entering the medium. Influent and effluent samples were prepared immediately for analysis. The pH value was determined immediately after sampling. Samples for analysis of As speciation, NO3− and NO2− were centrifuged (10,000 rpm. 10 min) or filtered (0.45 μm membrane filter) prior to dilution. The samples for analysis of As speciation were stored at −20°C, and NO3− and NO2− samples were stored at 4 °C.
Figure 1.
Schematic diagram of the bioreactors utilized in this study. Panel A – Bioreactors R1 and R2 used in the first continuous experiment. Panel B – Bioreactor R3 used in the second continuous experiment.
A second continuous experiment using a larger volume bioreactor (R3) was initiated with a methanogenic granular sludge with no prior exposure to As(III). R3 was operated for a period of 1,045 days and the operation was divided into fourteen periods, which were distinguished based on the concentration of As and the inclusion of NO3− as shown in Table S2. The volumetric loadings of As(III) ranged from 0.38–7.20 mmol As Lreactor−1 d−1 during the different periods. The NO3− concentration supplied (6.4 mM) was in excess of the concentration required for stoichiometric conversion of As(III) to As(V). The reactor monitoring was as described above for R1 and R2.
Batch bioassay
Batch bioassays were performed in shaken flasks, which were incubated in a dark climate-controlled room at 30±2°C (see Supporting Information for details of protocol).
Analytical methods
As(III) and As(V) species were analyzed by high performance liquid chromatography–inductively coupled plasma–mass spectroscopy (HPLC-ICP-MS) (see Supporting Information for details of analysis). Nitrate, nitrite and arsenate (As(V)) were analyzed by suppressed conductivity ion chromatography using a Dionex 500 system (Sunnyvale, CA, USA) fitted with a Dionex IonPac AS11 analytical column (4 × 250 mm) a AG16 guard column (4 mm × 40 mm). During each injection the eluent (20 mM KOH) was used for 20 min. N2 and N2O were analyzed using a Hewlett Packard 5890 Series II gas chromatograph fitted with a Carboxen™ 1010 Plot column (30 m × 0.32 mm) and a thermal conductivity detector. The temperatures of the column, the injector port and the detector were 220, 110 and 100°C, respectively. Helium was used as the carrier gas and the injection volume was 100 μl. Ammonia was analyzed spectrophotometrically at a wavelength of 510 mm. The analytical determination was conducted according to Standard Methods (APHA 1999). Other analytical determinations (e.g. pH, TSS, VSS, etc.) were conducted according to Standard Methods (APHA 1999).
Results
Nitrate-dependent oxidation of As(III) to As(V) in continuous laboratory bioreactors
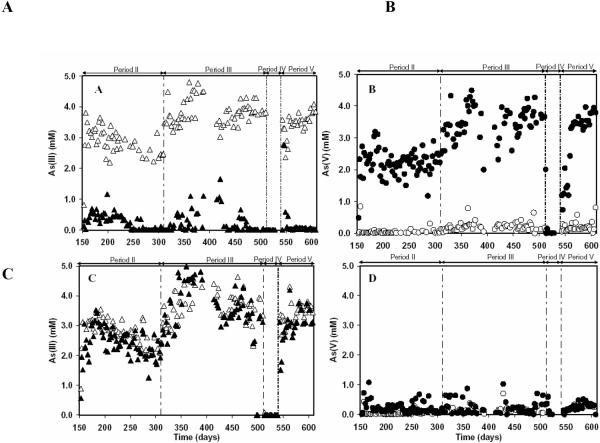
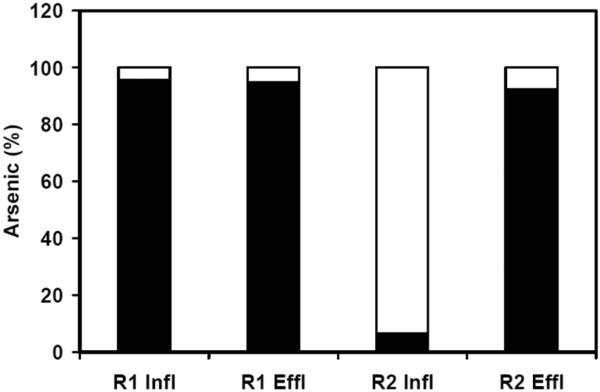
A summary of performance data for the two laboratory continuous bioreactors (R1 and R2) is presented in Table 1. The microbial oxidation of As(III) and the concomitant formation of As(V) is illustrated in Figure 2A and 2B for R1. The poor conversion of As(III) to As(V) in R2 is shown in Figure 2C and 2D. The results indicated that the microbial oxidation of As(III) in R1 could be attributed to the addition of nitrate, and its use as an electron acceptor for As(III) oxidation. Likewise, the absence of contaminating O2 in the influent was verified by the lack of any significant As(III) oxidation in R2. The initial period (period I, d 0–153) was utilized for the cultivation of an enrichment in the sludge capable of linking As(III) oxidation to denitrification. In period II (Day 154–309) and III (d 310–511), the influent concentration of As(III) was raised from 0.5 mM to 2.9 and 3.8 mM, respectively. The dominant As species in the effluent of R1 was As(V), indicating the occurrence of As(III) oxidation under denitrifying conditions. The formation of As(V) in the effluent corresponded almost stoichiometrically with the removal of As(III) in the influent, indicating that As(V) was the main product of the conversion. As(III) was efficiently oxidized in R1 with removal efficiencies of 87.6±8.8 and 92.7±10.6%, respectively, compared with nearly no oxidation in the control column (R2) during the period II and III. The volumetric loadings of As(III) in R1 was 2.39±0.47 and 3.56±0.71 mmol As Lr−1 d−1 for period II and III, respectively, where Lr refers to the empty bed volume of the reactors. In period IV (d 512–540), the As(III) feed was interrupted. In period V (d 541–608), feeding of As(III) was reestablished at 3.6 mM. After 28 d of operation without As(III), the anaerobic denitrifying microbes in the R1 column readily oxidized As(III) to As(V). The reactor became fully stable after a recovery period of 3 weeks. The As(III) removal efficiency of 98.6% was similar to the previous efficiency in period III. Figure 3 illustrates the oxidation efficiency of As(III) to As(V) during the whole operation of both columns. The As(III) removal efficiencies for the R1 were very stable with average value of 93.0% and the production yield of As(V) formed to As(III) removed was more than 90%. In comparison, negligible oxidation of As(III) to As(V) was observed in the R2.
Table 1.
Summary of As(III) oxidation linked to denitrification in bioreactor R1
| Parameters | Units | Period |
|||
|---|---|---|---|---|---|
| II | III | IV | V | ||
| As(III) volumetric load | mg As/Lr·d | 178.9±34.8 | 259.2±52.8 | — | 242.9±14.8 |
| As(III) removal efficiency | % | 88.38±7.2 | 92.71±10.6 | — | 98.58±1.8 |
| As(III) removed | mM | 2.52±0.38 | 3.56±0.57 | — | 3.55±0.22 |
| As(V) formed | mM | 2.39±0.31 | 3.32±0.48 | — | 3.33±0.27 |
| As(V) formed /As(III) removed | mol/mol | 0.90±0.15 | 0.95±0.15 | — | 0.94±0.06 |
| NO3− removed | mM | 2.36±0.53 | 2.63±0.57 | 1.18±0.31 | 2.56±0.41 |
| Corrected NO3− removed | mM | 1.24±0.49 | 1.45±0.57 | — | 1.38±0.41 |
| As(III) removed/corrected* NO3− removed | mol/mol | 2.49±0.98 | 2.75±0.90 | — | 2.83±0.74 |
| As(V) formed/corrected* NO3− removed | mol/mol | 2.32±0.95 | 2.50±0.15 | — | 2.65±0.71 |
Corrected for endogenous nitrate consumption measured in period IV
Figure 2.
The influent and effluent concentrations of As(III) and As(V) in the continuous bioreactors R1 and R2 as a function of time. Panel A and C - As(III) in R1 and R2, respectively: (△) Influent, (▲) Effluent. Panel B and D: As(V) in R1 and R2, respectively: (◯) Influent, (●) Effluent.
Figure 3.
The total average As(III) and As(V) concentrations in the influent and effluent of bioreactors R1 and R2 during Period II, III and V. As(III): solid block; As(V): empty block.
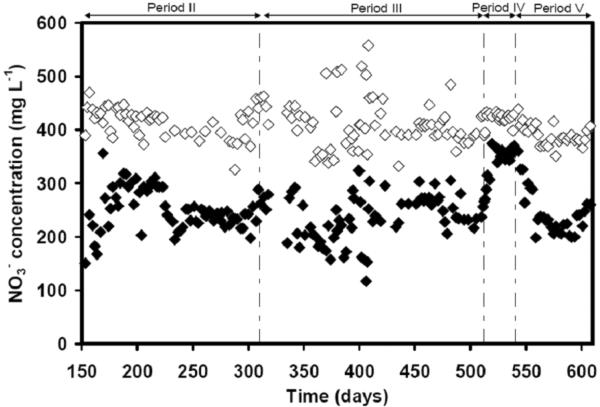
Figure 4 shows the NO3− consumption in column R1. The NO3− supplied (6.4 mM) was in excess of the concentration required for stoichiometric conversion of As(III) to As(V). The stoichiometric requirement is 1.0 and 1.5 mM NO3− for 2.5 and 3.8 mM As(III), respectively. In period IV (d 512–540), the endogenous consumption of NO3− in the absence of As(III) was measured as 1.18±0.31 mM. In those periods, the effluent concentration of NO3− increased. When As(III) was fed again, the gradual increase in the As(III) oxidation to As(V) was reflected by a gradual decrease of NO3− concentration in the effluent as a result of an increase in the NO3−-consumption. The results indicate that a large fraction of the NO3− consumption was dependent on As(III) oxidation. The molar ratio of As(V) formed compared to NO3− consumed involved in the coupled reactions was calculated from the formation of As(V) (ΔAs(V)) and the corrected NO3− (ΔNO3−) consumption. The background NO3− consumption in periods without As(III) is referred to as the “endogenous” consumption of NO3− and it is most likely due to native biomass being used as electron donor. To calculate the NO3−-consumption linked to As(III) oxidation, the total nitrate consumption was “corrected” by subtracting, the endogenous NO3−-consumption.
Figure 4.
The influent and effluent concentrations of nitrate determined in the continuous bioreactor R1 as a function of time: (◇) Influent, (◆) Effluent. Note: Phase IV is the endogenous period without feeding of As(III).
For the periods II, III and V, the calculated molar ratios of ΔAs(V): ΔNO3− are presented in Table 1. These ratios are very close to the theoretical stoichiometry ratio of 2.5 for As(III) oxidation linked to complete denitrification of NO3− to N2 as shown in eq. 1. The production of nitrite (NO2−) and ammonium (NH4+), two possible products from the microbial degradation of NO3−, were never detected in the effluent of R1 and R2. These findings indicate that nitrate was completely denitrified to the benign end product, N2.
| [eq. 1] |
Kinetics of chemolithotrophic As(III) oxidizers in the sludge in the column R1
To study the specific activity of sludge taken from R1, a batch assay was conducted with basal medium amended with 0.5 and 1.5 mM As(III) as the electron donor, and 5 mM NO3− as the electron acceptor. The maximum specific activities normalized to volatile suspended solids (VSS) are 0.98±0.04 and 0.73±0.02 mmol As(V) formed g−1 VSS d−1 for 0.5 and 1.5 mM As(III), respectively. To better understand the growth kinetics, batch assays were established with a very low inoculum concentration of 0.05 g VSS l−1. The growth rate of the population of As(III)-oxidizing bacteria with an initial As(III) concentration of 1 mM was estimated from ln(ΔAs(V) and ln(ΔN2) versus time graphs (not shown), indicating doubling times of 9.7 to 13.5 d.
Terminal products of autotrophic denitrification linked to As(III) oxidation to As(V)
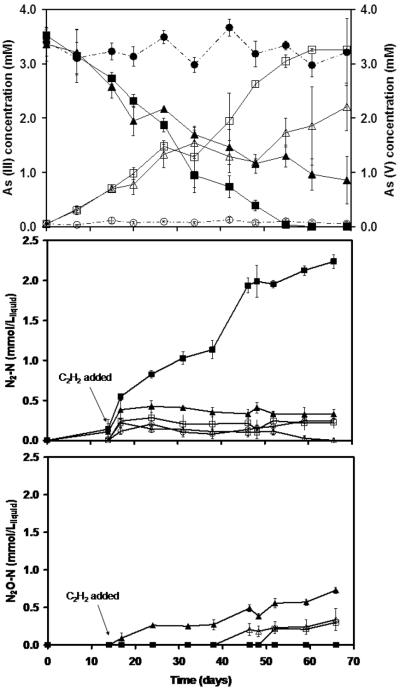
A batch experiment was set up with the biofilm obtained from R1 in order to confirm that N2 gas was the end product of nitrate reduction. The results shown in Figure 5A demonstrate the time course of As(III) removal and As(V) formation in the presence of nitrate, as well as in control treatments without nitrate, abiotic controls and an additional treatment amended with acetylene (C2H2) in the headspace to inhibit the final step of denitrification and favor accumulation of N2O. The C2H2 was injected on day 15 to avoid an initial inhibition of the reaction. In all treatments, the formation of As(V) corresponded to an almost stoichiometric elimination of As(III). The results indicated that the enriched microbial consortium could readily oxidize all As(III) to As(V) within 60 days in the presence of nitrate as electron acceptor without any lag phase. Before C2H2 was added, there was already 31.7% of As(III) oxidized to As(V). By the end of the experiment, only 52.0±13.9% of the initial As(III) in the treatments with C2H2 was oxidized to As(V), compared with 99.8±0.24% conversion in the treatment lacking C2H2. The possible products from NO3− reduction, including NO2−, NO, N2O, N2 and NH4+, were also monitored to determine the fate of NO3− in the anoxic oxidation of As(III). Figures 5B and 5C demonstrate that N2 was the only end product in the treatment without C2H2. In the treatment with C2H2, N2O had accumulated and no further reduction was observed. The results provide direct evidence that N2 was formed from nitrate reduction linked to oxidation of As(III) to As(V). In the treatment without C2H2, the formation of N2-N corresponded to 100.3±12.0% of the measured net removal of NO3−-N, also confirming that N2 was the only product from NO3−. The As and N mass balances determined in this experiment are shown in Table 2. The molar ratio of As: NO3− involved in the reaction was calculated from the As(V) formed (ΔAs(V)) to the NO3− (ΔNO3−) consumption corrected for NO3− removed in the endogenous control and N2-N (ΔN2-N) formation (corrected for the endogenous N2 formation). The calculated ratios of ΔAs(V) to ΔNO3− and ΔN2-N were 2.41±0.05 and 2.42±0.25, respectively, which are very close to the theoretical stoichiometric ratio of 2.5 (eq. 1) for As(III) oxidation linked to complete denitrification. Accumulation of NO2−, NO, or NH4+ was not detected in any of the treatments. These results confirm that R1 biofilm is capable of denitrification to N2 when utilizing As(III) as the electron donor.
Figure 5.
Batch experiment demonstrating linkage of As(III) oxidation to complete denitrification to dinitrogen gas. Panel A - Elimination of As(III) and formation of As(V): As(III) (●) and As(V) (◯) in the abiotic treatment; As(III) (■) and As(V) (□) in assay with R1 sludge supplied with As(III) and NO3−, but lacking C2H2; As(III) (▲) and As(V) (△) in assay with R1 sludge supplied with As(III), NO3− and C2H2. Panel B and C - Formation of N2 and N2O, respectively, in: Abiotic control (◯); R1 sludge supplemented with As(III)/nitrate and no C2H2 (■), R1 sludge with As(III)/nitrate and C2H2 (◆); R1 sludge with nitrate and no C2H2 (▲); R1 sludge with nitrate and C2H2 (X), in all the treatments under denitrifying conditions. The arrow indicates that the C2H2 was injected into the headspace of bottles on day 14.
Table 2.
Nitrate-N balance for the denitrification linked to As(III) oxidation in the batch experiment to determine terminal products of N
| Parameters | Abiotic | As(III)+Nitrate | As(III)+nitrate +Acetylene | Nitrate | Nitrate+ Acetylene |
|---|---|---|---|---|---|
| As(III) removed (mM) | — | 3.51±0.11 | 2.50±0.31 | — | — |
| As(V) formed (mM) | — | 3.20±0.06 | 2.16±0.43 | — | — |
| NO3− consumed (mM) | 0.06±0.05 | 1.74±0.02 | 1.44±0.13 | 0.41±0.03 | 0.51±0.05 |
| Corrected NO3− consumed (mM) | — | 1.33±0.06 | 1.10±0.19 | — | — |
| N2O-N formed (mmol/Lliquid) | 0.00±0.00 | 0.00±0.00 | 1.15±0.04 | 0.30±0.01 | 0.34±0.14 |
| N2-N formed (mmol/Lliquid) | 0.25±0.03 | 2.10±0.17 | 0.22±0.14 | 0.22±0.02 | 0.00±0.00 |
| Total gas-N formed (mmol/Lliquid) | 0.25±0.03 | 2.10±0.17 | 1.37±0.14 | 0.53±0.03 | 0.39±0.14 |
| Corrected total gas-N formed (mmol/Lliquid) | — | 1.43±0.09 | 1.03±0.12 | — | — |
| Corrected total gas-N formed/ Corrected NO3− consumed | — | 1.07±0.02 | 0.94±0.03 | — | — |
| Mol As(III) removed/corrected* mol NO3− | — | 2.65±0.09 | 2.29±0.22 | — | — |
| Mol As(V) formed/corrected* mol NO3− | — | 2.41±0.05 | 1.96±0.26 | — | — |
| Mol As(III) removed/corrected* mol total gas-N formed | — | 2.66±0.27 | 2.43±0.30 | — | — |
| Mol As(V) formed/corrected* mol total gas-N formed | — | 2.42±0.25 | 2.09±0.42 | — | — |
Corrected for endogenous nitrate consumption measured in nitrate treatment
Nitrate-dependent oxidation of As(III) to As(V) in continuous bench-scale bioreactor
The anoxic oxidation of As(III) and the concomitant formation of As(V) in reactor R3 as a function of time are illustrated in Figures 6A and 6B, respectively. Table 3 summarizes the speciation of the As in the reactor effluent. During the first 477 d (periods I–VI), the influent As(III) concentration was gradually incremented from 0.5 mM until reaching 5.0 mM in the period VII, while the concentration of NO3− was constant at 6.4 mM. The denitrifying bacteria in the bioreactor were able to tolerate up to 5 mM As(III) in the influent. The highest successful loading of As(III) was 5.14 mmol As Lr−1 d−1 with an As(III) removal efficiency of 92.0±5.9%. When 7.5 mM or more As(III) was applied during periods VIII and IX, there was a steady decline from 91.7±6.7% to 57.2±20.2% in the As(III) removal efficiency. R3 was remedied by lowering the influent As(III) concentration back to 3.75 mM in periods IX to X (Figure 6A and 6B). It took 2–3 weeks for the removal efficiency to recover back to 94.8±3.4%. A gradual increase in the As(V) formation (Fig 6B) was paralleled by a gradual decrease in effluent NO3− concentration (Fig 6C). In period V (day 349–403) and XI (day 800–927), the As(III) feed to R3 was interrupted. When no As(III) was added, the endogenous consumption of nitrate was measured as 0.79±0.18 and 0.30±0.24 mM respectively. Figure 6C shows the NO3− consumption in the bioreactor. In endogenous consumption periods, the effluent NO3− concentration started to increase, indicating a decrease in NO3−-consumption when As(III) was omitted from the feed. For the period of VI and XII when As(III) was fed again following the feed interruptions, the anaerobic denitrifying microbes readily oxidized As(III) to As(V) again. The bioreactor fully recovered in less than 2 weeks. During the recovery process, a gradual increase in the As(III) oxidation to As(V) (Fig 6A and 6B) co-occurred with a gradual increase of the NO3−-consumption (Fig 6C).
Figure 6.
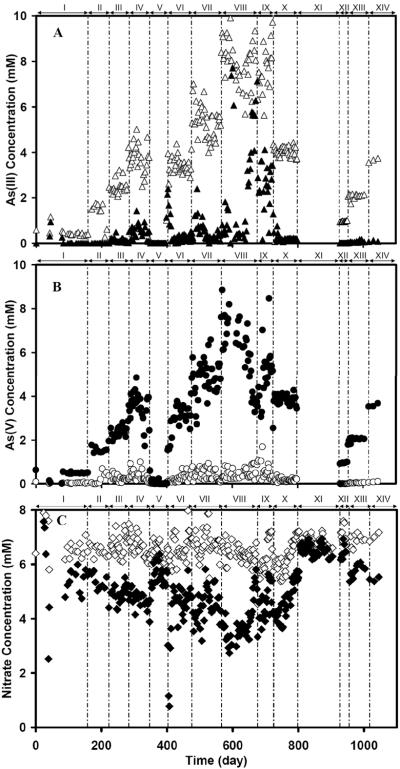
The influent and effluent concentrations of As(III), As(V) and nitrate during the operation of a 2-L bench-scale up-flow anaerobic sludge blanket reactor (R3) as a function of time. Panel A - As(III) in: (△) Influent and (▲) Effluent. Panel B - As(V) in:, (◯) Influent and (●) Effluent. Panel C - Nitrate in: (◇) Influent and (◆) Effluent.
Table 3.
Results summary of operation periods for the R3 bioreactor
| Period | Days | As(III) load mg As/Lr.d | As(III) removed mM | As(III) removal efficiency % | As(V) formed mM | As(V) formed/As(III) removed mol mol−1 | Corrected‡ NO3− consumed mM | As(III) formed/corrected NO3− consumed mol mol−1 | As(V) formed/corrected NO3− consumed mol mol−1 |
|---|---|---|---|---|---|---|---|---|---|
| I | 0–158 | 71.7±8.1 | 0.41±0.05 | 99.5±0.9 | 0.46±0.04 | 1.13±0.16 | 0.43±0.41 | 2.73±2.84 | 3.08±3.01 |
| II | 160–223 | 258.8±25.9 | 1.60±0.15 | 99.9±0.1 | 1.45±0.32 | 0.88±0.21 | 1.17±0.19 | 2.93±0.67 | 2.38±0.61 |
| III | 225–284 | 441.0±53.0 | 2.34±0.33 | 94.8±4.7 | 2.08±0.30 | 0.91±0.15 | 1.00±0.41 | 2.83±1.43 | 2.60±1.29 |
| IV | 286–347 | 674.7±106.7 | 3.27±0.64 | 87.2±7.5 | 3.18±0.69 | 0.98±0.18 | 1.17±0.34 | 3.06±1.29 | 2.97±1.21 |
| V | 349–403 | — | — | — | — | — | 0.79±0.18† | — | — |
| VI | 405–475 | 617.7±66.2 | 3.25±0.40 | 93.9±4.1 | 2.96±0.41 | 0.91±0.09 | 1.26±0.31 | 2.65±0.82 | 2.45±0.75 |
| VII | 477–566 | 935.6±113.8 | 4.81±0.60 | 92.0±5.9 | 4.40±0.70 | 0.96±0.19 | 1.86±0.65 | 2.53±0.72 | 2.36±0.69 |
| VIII | 568–636 | 1362.7±454.9 | 7.48±1.07 | 91.7±6.7 | 6.93±1.35 | 0.93±0.12 | 2.65±0.36 | 2.75±0.41 | 2.53±0.40 |
| IX | 638–725 | 1443.3±249.7 | 4.63±1.87 | 57.2±20.2 | 4.13±1.24 | 0.85±0.16 | 1.37±0.60 | NA* | NA |
| X | 727–798 | 721.1±35.4 | 3.83±0.25 | 94.8±3.4 | 3.68±0.23 | 0.96±0.05 | 1.33±0.26 | 2.85±0.71 | 2.75±0.71 |
| XI | 800–927 | — | — | — | — | — | 0.30±0.24† | — | — |
| XII | 929–954 | 174.8±78.7 | 0.97±0.44 | 99.3±0.2 | 0.94±0.38 | 0.97±0.04 | 0.53±0.23 | 2.36±0.91 | 2.54±1.09 |
| XIII | 956–1017 | 392.5±79.7 | 2.13±0.45 | 96.6±2.4 | 2.11±0.43 | 1.00±0.04 | 0.88±0.18 | 2.43±0.47 | 2.44±0.51 |
| XIV | 1019–1045 | 653.7±16.2 | 3.57±0.09 | 97.4±0.9 | 3.49±0.06 | 0.98±0.01 | 1.32±0.13 | 2.72±0.25 | 2.67±0.27 |
The endogenous consumption of nitrate.
NA means not applicable because of toxicity period.
Endogenous nitrate consumption of period V and XI were used to correct period I–IV, VI–VII; and VIII–X, XII–XIV; respectively.
The dominant As species in the effluent was As(V) (Figure 6B), indicating the occurrence of microbial As(III) oxidation under denitrifying conditions. The formation of As(V) in the effluent corresponded with the almost stoichiometric removal of As(III) in the influent, indicating that As(V) was the main product of the conversion. The yield of As(V) formed to As(III) converted averaged at 94.3±16.6%.
For all the experimental periods, the calculated molar ratios of ΔAs(V): ΔNO−3 are presented in Table 3. The molar ratio of As(III) removed and As(V) formed compared to NO−3-consumed averaged 2.62±0.24 and 2.71±0.21, respectively for all experimental periods with 5 mM As(III) or less. These values are very close the theoretical stoichiometry of 2.5 for complete denitrification as shown in eq. 1. They also clearly illustrate that the calculated molar ratios of ΔAs(V): ΔNO−3 does not coincide at all with the theoretical ratio of 1 expected for the partial reduction of NO−3 to NO−2, nor the ratio of 4 expected for the dissimilatory NO−3 reduction to NH+4. The production of NO−2 and NH+4, the possible products from the microbial degradation of NO−3 by alternative pathways, were never detected in the effluent of R3. These findings taken together indicate that NO−3 was completely denitrified to the benign end product, N2.
Discussion
Microbial oxidation of As(III) linked to denitrification in the continuous bioreactors
The anoxic oxidation of As(III) in the continuous bioreactors observed in this study showed a significant dependence on the presence of NO−3 in the feed, confirming that the conversion was linked to denitrification. During the continuous studies with bioreactors R1 and R3, the observed biological NO−3-consumptions in periods fed with As(III) were much higher than the endogenous background NO−3-consumption in periods when As(III) was omitted. The molar ratio of As(III) removed (or As(V) formed) to NO−3-consumed (corrected for endogenous consumption) was approximately equal to the theoretically expected value of 2.5 for complete denitrification of NO−3 to N2 as shown in eq. 1. The production of nitrite (NO−2) and ammonium (NH+4), two possible products from the microbial degradation of NO−3, were never detected in the effluent of R1 and R2. These findings indicate that nitrate was completely denitrified to the benign end product, N2.
| [eq. 1] |
Furthermore, the batch bioassays with biofilm sampled from the bioreactors revealed that NO−3-consumption and N2-production were significantly higher in the biologically active treatments containing As(III) compared to endogenous controls without As(III). These results taken together provide compelling evidence that the chemolithotrophic denitrification process was responsible for the microbial oxidation of As(III) to As(V).
Microbial nitrate-dependent oxidation of As(III) was first reported by Oremland and collaborators (Oremland et al. 2002). Several microorganisms capable of As(III) oxidation with nitrate have been identified recently. Alkalilimnicola ehrlichii sp. strain MLHE-1 from an As-rich, alkaline hypersaline soda lake in California (Mono Lake) (Hoeft et al. 2007), and strains DAO1 and DAO10 were isolated from As-polluted industrial soil (Hoeft et al. 2007; Rhine et al. 2006). As(III)-oxidizing denitrifying bacteria were also indentified in mixed cultures established from sludges and sediments with no prior exposure to As (Sun et al. 2008). A 16S rRNA gene clone library characterization of enrichment cultures derived from the sediments and sludges indicated that the predominant phylotypes were from the genus Azoarcus and the family Comamonadaceae (Sun et al. 2009).
End products of denitrification linked to As(III) oxidation in the continuous bioreactors
In this study, multiple lines of evidence point to the fact that microorganisms in the biofilm of R1 and R3 reactor linked the anoxic oxidation of As(III) to complete denitrification as opposed to partial denitrification to NO−2 or dissimilatory NO−3 reduction to NH+4. Firstly, this was confirmed by the lack of any accumulation of NO−2 or NH+4. Secondly, the stoichiometric ratio of As(III) oxidation to NO−3/NO−2, NO3−/N2 and NO−3/NH+4 would be 1, 2.5 and 4, respectively; and the observed ratios in this study were consistently approximating 2.5 (Table 1 and 3), corresponding to complete denitrification to N2. Thirdly, the batch study with biofilm granules sampled from bioreactor R1 confirmed the production of N2 due to the oxidation of As(III) and the yield of N2 corresponded to the expected stoichiometry from the electron equivalents in As(III). The measured N2 was not an artifact of sample contamination due to leakage of atmospheric N2, because only negligible background levels of N2 were measured in controls handled in the same fashion. Furthermore, inhibiting the reaction with C2H2 generated N2O in lieu of N2, and N2O has no major atmospheric source to cause sample contamination.
Although the first chemolithoautotrophic As(III)-oxidizing denitrifying bacterium isolated, Alkalilimnicola ehrlichii sp. strain MLHE-1, only partially denitrifies NO−3 to NO−2 (Hoeft et al. 2007; Oremland et al. 2002), it has already been demonstrated that some microorganisms can denitrify nitrate to N2 when oxidizing As(III) to As(V). Evidence for Azoarcus strain DAO1 and Sinorhizobium strain DAO10 was obtained based on the stoichiometry of the reaction and the amplification of the nitrous oxide reductase gene, nosZ (Rhine et al. 2006). In our previous study, As(III) adsorbed on activated aluminum to alleviate toxicity, was effectively utilized as an electron donor for the denitrification to N2 gas with natural mixed cultures (Sun et al. 2008).
As(III) substrate inhibition on anoxic oxidation of As(III) in continuous bioreactors
The toxicity of As(V) is due mainly to its similarity in structure with phosphate. As(V) can replace phosphate and inhibit oxidative phosphorylation (Oremland and Stolz 2003; Silver and Phung 2005). As(III) is more toxic than As(V) according to various studies (Abdullaev et al. 2001; Sierra-Alvarez et al. 2004). As(III) can enter cells through aqua-glycerolporins and bind to thiols or vicinal sulfhydryl groups and inactivate/denature many proteins and enzymes, causing cell damage (Mukhopadhyay et al. 2002). Although As is toxic to many bacteria, some bacteria are resistant to arsenic due to an efflux system mediated by the plasmid- or chromosomally-encoded ars operon (Silver and Phung 2005).
The microbial population in the continuous bioreactor R1 acclimated to high concentration of As(III) up to 3.8 mM in the influent. The R3 reactor was operated with a range of As(III) concentrations in the influent from 0.5 to 8.1 mM. R3 reactor tolerated As(III) up to 5.2 mM, However, when the As(III) concentration was increased to the range of 7.6 to 8.1 mM, inhibition of As(III) oxidation became evident and the efficiency of As(III) conversion dramatically decreased. Such high concentrations of As(III) are highly toxic to microorganisms (Stasinakis et al. 2003). The 50% inhibitory concentrations of As(III) to methanogenic activity are reported to be as low as 15 μM (Sierra-Alvarez et al. 2004). Nine arsenic resistant strains exhibited an effective 50%-inhibiting concentration in the range of 0.27–9.73 mM (Takeuchi et al. 2007). In our previous batch study, mixed consortia from sludge and sediments without prior exposure to As demonstrated the capacity to oxidize As(III) linked to denitrification up to 3.5 mM, but at that concentration, the rate was severely inhibited (Sun et al. 2008). In the unadapted natural mixed cultures, the denitrification was inhibited as reflected by the N2O accumulation at 3.5 mM of As(III) (Sun et al. 2008). However in this study, the highly enriched biofilm from R1 reactor was able to readily link oxidation of 3.5 mM As(III) to complete denitrification of NO−3 to N2 without any evidence of inhibition.
Supplementary Material
Acknowledgments
The work presented here was funded by a USGS, National Institute for Water Resources 104G grant (2005AZ114G), and by a grant of the NIEHS-supported Superfund Basic Research Program (NIH ES-04940).
References
- Abdullaev FI, Rivera-Luna R, Garcia-Carranca A, Ayala-Fierro F, Espinosa-Aguirre JJ. Cytotoxic effect of three arsenic compounds in HeLa human tumor and bacterial cells. Mutat. Res.-Gen. Tox. En. 2001;493(1–2):31–38. doi: 10.1016/s1383-5718(01)00161-9. [DOI] [PubMed] [Google Scholar]
- APHA . Standard methods for the examination of water and wastewater. American Public Health Association; Washington D. C.: 1999. [Google Scholar]
- Battaglia-Brunet F, Itard Y, Garrido F, Delorme F, Crouzet C, Greffie C, Joulian C. A simple biogeochemical process removing arsenic from a mine drainage water. Geomicrobiol. J. 2006;23(3–4):201–211. [Google Scholar]
- Hoeft SE, Blum JS, Stolz JF, Tabita FR, Witte B, King GM, Santini JM, Oremland RS. Alkalilimnicola ehrlichii sp nov., a novel, arsenite-oxidizing haloalkaliphilic gammaproteobacterium capable of chemoautotrophic or heterotrophic growth with nitrate or oxygen as the electron acceptor. Int. J. Syst. Evol. Microbiol. 2007;57(3):504–512. doi: 10.1099/ijs.0.64576-0. [DOI] [PubMed] [Google Scholar]
- Inskeep WP, Macur RE, Hamamura N, Warelow TP, Ward SA, Santini JM. Detection, diversity and expression of aerobic bacterial arsenite oxidase genes. Environ. Microbiol. 2007;9(4):934–943. doi: 10.1111/j.1462-2920.2006.01215.x. [DOI] [PubMed] [Google Scholar]
- Kleerebezem R, Mendez R. Autotrophic denitrification for combined hydrogen sulfide removal from biogas and post-denitrification. Water Sci. Technol. 2002;45(10):349–356. [PubMed] [Google Scholar]
- Lee KC, Rittmann BE. Applying a novel autohydrogenotrophic hollow-fiber membrane biofilm reactor for denitrification of drinking water. Water Res. 2002;36(8):2040–2052. doi: 10.1016/s0043-1354(01)00425-0. [DOI] [PubMed] [Google Scholar]
- Lievremont D, N'Negue MA, Behra P, Lett MC. Biological oxidation of arsenite: batch reactor experiments in presence of kutnahorite and chabazite. Chemosphere. 2003;51(5):419–428. doi: 10.1016/S0045-6535(02)00869-X. [DOI] [PubMed] [Google Scholar]
- Lin TF, Wu JK. Adsorption of arsenite and arsenate within activated alumina grains: Equilibrium and kinetics. Water Res. 2001;35(8):2049–2057. doi: 10.1016/s0043-1354(00)00467-x. [DOI] [PubMed] [Google Scholar]
- Manning BA, Goldberg S. Adsorption and stability of arsenic(III) at the clay mineral-water interface. Abstracts of Papers of the American Chemical Society. 1997;213 221-GEOC. [Google Scholar]
- Mukhopadhyay R, Rosen BP, Pung LT, Silver S. Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol. Rev. 2002;26(3):311–325. doi: 10.1111/j.1574-6976.2002.tb00617.x. [DOI] [PubMed] [Google Scholar]
- Nolan BT, Ruddy BC, Hitt KJ, Helsel DR. Risk of nitrate in groundwaters of the United States - A national perspective. Environ. Sci. Technol. 1997;31(8):2229–2236. [Google Scholar]
- Oremland RS, Hoeft SE, Santini JA, Bano N, Hollibaugh RA, Hollibaugh JT. Anaerobic oxidation of arsenite in Mono Lake water and by facultative, arsenite-oxidizing chemoautotroph, strain MLHE-1. Appl. Environ. Microbiol. 2002;68(10):4795–4802. doi: 10.1128/AEM.68.10.4795-4802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland RS, Kulp TR, Blum JS, Hoeft SE, Baesman S, Miller LG, Stolz JF. A microbial arsenic cycle in a salt-saturated, extreme environment. Science. 2005;308(5726):1305–1308. doi: 10.1126/science.1110832. [DOI] [PubMed] [Google Scholar]
- Oremland RS, Stolz JF. The ecology of arsenic. Science. 2003;300(5621):939–944. doi: 10.1126/science.1081903. [DOI] [PubMed] [Google Scholar]
- Rhine ED, Ni Chadhain SM, Zylstra GJ, Young LY. The arsenite oxidase genes (aroAB) in novel chemoautotrophic arsenite oxidizers. Biochem. Biophys. Res. Commun. 2007;354(3):662–667. doi: 10.1016/j.bbrc.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Rhine ED, Phelps CD, Young LY. Anaerobic arsenite oxidation by novel denitrifying isolates. Environ. Microbiol. 2006;8(5):899–908. doi: 10.1111/j.1462-2920.2005.00977.x. [DOI] [PubMed] [Google Scholar]
- Sierra-Alvarez R, Beristain-Cardoso R, Salazar M, Gomez J, Razo-Flores E, Field JA. Chemolithotrophic denitrification with elemental sulfur for groundwater treatment. Water Res. 2007;41(6):1253–1262. doi: 10.1016/j.watres.2006.12.039. [DOI] [PubMed] [Google Scholar]
- Sierra-Alvarez R, Cortinas I, Yenal U, Field JA. Methanogenic inhibition by arsenic compounds. Appl. Environ. Microbiol. 2004;70(9):5688–5691. doi: 10.1128/AEM.70.9.5688-5691.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S, Phung LT. Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic. Appl. Environ. Microbiol. 2005;71(2):599–608. doi: 10.1128/AEM.71.2.599-608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeonova DD, Micheva K, Muller DAE, Lagarde F, Lett MC, Groudeva VI, Lievremont D. Arsenite oxidation in batch reactors with alginate-immobilized ULPAs1 strain. Biotechnol. Bioeng. 2005;91(4):441–446. doi: 10.1002/bit.20530. [DOI] [PubMed] [Google Scholar]
- Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002;17(5):517–568. [Google Scholar]
- Stasinakis AS, Thomaidis NS, Giannes AS, Lekkas TD. Effect of arsenic and mercury speciation on inhibition of respiration rate in activated sludge systems. Environ. Sci. Pollut. Res. 2003;10(3):177–182. doi: 10.1065/espr2002.05.121. [DOI] [PubMed] [Google Scholar]
- Stolz JE, Basu P, Santini JM, Oremland RS. Arsenic and selenium in microbial metabolism. Annu. Rev. Microbiol. 2006;60:107–130. doi: 10.1146/annurev.micro.60.080805.142053. [DOI] [PubMed] [Google Scholar]
- Sun WJ, Sierra-Alvarez R, Fernandez N, Sanz JL, Amils R, Legatzki A, Maier RM, Field JA. Molecular characterization and in situ quantification of anoxic arsenite-oxidizing denitrifying enrichment cultures. FEMS Microbiol. Ecol. 2009;68(1):72–85. doi: 10.1111/j.1574-6941.2009.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WJ, Sierra R, Field JA. Anoxic oxidation of arsenite linked to denitrification in sludges and sediments. Water Res. 2008;42(17):4569–4577. doi: 10.1016/j.watres.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Kawahata H, Gupta LP, Kita N, Morishita Y, Ono Y, Komai T. Arsenic resistance and removal by marine and non-marine bacteria. J. Biotechnol. 2007;127(3):434–442. doi: 10.1016/j.jbiotec.2006.07.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.