Abstract
Although parents and children must adhere to five primary treatments for cystic fibrosis (CF), and their roles transition over time, the scope of CF studies often has been limited to one treatment regimen or to children within a specified age range. The purpose of this mixed research synthesis study is to integrate findings from qualitative and quantitative studies addressing the transition of CF management from parent to child, as well as factors related to adherence across treatments and over time. An existing grounded theory was used as a framework to synthesize findings in 17 reports from 16 studies. The results confirm the theory and posit three additional factors that may influence parent-to-child transition of care management.
Keywords: Cystic fibrosis, disease management, family, grounded theory, research synthesis, transition to adult care
Cystic fibrosis (CF) affects approximately 30,000 individuals in the United States and 70,000 worldwide (Cystic Fibrosis Foundation, 2014; Ernst, Johnson, & Stark, 2010). A diagnosis of CF has been referred to as a “family diagnosis” (Ernst et al., 2010, p. 264), because managing it involves daily treatments that are complex and time consuming, and require children and their families to manage multiple regimens. Families living with a child with CF must balance the demands of daily CF care with the needs of the family, and transition this balancing act to accommodate the child’s developmental changes (Glasscoe & Quittner, 2008). Until recently, most children with CF died by the age of 6 years, but many now are living into their 30s and even 40s. As a result, parents and children must negotiate transitions in care across the full developmental spectrum from infancy to adulthood. A major challenge for families is how best to balance adherence to daily CF care with the needs of the affected child and the needs of other family members and the family as a whole.
Managing CF involves five primary treatments: (a) airway clearance (e.g., chest physiotherapy), (b) nebulized medications, (c) antibiotics (oral or intravenous), (d) enzyme replacement pills, and (e) increased calorie intake (Glasscoe & Quittner, 2008). Adherence to these treatments slows disease progression and increases the life spans of individuals with CF (Mogayzel et al., 2013). Although these treatments are central to CF management, reported adherence levels vary across treatment regimens and full compliance generally occurs less than 50% of the time (Glasscoe & Quittner, 2008; Latchford, Duff, Quinn, Conway, & Conner, 2009; Modi et al., 2010). Rates of adherence tend to be higher for replacing pancreatic enzymes and oral antibiotics and lower for chest physiotherapy and nebulized medications, regardless of family relationship factors (Abbott, Havermans, & Hart, 2009). Understanding factors that influence adherence to CF treatment regimens, however, is critical to developing more effective adherence interventions, and thereby improving the health and well-being of children with CF and their families (Abbott et al., 2009).
Parents and children share responsibility for managing a child’s chronic disease, with the parents transitioning responsibility to the child over time. Transitions in the parent–child roles in care management may lead to conflict between the parent and child and to lower levels of adherence (Schilling, Knafl, & Grey, 2006). Thus, understanding the parent-to-child transition is essential to understanding how best to intervene to improve disease management.
Numerous researchers have studied the factors that influence the transition of CF management from parent to child and its effects on adherence to treatment regimens. The scope of these studies is typically limited to one treatment regimen (e.g., caloric intake) or to children in a specified age range (e.g., adolescents). Synthesizing findings from these studies therefore offers the potential to explore variations in the parent–child division of responsibility for CF management across regimens and over the child’s developmental trajectory. Accordingly, the purpose of this research synthesis study is to integrate findings from qualitative and quantitative studies addressing family management of CF with a focus on the transition in management from parent to child and on factors related to adherence across treatments and over time.
Method
The analysis reported here was conducted as part of a larger, ongoing mixed-methods mixed research synthesis study, the purpose of which is to explore the intersection between family life and childhood chronic physical conditions, such as anemia, arthritis, asthma, cancer, cystic fibrosis, diabetes, end-stage renal disease, heart problems, muscular dystrophy, and seizure disorders. The analysis featured in this article included all reports addressing families with children with cystic fibrosis. This study entails the use of qualitative and quantitative research techniques to synthesize findings from reports of primary qualitative, quantitative, and mixed-methods studies (Sandelowski, Voils, Crandell, & Leeman, 2013).
Literature Search
The literature search for the present study was designed to be broadly inclusive, with the goal of identifying the full breadth of empirical research findings related to the intersection of family life and management of a child’s cystic fibrosis. The following electronic databases were searched: Academic Search Premier, CINAHL, EMBASE, ERIC, Family & Society Worldwide, PsycINFO, PubMed, Social Work Abstracts, and Sociological Abstracts. The search terms combined family terms (family, caregiver, mother, father, sibling, brother, sister, grandparent, and parent), child terms (child, infant, newborn, adolescent, and teenager), and cystic fibrosis. We limited the search to English-language reports published between 2000 and 2011. No limits were placed on the types of research designs or methodologies. Two members of the research team reviewed titles and abstracts and, as needed, the full manuscript. Child was defined as an individual no older than 18 years. All reports of studies regardless of methodology were conceptualized as addressing the intersection of family life and childhood chronic physical conditions if they included high-relevance family findings as defined and described in Knafl, Leeman, Havill, Crandell, and Sandelowski (2014), that is, if they addressed links among aspects of family functioning, relationships, and structure; family member well-being, competence, or role performance; and/or family member understandings or management of the condition. Reports of intervention studies in particular were included if they met the following criteria: presence of comparison group, random assignment to groups, and testing of the effects of an intervention on one or more of these aspects or relationships. Examples of types of reports excluded were those focused on instrument development or validation, health-care utilization, or cost analyses. Further details about the search and retrieval process, and inclusion and exclusion criteria for the full study, are reported elsewhere (Havill et al., 2013; Knafl et al., 2014).
Data Extraction
Standardized forms developed for the larger study were used to extract data from each report in the present study, including research purpose, theoretical framework, study design, demographics of index children and other family members, inclusion and exclusion criteria, recruitment methods, data collection methods and measures, and findings. To facilitate efforts to synthesize primary qualitative and quantitative study findings, we transformed findings into statements that anchored results to relevant information about the sample, source of information, time, comparative reference point, magnitude, and significance (Sandelowski, Leeman, Knafl, & Crandell, 2013). One member of the research team extracted data from each report and a second team member verified the accuracy of the extraction. As needed, a third team member was enlisted to resolve discrepancies. Information pertinent to study quality also was extracted. Studies were coded as “noisy” (Edwards, Elwyn, Hood, & Rollnick, 2000) if key study features had the potential to undermine the credibility of findings. For qualitative studies, such factors included (a) findings not demonstrably plausible and/or insufficiently substantiated with data, (b) sample size and configuration insufficient to support the findings, (c) features of the sample critical to the understanding of findings not described, and (d) variations in findings by relevant sample and event characteristics not addressed (Sandelowski & Barroso, 2007). For quantitative observational studies, factors included (a) internal validity threats of confounding, reporting bias, and significant amounts of missing data, and (b) external validity threats of low participation rate and lack of representativeness of the target population (STROBE Statement, 2007). For intervention studies, factors included (a) internal validity threats of selection, performance, detection, attrition, and reporting bias, and (b) external validity threats of large proportion of participants declining to participate or ineligible, and evidence that the intervention was not implemented or difficult to implement and/or maintain as intended (Higgins & Green, 2011). The standard now in systematic reviews is to use quality criteria not to exclude reports or findings a priori but to characterize them for use in a posteriori analyses (Pawson, 2006)—for example, to determine for each set of synthesized findings whether they derived from largely high-signal, low-noise or low-signal, high-noise studies (Sandelowski, 2012). Although no reports were excluded on the basis of quality, we considered quality in our judgments concerning the strength of evidence for a theme. The synthesis was judged to support a theme when findings were consistent across two or more “non-noisy” studies.
Synthesis of Findings
We synthesized findings by configuration, which “entails the arrangement of thematically diverse individual findings … into a coherent theoretical rendering of them” to ascertain their relationships to each other (i.e., whether they contradict, extend, or otherwise modify each other; Sandelowski et al., 2012, p. 352). Two members of the research team reviewed the extracted findings to identify those findings relevant to the research purpose and to group similar findings together. In the process of this review, they identified in one of the included reports a grounded theory of CF management transition (Williams, Mukhopadhyay, Dowell, & Coyle, 2007a) that seemed appropriate to use as a placeholder to configure the relevant findings from the other reports included in the synthesis. The use of a theory to hold in place the findings of other studies is an example of a top-down approach to synthesis by configuration (Sandelowski, Voils, Leeman, & Crandell, 2012). The results of such a synthesis may be the confirmation or refutation of theory, or its further refinement or extension.
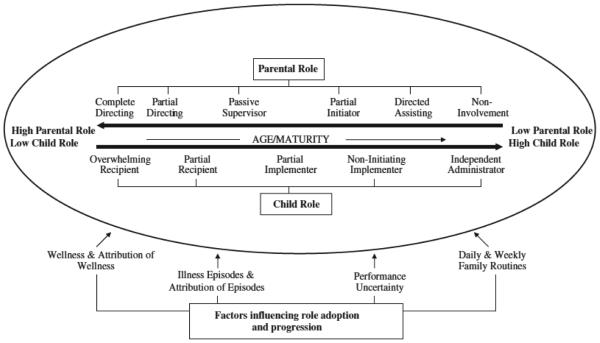
Figure 1 shows the theory as originally displayed in Williams et al. (2007a). Williams et al. (2007a, 2007b) developed this theory from in-depth interviews conducted with 63 children with CF and their parents. The theory posits that the role of parents and children in managing physiotherapy varies from day to day and over time as the child ages, and our initial review of the literature suggested that the theory’s constructs would transfer to other CF treatment regimens.
Figure 1.
Parental and child roles in the initiation and implementation of physiotherapy over time.
Source. Williams et al. (2007a). Copyright 2007 Elsevier Ltd.
We used a directed-content analysis approach (Hsieh & Shannon, 2005) to code all relevant findings from the other reports, according to the components of the theory pertinent to them (Hsieh & Shannon, 2005); that is, we grouped findings related to parent–child transitions in management over time related to any or all five primary treatment modalities of CF, not just physiotherapy (i.e., airway clearance or physiotherapy, nebulized medications, antibiotics, pancreatic enzymes, and increased calorie intake). We placed findings addressing (a) parent–child transition in management as the child ages, (b) child’s wellness and illness episodes, (c) child and parent attribution of wellness and illness, (d) parental uncertainty about the child’s performance of CF treatments, (e) daily and weekly family routines, and (f) parent and child perceptions of the benefits of transitioning care to the child. Although not explicitly included in the original Williams et al. (2007a) display of the theory, we added the (f) benefits grouping, as it was clearly stated in their report. To further assess the theory’s applicability across treatment modalities, we also identified (g) findings related to differences in family adherence across treatment regimens.
Using Atlas.ti, two members of the research team independently placed study findings into groups (a–g) and integrated common findings within groupings. They then compared their results and resolved discrepancies until they achieved consensus.
Findings
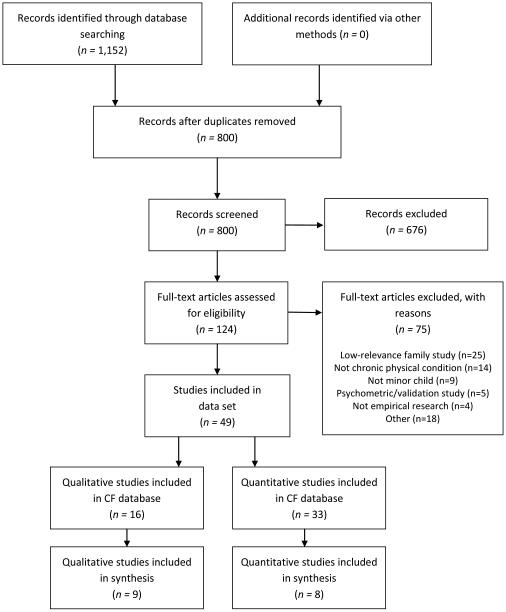
As Figure 2 details, the electronic search yielded 800 reports of studies addressing children with CF. Following screening, 49 reports from 44 studies were included in the initial review: 5 intervention, 16 qualitative, and 28 quantitative observational reports. Of the 49 reports of CF included in the parent study, 17 reports from 16 studies (Table 1) addressed findings relevant to the purpose of this analysis, namely the transition of condition management in families with children with CF over time. With the exception of Williams et al. (2007a, 2007b), none of the studies had more than one report included in the review. Eight of the included studies were qualitative, seven were quantitative observational, and one was a randomized controlled trial. Six of the studies were identified as methodologically noisy, including three qualitative, two quantitative observational, and one intervention study. In half of the studies, researchers addressed adherence without specifying adherence to a particular regimen (n = 8). In the remaining studies, researchers addressed adherence to multiple regimens (n = 3) or to one specific regimen, such as airway clearance (n = 2), mediations (n = 2), or dietary intake (n = 1). Most studies were conducted in the United States (n = 7) or the United Kingdom (n = 6); two studies were conducted in Australia and one in Canada.
Figure 2.
PRISMA flow diagram for research synthesis
Table 1.
Description of Studies Included in the Review
| Citation | Metho d |
Location | Statement of purpose | Participants | Age (years) of index child |
Data collection | CF regimen |
|---|---|---|---|---|---|---|---|
| Carpenter & Narsavage (2004) | QL | US | Describe lived experiences of families caring for a child with cystic fibrosis at time of initial diagnosis |
9 family members |
NS | Focus group and written narratives |
NS |
| Cronin (2004) | QL | US | Explore how type of hidden impairment in a child influences family routines and occupations |
22 mothers of CF; 22 mothers of ADHD |
5–18; × = 11 |
Individual interview | NS |
| DeLambo et al. (2004) | QN | US | Examine associations between observations of the quality of family relationships and problem-solving skills and reported adherence to medical treatments for older children and adolescents with cystic fibrosis (CF) |
96 children and parents (actual number of parents NS) |
9–17 | Treatment Adherence Rating Scale (TARS), Issues Checklist (IC), CF Issues Checklist (CF-IC), observed family discussion, Iowa Family Interaction Rating Scales (IFIRS), |
Airway clearance, nutrition, enzymes, antibiotics |
| Duff et al. (2003) | QN | UK | Establish the prevalence and nature of disruptive mealtime behaviors and inappropriate parental responses in children with CF |
108 parents | 1–17 | Behavioural Paediatric Feeding Assessment Scale (BPFAS-UK) |
Nutrition |
| Dupuis et al. (2011) | QL | Canada | Explore experience of parents and adolescents living with cystic fibrosis prior to the transfer of the adolescent’s care from a pediatric to an adult health care facility. |
7 adolescents; 7 mothers; 4 fathers; 8 health-care workers |
15–18 | Individual interviews, group interview with health-care team |
NS |
| Foster et al. (2001) | QL | UK | Investigate impact of CF and treatment on patients and family members |
8 patients; 8 siblings; 8 mothers; 1 father |
10–18 | Individual interviews | NS |
| Gayer & Ganong (2006) | QN | US | Examine differences in the experiences of mothers of children with cystic fibrosis who are in diverse family structures |
318 mothers | NS: × = 9.5 | Family Time and Routines Inventory (FTRI), Family Sense of Coherence Scale, Quality of Coparental Communication Instrument, |
NS |
| Graetz et al. (2000) | QN | Australia | Identify and compare perceived supportive and nonsupportive behaviors exhibited by family members and friends toward adolescents with CF, and examine the relationships between supportive and nonsupportive behaviors and adolescents’ psychological adjustment |
35 adolescent | 11–18; × = 14.5 | Chronic Disease Support Interview (CDSI), Youth Self Report Form (YSR), |
NS |
| Grasso et al. (2000) | RCT | Australia | Evaluate the effect of recorded music as an adjunct to CPT |
20 parents | 4.5–24 | Enjoyment: 7-point Likert- type scale, perception of time question |
Airway clearance |
| Hayes & Savage (2008) | QL | UK | Examine fathers’ perspectives on the emotional impact of managing the care of their children with CF |
8 fathers | 1.5–6 | Individual interviews | NS |
| Hobbs et al., (2003) | QN | US | Examine mothers’ attributions related to their children’s compliance with various components of medical treatment for CF |
27 mothers | 2–18 | Medical and Nonmedical Compliance Questionnaire (MNCQ), CF Parent Attribution Questionnaire (CF-PAQ) |
Airway clearance, nebulized medications , enzymes, nutrition |
| Hodgkinson & Lester (2002) | QL | UK | Explore current stresses and coping strategies used by mothers, identify roles and strategies that nursing professionals could extend or adopt to support them and families of children with CF |
17 mothers | 2–13 | Semistructured individual interviews |
NS |
| Modi et al. (2008) | QN | US | Evaluate relationship between patient- reported parental supervision and adherence |
103 adolescents and preadolescent |
10–17; × = 13.4 |
Daily phone diary, electronic monitors, prescribed treatment plan, pulmonary function tests |
Nebulized medications |
| Slatter et al. (2004) | QL | UK | Examine parents’ medication-related roles and problems |
15 mothers; 2 fathers |
2–12 | Semistructured individual interviews |
Medication |
| White et al. (2009) | QN | US | Investigate the association between psychopathology and treatment adherence in children and adolescents with CF |
53 child and primary caregivers |
9–17 | Shwachman Clinical Evaluation, Confidential Cystic Fibrosis Management Profile (CCFMP), Computer- Aided Diagnostic Interview Schedule for Children (C- DISC), Family Adaptability and Cohesion Evaluation Scales, Version II (FACES II), |
Airway clearance, nutrition, enzymes, antibiotics, nebulized medication |
| Williams et al. (2007a) | QL | UK | (1) What are the roles of family members in the initiation and implementation of home exercises? (2) How is the responsibility for physiotherapy exercises transferred from the parent or family to the child, and what factors help this process? |
32 children; 31 parents |
7–17 | Individual interviews | Airway clearance |
| Williams et al. (2007b) | QL | UK | Explore the experiences and strategies used by children and parents to adhere to routine home physiotherapy and to generate potential lessons for pediatric adherence in other clinical areas |
32 children; 31 parents |
7–17 | Individual interviews | Airway clearance |
Note. QL= Report of qualitative research; QN= report of quantitative research; RCT= report of randomized controlled trial; NS = not specified.
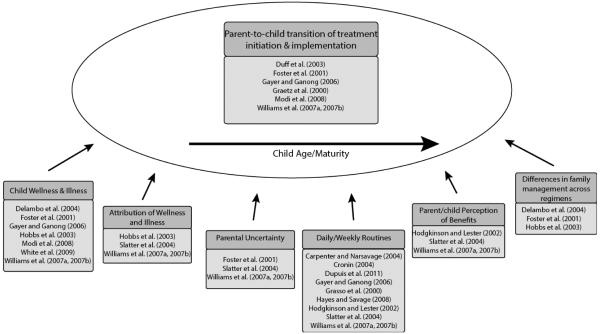
Figure 3 provides an overview of how findings were grouped within components of the theory. The results of our research synthesis confirm and extend the Williams et al. (2007a, 2007b) grounded theory. We were able easily to place findings within the theory components, indicating fit between theory and empirical data.
Figure 3.
Findings grouped by components of the Williams et al. theory.
Parent–Child Transitions in Management as the Child Ages
Williams et al. (2007a, 2007b) described how responsibility for chest physiotherapy (airway clearance) transitions from parent to child as the child ages. When children are young, parents both initiate and implement physiotherapy. Although the child’s role is that of recipient, he or she may not be a passive recipient. Findings indicate that administering physiotherapy was particularly problematic for parents of toddlers who were difficult to reason with, threw tantrums, and often refused to lie still. As the child ages, parents may continue to initiate the therapy, for example, by reminding the child to do it, but they will begin to transfer responsibility for implementation to the child by initially supervising the child’s implementation. The transition is complete when children administer therapy independently and take full responsibility for both initiating and implementing treatment.
Reports from five other studies in addition to those by Williams et al. (2007a, 2007b) contain findings pertaining to the transition in responsibility from parent to child as the child aged and the transition’s effects on adherence. In three of these reports, researchers found that parents had greater responsibility for treatment regimens when children were younger and that this resulted in greater adherence (Foster et al., 2001; Gayer & Ganong, 2006; Modi, Marciel, Slater, Drotar, & Quittner, 2008). Modi et al. (2008) specifically assessed the relationship between parental supervision and adherence to nebulized medications in a study of 103 adolescents and preadolescents and found that parents provided less supervision to older adolescents and that youth who spent more of their treatment time supervised by parents, particularly mothers, showed better adherence. Foster et al. (2001) provided further explanation for how the transition of roles over time may affect adherence. In interviews with eight children between the ages of 10 and 18 and their parents, they found that parents generally intervened when younger (as compared to older) children did not adhere by requiring children to perform treatments. Older (as compared to younger) children had greater independence in deciding whether to perform treatments, which generally led to lower adherence, especially with time-consuming treatments (i.e., nebulized medications and airway clearance).
In three studies, researchers addressed also the nature of parent–child interactions related to adherence in children at different stages of development. Their findings suggest that levels of conflict may vary across developmental stages and type of treatment. In a study of 108 parents, Duff, Wolfe, Dickson, Conway, and Brownlee (2003) found that children’s mealtime behaviors (i.e., managing dietary intake) were significantly more disruptive when they were between the ages of 5 and 8 than for either younger or older children. Parents also showed a higher frequency of inappropriate responses (e.g., getting frustrated or coaxing them to take bites) to their 5- to 8-year-old children during mealtimes than with their older (but not younger) children. In two additional studies, researchers found that conflict increased during the adolescent as compared to preadolescent years. In a study of 35 youth between the ages of 11 and 18, Graetz, Shute, and Sawyer (2000) found that younger children rated their family’s supportive behaviors more highly than older youth. In a study of 10- to 18-year-olds, Foster et al. (2001) found that parents reported increased difficulty and conflict trying to enforce treatment as their children grew older.
Child’s Wellness and Illness
The Williams et al. (2007a, 2007b) theory posits that changes in the child’s health influence the transition of responsibility for treatments from parent to child. As symptom severity increases, parents experience anxiety about allowing children to self-administer physiotherapy, which in turn leads to parents and children renegotiating management roles.
A report from one other study addressed findings related to the influence of the child’s health on the parent–child transition and reports from five other studies addressed the relationship of the child’s health to adherence. The Foster et al. (2001) study provides further support for the Williams et al. (2007a, 2007b) grounded theory and its finding that parents tended to help their older children with physiotherapy when the child’s illness severity was worse (e.g., when they had a chest infection).
Illness severity can both affect and be affected by adherence, and the effects of severity appear to vary across treatment regimens and possibly family structures. In a survey study of 53 children and their caregivers, White, Miller, Smith, and McMahon (2009) found that lower (versus higher) levels of disease severity trended toward being associated with lower overall adherence. Similarly, in a study of 96 children and their parents, DeLambo, Ievers-Landis, Drotar, and Quittner (2004) found that greater disease severity was associated with mothers’ reports of greater adherence to airway clearance and aerosolized medications, but not with adherence to antibiotic, enzyme, or nutrition treatments. In several studies, researchers found the opposite relationship between disease severity and adherence, with greater adherence when disease severity was lower, and vice versa. In a study of 108 youth, Modi et al. (2008) found that less disease severity, as measured by forced expiratory volume (FEV1%, a measure of pulmonary function), predicted significantly higher adherence to the frequency of electronically monitored nebulized medications than did greater severity. In a study of 27 mothers, Hobbs et al. (2003) found that one of the reasons mothers gave for child adherence to medical tasks was that their child felt well enough. Finally, in a study of 318 mothers, Gayer and Ganong (2006) found that mothers living in first-marriage, two-parent households reported that significantly more treatments were done when children were healthier, whereas mothers living in single-parent households reported that significantly more treatments were done when the child’s health was perceived to be poorer.
Attribution of Illness and Wellness
One of the factors that Williams et al. (2007a, 2007b) identified as influencing the transition of physiotherapy from parent to child was the extent to which parents and children attributed the child’s health to physiotherapy. Williams et al. (2007b) found that parents’ motivation to overcome the problems they perceived and experienced with initiating and implementing home physiotherapy was influenced by their perceptions of physiotherapy’s effectiveness. Perceptions of effectiveness were, in turn, influenced by direct experience of benefits (i.e., size and timing of improvement, sputum production, experienced consequences of missed sessions), trust in health professionals, and understanding of the physiology underlying physiotherapy effects. Children relied on symptoms to evaluate physiotherapy, as compared to their parents, who relied on signs (e.g., coughing, energy level, general appearance, peak flow readings). Children with mild symptoms were often less certain of the benefits of physiotherapy by virtue of the mildness of their symptoms. For parents of children with CF with mild symptoms, their beliefs about the effectiveness of therapy were more dependent on trust in the provider than on signs indicating change.
Two other reports provide support for the role of attribution in explaining adherence. In interviews with 17 parents, Slatter, Francis, Smith, and Bush (2004) found that parents were more likely to report missing nebulized therapy and oral antibiotics than pancreatic enzymes, because they attributed flatulence and diarrhea in their child to nonadherence to the enzyme regimen, whereas they saw no ill effects resulting from variations in adherence to nebulized therapy or oral antibiotics. Slatter et al. (2004) further found that parents adhered to IV antibiotics despite the child’s pain and trauma because of their fears that nonadherence would lead to hospitalization. In a survey of 27 mothers, Hobbs et al. (2003) found that nearly 80% of mothers reported that the physical benefits to the child were one of the reasons they complied with medical treatments.
Parental Uncertainty About Child’s Physiotherapy Performance
The Williams et al. (2007a, 2007b) theory posits that parents’ uncertainty over the child’s performance of physiotherapy influenced their willingness to transfer responsibility to the child. Parents were uncertain about the child’s ability and/or willingness to carry out physiotherapy, although both parents and children felt that children were more effective at administering the physiotherapy and bringing up mucus than parents were. Uncertainty played a particularly large role when parents were still initiating, but no longer implementing, therapy, because they no longer had direct involvement in therapy and were unable to verify whether the child was performing physiotherapy effectively or doing it at all.
Two other studies offered findings related to parental uncertainty. Foster et al. (2001) reported that parents were concerned about whether the child would adhere and that these concerns increased as the child aged. Slatter et al. (2004) found that parents varied in their beliefs about when it would be appropriate to hand over therapy and were reluctant to make the transfer because of fear of deterioration in the child’s condition and practical problems regarding administration.
Daily and Weekly Family Routines
Williams et al. (2007a, 2007b) identified daily and weekly family routines as among the factors necessitating temporary shifts forward or backward along a continuum of shared responsibility. They highlighted the role of routine in establishing a normal life for the child with CF, other family members, and the family as a whole. Whether administered by parent or child, physiotherapy took time away from non-CF-related activities. Moreover, physiotherapy entailed practices (e.g., spitting) that children found embarrassing, thereby threatening the creation and maintenance of a nondifferent, or normal, identity. The maintenance of routines and normalcy was harder for children with more severe symptoms. Even when children were independently performing physiotherapy, they reported that overnights away from home were too much trouble because of the amount of required medication and equipment and because they were embarrassed to be seen doing physiotherapy.
Reports from eight other studies included findings related to daily and weekly routines. In four of these studies, researchers found that parents focused on the normal aspects of their children’s lives as a way of coping and remaining positive, and they worked hard to normalize the lives of children and to ensure family participation in typical activities (Carpenter & Narsavage, 2004; Dupuis, Duhamel, & Gendron, 2011; Hayes & Savage, 2008; Hodgkinson & Lester, 2002). Parents reported that managing CF dominated family life and impeded efforts to engage in leisure activities and affected their struggle to maintain a balance between attention directed to the child with CF and their siblings (Carpenter & Narsavage, 2004; Hodgkinson & Lester, 2002). In a study that included 22 mothers of children with CF, Cronin (2004) found that mothers reported that they felt more like good mothers and had a greater sense of personal control and well-being when their children were able to participate successfully in normal childhood activities such as school, worship, and parties. In two studies, an explicit link was made between routines and adherence; Slatter et al. (2004) found that parents’ difficulties accommodating medication regimens to everyday activities or special occasions contributed to deviations from the prescribed medication regimen. In a survey of 318 mothers, Gayer and Ganong (2006) found that single mothers reported that significantly more treatments were done when family behaviors were more routine, and Dupuis et al. (2011) reported the importance of routines to children.
Findings from one intervention study also support the importance of routine. Grasso, Button, Allison, and Sawyer (2000) found that one of parents’ central reasons for not using the intervention’s music tapes during physiotherapy intervention was that it interfered with their established routine.
Parent and Child Perceptions of the Benefits of Transitioning Care
Williams et al. (2007a, 2007b) found that children viewed the benefits of transfer as including increased social status and independence, reduced parental scrutiny of their behavior, reduced burden to parents and siblings, and a general increase in the amount of time for and flexibility in their social lives. Parents perceived the transfer of responsibility as increasing the child’s independence, which allowed the child to engage in social activities away from home and adopt more normal roles. Independence allowed a more mature identity for the child and therefore an increased sense of responsibility for and thus adherence to therapy. Parents also appreciated the respite from the demands, intensity, and stress of the parental role in enforcing therapy.
Two additional reports provide support for the role of parental perception of benefits. Hodgkinson and Lester (2002) found that parents hoped their children would take responsibility for their own health and would become socially and financially independent. Slatter et al. (2004) reported that parents recognized that transitioning responsibility for medication to their child increased the child’s independence and opportunities to engage in social activities.
Differences in Family Adherence Across Treatment Regimens
In three reports, researchers compared differences in family adherence across treatment regimens (DeLambo et al., 2004; Foster et al., 2001; Hobbs et al., 2003). Findings from these three reports suggest that families may play a larger role in adherence to airway clearance and nebulized medications than to other treatment regimens. In a survey study of 96 children and their parents, DeLambo et al. (2004) found that the quality of the family relationship was associated with greater adherence to airway clearance and nebulized medications, but not adherence to antibiotics, enzymes, or nutrition. In a study reporting the findings from interviews with eight children and their parents and siblings, Foster et al. (2001) found that parents may forget younger children’s antibiotics and enzymes, but not their airway-clearance treatments. Time pressures, however, led parents sometimes intentionally to skip airway-clearance treatments and nebulized medications, especially if the family included younger siblings. In Hobbs et al.’s (2003) survey study of 27 mothers of children aged 2–18, mothers reported that they had greater control over whether children adhered to airway clearance and nebulized or oral medications and less control over adherence to calorie intake. Mothers also reported that the reasons for adherence to calorie intake were significantly more variable than for medication compliance.
Discussion
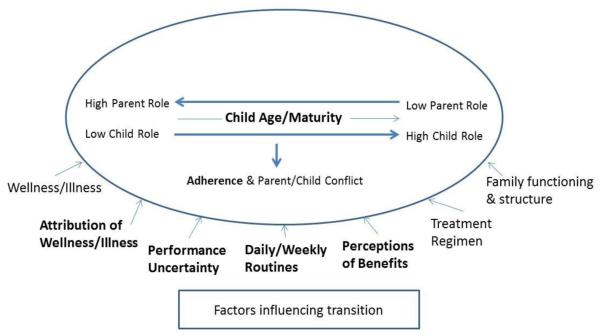
The results of the literature synthesis largely confirm and extend the grounded theory proposed by Williams et al. (2007a, 2007b). Figure 4 presents a refinement of the theory constituting our synthesis of the findings of the 17 reports of 16 studies addressing the transition of CF management from parent to child and on factors related to adherence across treatments and over time. In Figure 4, the components of the revised theory that were supported by the review are in bold; support is defined as consistent findings across two or more “non-noisy” studies. Noisy studies were included in the review because even studies with methodological limitations contribute to the development of beginning theories, such as the one described here (Pawson, Greenhalgh, Harvey, & Walshe, 2005).
Figure 4.
Revised theory of parent-to-child transition of CF condition management.
Note. Boldface items were supported by the literature review.
The revision shown in Figure 4 expands the Williams et al. (2007a, 2007b) theory to include factors that influence not only the parent–child transition of care management but also adherence and parent–child conflict. Three studies (one of which was methodologically noisy) provide support for the relationship between the parent–child role transition and adherence. The larger role of parents when children are younger promotes adherence, as compared to when parents shift responsibility to their maturing child and adherence levels drop. Support for the relationship between role transition and parent–child conflict was less strong, with two noisy studies among the four that provided support and all four studies addressing conflict at different developmental stages.
The revised theory retains all of the factors that Williams et al. (2007a, 2007b) described as influencing the role transition and adds three new ones: perceptions of benefits, treatment regimen, and family functioning and structure. To increase the model’s clarity, we combined wellness and illness into one factor and attribution of wellness and illness into another. Findings from seven studies support the role of wellness or illness as an influencing factor, and two of the studies (one coded as noisy) supported its influence on the parent–child role transition. Five of the studies supported the influence of wellness or illness on adherence, but the nature of its influence varied in opposite directions, with adherence found to be both more and less when children’s symptoms were less severe. The relationship between the child’s symptoms and adherence is complex. Higher levels of adherence to some treatments may reduce some symptoms, whereas more severe symptoms may either motivate or serve as a barrier to adherence. For example, a parent may be more lenient about performing all prescribed physiotherapy sessions when the child feels unwell. Additional research is needed to better understand when and how the child’s wellness or illness status influences adherence. Findings from three studies (one noisy) support a consistent role for both attribution and performance uncertainty as influencing factors. Nine studies (three noisy) provide support for the importance of daily or weekly routines.
Three influencing factors were added to the theory. Three studies (one noisy) supported perception of benefits, referred to in Williams et al. (2007a, 2007b) but not displayed in their model. Treatment regimen was added because findings from three studies (two noisy) suggested that differences in regimen are one factor influencing both role transition and adherence; Williams et al. (2007a, 2007b) addressed only physiotherapy. Findings from one study each suggest that family functioning (DeLambo et al., 2004) and structure (Gayer & Ganong, 2006) may be influencing factors. Of note, other factors are known to influence family management of conditions, such as financial resources and social networks (Knafl, Deatrick, & Havill, 2012). Although these factors were not identified in our review, they merit additional research.
The refined theory’s key elements are congruent with other conceptualizations of family management of a child with chronic illness. For example, in a study of youth aged 8–19 years with type 1 diabetes and their parents, Schilling et al. (2006) identified three patterns of youth self-management (parent dominant, transitional, and adolescent dominant) that reflect changes in the division of labor and responsibility and the level and focus of conflict between parent and child. Beacham and Deatrick’s (2013) model of health-care autonomy in children posits that the child’s age (developmental level) and maturity (physical, cognitive, psychosocial) shape the parent–child interaction that in turn influences family management of the illness. Although not focused on transfer of responsibility, the family management style framework (Knafl et al., 2012) includes management routine as a key factor shaping family life in the context of childhood chronic conditions, and thus is consistent with the daily or weekly routines factor identified in the current synthesis. These studies provide support for the current framework but also point to the importance of situating the transition process in the broader context of overall family life. Thus, what we found potentially transferable to any treatment regimen—whether for CF or any other child-to-adult chronic condition—were the concepts and propositions regarding transitions. Indeed, that turned out to be the case with additional refinements to the theory. We plan on using our revised theory to synthesize findings about parent-to-child transitions in other chronic conditions in our data set.
As with all research synthesis studies, the present study was constrained by the amount and quality of research conducted on life in families with children with CF. Relatively few studies have been conducted on the parent-to-child transition of CF management or the effects of this transition on adherence. Six of the 16 studies included in our review had features that potentially undermine the validity of their findings, and seven provided limited detail on the treatment regimens being studied. Despite these limitations, the revised framework suggests the practice relevance of situation-specific theories (Im & Meleis, 1999) and the utility of using existing frameworks as a means to synthesize findings across studies (Sandelowski & Barroso, 2007). The revised framework presented here offers a more refined understanding of the problems entailed in parent-to-child transitions in CF condition management and the influencing factors that might be targeted in interventions designed to improve condition management and child and family well-being across chronic physical conditions. Although we were unable to synthesize findings on conflict related to the transition because the age of children, and the focus of research was too varied across studies, more research needs to be done in this area. Further research also is needed to develop this theory, particularly on (a) differences in the parent-to-child transition of management across different treatment regimens, (b) the effect of the child’s health on adherence, and (c) the role of family functioning and structure in the transition of care.
Acknowledgments
This article was supported by the National Institute of Nursing Research and National Institutes of Health under award number R01 NR012445: “ Mixed-Methods Synthesis of Research on Childhood Chronic Conditions and Family.”
References
- Abbott J, Havermans T, Hart A. Adherence to the medical regimen: Clinical implications of new findings. Current Opinion in Pulmonary Medicine. 2009;15(6):597–603. doi: 10.1097/MCP.0b013e3283310859. doi: 10.1097/MCP.0b013e3283310859. [DOI] [PubMed] [Google Scholar]
- Beacham B, Deatrick J. Health care autonomy in children with chronic conditions: Implications for self-care and family management. Nursing Clinics of North America. 2013;48(2):305–317. doi: 10.1016/j.cnur.2013.01.010. http://dx.doi.org/10.1016/j.cnur.2013.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter DR, Narsavage GL. One breath at a time: Living with cystic fibrosis. Journal of Pediatric Nursing. 2004;19(1):25–32. doi: 10.1016/j.pedn.2003.09.004. * doi: 10.1016/j.pedn.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Cronin AF. Mothering a child with hidden impairments. American Journal of Occupational Therapy. 2004;58(1):83–92. doi: 10.5014/ajot.58.1.83. * doi: 10.5014/ajot.58.1.83. [DOI] [PubMed] [Google Scholar]
- Cystic Fibrosis Foundation About CF. 2014 Retrieved from http://www.cff.org/aboutcf/
- DeLambo KE, Ievers-Landis CE, Drotar D, Quittner AL. Association of observed family relationship quality and problem-solving skills with treatment adherence in older children and adolescents with cystic fibrosis. Journal of Pediatric Psychology. 2004;29(5):343–353. doi: 10.1093/jpepsy/jsh038. * doi: 10.1093/jpepsy/jsh038. [DOI] [PubMed] [Google Scholar]
- Duff AJ, Wolfe SP, Dickson C, Conway SP, Brownlee KG. Feeding behavior problems in children with cystic fibrosis in the UK: Prevalence and comparison with healthy controls. Journal of Pediatric Gastroenterology and Nutrition. 2003;36(4):443–447. doi: 10.1097/00005176-200304000-00004. * doi: 10.1097/00005176-200304000-00004. [DOI] [PubMed] [Google Scholar]
- Dupuis F, Duhamel F, Gendron S. Transitioning care of an adolescent with cystic fibrosis: Development of systemic hypothesis between parents, adolescents, and health care professionals. Journal of Family Nursing. 2011;17(3):291–311. doi: 10.1177/1074840711414907. * doi: 10.1177/1074840711414907. [DOI] [PubMed] [Google Scholar]
- Edwards A, Elwyn G, Hood K, Rollnick S. Judging the “weight of evidence” in systematic reviews: Introducing rigour into the qualitative overview stage by assessing Signal and Noise. Journal of Evaluation in Clinical Practice. 2000;6(2):177–84. doi: 10.1046/j.1365-2753.2000.00212.x. doi: 10.1046/j.1365-2753.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- Ernst MM, Johnson MC, Stark LJ. Developmental and psychosocial issues in cystic fibrosis. Pediatric Clinics of North America. 2010;58(4):865–85. doi: 10.1016/j.pcl.2011.06.004. doi: 10.1016/j.pcl.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Foster C, Eiser C, Oades P, Sheldon C, Tripp J, Goldman P, Trott J. Treatment demands and differential treatment of patients with cystic fibrosis and their siblings: Patient, parent and sibling. Child: Care, Health and Development. 2001;27(4):349–364. doi: 10.1046/j.1365-2214.2001.00196.x. * doi: 10.1046/j.1365-2214.2001.00196. [DOI] [PubMed] [Google Scholar]
- Gayer D, Ganong L. Family structure and mothers’ caregiving of children with cystic fibrosis. Journal of Family Nursing. 2006;12(4):390–412. doi: 10.1177/1074840706294510. * doi: 10.1177/1074840706294510. [DOI] [PubMed] [Google Scholar]
- Glasscoe CA, Quittner AL. Psychological interventions for people with cystic fibrosis and their families. Cochrane Database Systematic Reviews. 2008 doi: 10.1002/14651858.CD003148.pub2. (3): art. CD003148. doi: 10.1002/14651858.CD003148.pub2. [DOI] [PubMed] [Google Scholar]
- Graetz BW, Shute RH, Sawyer MG. An Australian study of adolescents with cystic fibrosis: Perceived supportive and nonsupportive behaviors from families and friends and psychological adjustment. Journal of Adolescent Health. 2000;26(1):64–69. doi: 10.1016/s1054-139x(99)00026-9. * doi: 10.1016/S1054-139X(99)00026-9. [DOI] [PubMed] [Google Scholar]
- Grasso MC, Button BM, Allison DJ, Sawyer SM. Benefits of music therapy as an adjunct to chest physiotherapy in infants and toddlers with cystic fibrosis. Pediatric Pulmonology. 2000;29(5):371–381. doi: 10.1002/(sici)1099-0496(200005)29:5<371::aid-ppul6>3.0.co;2-k. * doi: 10.1002/(SICI)1099-0496(200005)29:5<371::AID-PPUL6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Havill NL, Leeman J, Shaw-Kokot J, Knafl K, Crandell J, Sandelowski M. Managing large-volume literature searches in research synthesis studies. Nursing Outlook. 2013 doi: 10.1016/j.outlook.2013.11.002. doi: S0029-6554(13)00229-7 [pii]10.1016/j.outlook.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes CC, Savage E. Fathers’ perspectives on the emotional impact of managing the care of their children with cystic fibrosis. Journal of Pediatric Nursing. 2008;23(4):250–256. doi: 10.1016/j.pedn.2007.09.002. * doi: 10.1016/j.pedn.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions (Version 5.1.0) Cochrane Collaboration; Oxford, UK: Mar, 2011. Retrieved from http://www.cochrane-handbook.org. [Google Scholar]
- Hobbs SA, Schweitzer JB, Cohen LL, Hayes AL, Schoell C, Crain BK. Maternal attributions related to compliance with cystic fibrosis treatment. Journal of Clinical Psychology in Medical Settings. 2003;10(4):273–277. * doi: 10.1023/A:1026349303930. [Google Scholar]
- Hodgkinson R, Lester H. Stresses and coping strategies of mothers living with a child with cystic fibrosis: Implications for nursing professionals. Journal of Advanced Nursing. 2002;39(4):377–383. doi: 10.1046/j.1365-2648.2002.02299.x. * [DOI] [PubMed] [Google Scholar]
- Hsieh H, Shannon S. Three approaches to qualitative content analysis. Qualitative Health Research. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- Im E-O, Meleis A. Situation-specific theories: Philosophical roots, properties, and approach. Advances in Nursing Science. 1999;22(2):11–24. doi: 10.1097/00012272-199912000-00003. [DOI] [PubMed] [Google Scholar]
- Knafl K, Deatrick J, Havill N. Continued development of the family management style framework. Journal of Family Nursing. 2012;18(1):11–34. doi: 10.1177/1074840711427294. doi: 10.1177/1074840711427294. [DOI] [PubMed] [Google Scholar]
- Knafl K, Leeman J, Havill N, Crandell JL, Sandelowski M. Delimiting family in syntheses of research on childhood chronic conditions and family life. Manuscript submitted for publication. 2014 doi: 10.1111/famp.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchford G, Duff A, Quinn J, Conway S, Conner M. Adherence to nebulised antibiotics in cystic fibrosis. Patient Education and Counseling. 2009;75(1):141–144. doi: 10.1016/j.pec.2008.08.027. doi: 10.1016/j.pec.2008.08.027S0738-3991(08)00478-3 [pii] [DOI] [PubMed] [Google Scholar]
- Modi AC, Cassedy AE, Quittner AL, Accurso F, Sontag M, Koenig JM, Ittenbach RF. Trajectories of adherence to airway clearance therapy for patients with cystic fibrosis. Journal of Pediatric Psychology. 2010;35(9):1028–1037. doi: 10.1093/jpepsy/jsq015. doi: 10.1093/jpepsy/jsq015jsq015 [pii] [DOI] [PubMed] [Google Scholar]
- Modi AC, Marciel KK, Slater SK, Drotar D, Quittner AL. The influence of parental supervision on medical adherence in adolescents with cystic fibrosis: Developmental shifts from pre to late adolescence. Children’s Health Care. 2008;37(1):78–92. * doi: 10.1080/02739610701766925. [Google Scholar]
- Mogayzel PJ, Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB, Marshall B. Cystic fibrosis pulmonary guidelines. American Journal of Respiratory and Critical Care Medicine. 2013;187(7):680–689. doi: 10.1164/rccm.201207-1160oe. [DOI] [PubMed] [Google Scholar]
- Pawson R. Digging for nuggets: How “bad” research can yield “good” evidence. International Journal of Social Research Methodology. 2006;9:127–142. [Google Scholar]
- Pawson R, Greenhalgh T, Harvey G, Walshe K. Realist review: A new method of systematic review designed for complex policy interventions. Journal of Health Services Research and Policy. 2005;10(suppl. 1):21–34. doi: 10.1258/1355819054308530. doi: 10.1258/1355819054308530. [DOI] [PubMed] [Google Scholar]
- Sandelowski M. In: Metasynthesis of qualitative research. APA handbook of research methods in psychology: Vol. 2. Research designs: Quantitative, qualitative, neuropsychological, and biological. Cooper H, editor. American Psychological Association; Washington, DC: 2012. pp. 19–36. [Google Scholar]
- Sandelowski M, Barroso J. Handbook for synthesizing qualitative research. Springer; New York, NY: 2007. [Google Scholar]
- Sandelowski M, Leeman J, Knafl K, Crandell JL. Text-in-context: A method for extracting findings in mixed-methods mixed research synthesis studies. Journal of Advanced Nursing. 2013;69:1428–1437. doi: 10.1111/jan.12000. doi: 10.1111/jan.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelowski M, Voils CI, Crandell J, Leeman J. Synthesizing qualitative and quantitative research findings. In: Beck CT, editor. Routledge international handbook of qualitative nursing research. Routledge; New York, NY: 2013. pp. 347–356. [Google Scholar]
- Sandelowski M, Voils CI, Leeman J, Crandell JL. Mapping the mixed research synthesis terrain. Journal of Mixed Methods Research. 2012;6(4):317–331. doi: 10.1177/1558689811427913. doi: 10.1177/1558689811427913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling L, Knafl K, Grey M. Changing patterns of self-management in youth with type 1 diabetes. Journal of Pediatric Nursing. 2006;21(6):412–423. doi: 10.1016/j.pedn.2006.01.034. doi: 10.1016/j.pedn.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Slatter A, Francis S-A, Smith F, Bush A. Supporting parents in managing drugs for children with cystic fibrosis. British Journal of Nursing. 2004;13(19):1135–1139. doi: 10.12968/bjon.2004.13.19.16318. * [DOI] [PubMed] [Google Scholar]
- STROBE Statement STROBE Statement: Strengthening the reporting of observational studies in epidemiology. 2007 doi: 10.2471/BLT.07.045120. Retrieved from http://www.strobe-statement.org/PDF%20hochladen/index.php?id = available-checklists. [DOI] [PMC free article] [PubMed]
- White T, Miller J, Smith GL, McMahon WM. Adherence and psychopathology in children and adolescents with cystic fibrosis. European Child & Adolescent Psychiatry. 2009;18(2):96–104. doi: 10.1007/s00787-008-0709-5. * doi: 10.1007/s00787-008-0709-5. [DOI] [PubMed] [Google Scholar]
- Williams B, Mukhopadhyay S, Dowell J, Coyle J. From child to adult: An exploration of shifting family roles and responsibilities in managing physiotherapy for cystic fibrosis. Social Science & Medicine. 2007a;65(10):2135–2146. doi: 10.1016/j.socscimed.2007.07.020. * doi: 10.1016/j.socscimed.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Williams B, Mukhopadhyay S, Dowell J, Coyle J. Problems and solutions: Accounts by parents and children of adhering to chest physiotherapy for cystic fibrosis. Disability and Rehabilitation. 2007b;29(14):1097–1105. doi: 10.1080/09638280600948060. * doi: 10.1080/09638280600948060. [DOI] [PubMed] [Google Scholar]