Abstract
The objective of this study was to explore a bioremediation strategy based on injecting NO3− to support the anoxic oxidation of ferrous iron (Fe(II)) and arsenite (As(III)) in the subsurface as a means to immobilize As in the form of arsenate (As(V)) adsorbed onto biogenic ferric (Fe(III)) (hydr)oxides. Continuous flow sand filled columns were used to simulate a natural anaerobic groundwater and sediment system with co-occurring As(III) and Fe(II) in the presence (SF1) or absence (SF2) of nitrate, respectively. During operation for 250 days, the average influent arsenic concentration of 567 µg l−1 was reduced to 10.6 (±9.6) µg l−1 in the effluent of column SF1. The cumulative removal of Fe(II) and As(III) in SF1 was 6.5–10-fold higher than that in SF2. Extraction and measurement of the mass of iron and arsenic immobilized on the sand packing of the columns was close to the iron and arsenic removed from the aqueous phase during column operation. The dominant speciation of the immobilized iron and arsenic was Fe(III) and As(V) in SF1, compared with Fe(II) and As(III) in SF2. The speciation was confirmed by XRD and XPS. The results indicate that microbial oxidation of As(III) and Fe(II) linked to denitrification resulted in the enhanced immobilization of aqueous arsenic in anaerobic environments by forming Fe(III) (hydr)oxides coated sands with adsorbed As(V).
Introduction
Although arsenic (As) has a relatively low abundance in the earth’s crust, it is generally found as a contaminant in soil and water systems due to various anthropogenic activities, such as mining, discharge of industrial waste and agriculture, as well as from natural biogeochemical reactions (1–3). Arsenic is a known human carcinogen (1), and its contamination of drinking water sources is presently a worldwide concern (3).
The predominant species of As found in surface water and groundwater are arsenite (As(III), H3AsO3) and arsenate (As(V), H2AsO4− and HAsO42−). In natural soil and sediments, iron (Fe) (hydr)oxides strongly sorb both As(III) and As(V) in circumneutral pH environments (4, 5). In anaerobic environments, microorganisms play an important role in the mobilization of adsorbed arsenic (6). Dissimilatory reductive dissolution of ferric (Fe(III)) (hydr)oxides can lead to release of adsorbed As into the aqueous phase (6–8). The microbial reduction of As(V) to As(III) also increases the mobility of As (9) due to the lower sorption strength of non-iron metal oxides such as aluminum (hydr)oxide (10, 11). Thus, in As contaminated sites, anoxic conditions leading to the microbial reduction of As(V) and Fe(III) could enhance the mobility of As, posing a threat of As contamination in drinking water (3, 7).
One strategy to suppress As mobilization in anaerobic zones is to promote the microbial re-oxidation of Fe(II) and As(III). The oxidation of Fe(II) results in the formation of Fe(III) (hydr)oxides (12, 13) that are known to strongly adsorb both As(III) and As(V) and remove them from the aqueous phase (4). It is well-established that aluminum oxides have a higher adsorption capacity for As(V) compared to As(III) (11, 14). Likwise As(V) is adsorbed better by various clay minerals compared to As(III) (14, 15). Therefore prior oxidation of As(III) to As(V) could potentially enhance the adsorption of arsenic onto clays and aluminum oxide components of sediments
Fe(II) is subject to both spontaneous chemical oxidation (12, 16) and microbial catalyzed oxidation (17) in the presence of dissolved O2 to form Fe(III) (hydr)oxides at circumneutral pH conditions. Abiotic oxidation of As(III) by O2 is slow under circumneutral conditions (18). On the other hand, As(III) is readily oxidized to As(V) by a large diversity of microorganisms under aerobic conditions (19, 20). In fact, the biological oxidation of Fe(II) and As(III) by aeration has been utilized to remediate As contaminated mine drainage water (21). The same strategy could be considered for in situ groundwater applications. However, O2 has limited aqueous solubility and may be significantly consumed by organic matter, sulfides and other reducing compounds in submerged sediments. These limitations merit exploration of other routes for the oxidation of As(III) and Fe(II) with alternative oxidants. In a field study, injection of nitrate into Bangladesh sediments was shown to reduce the mobility of arsenic (22).
Recent evidence indicate that biological nitrate-dependent As(III) oxidation (23–25) as well as Fe(II) oxidation (26, 27) is catalyzed by diverse microorganisms in the absence of O2. Anoxic biological oxidation of As(III) by denitrifying microorganisms is now known to occur by several strains including bacteria isolated from a salt lake with naturally high levels (23, 28) and from As-polluted sediments (24). Natural mixed cultures from anaerobic sludges and pond sediments with no prior exposure to As were also shown to readily oxidize As(III) to As(V) linked to denitrification (25). Two pure cultures were isolated from enrichments originating from the pond sediments (29).
Microbial oxidation of both soluble and insoluble Fe(II) coupled to nitrate reduction has been demonstrated in various freshwater and saline environmental systems at neutral pH (27, 30). The biological oxidation of Fe(II) results in the formation of insoluble Fe(III) (hydr)oxide minerals in anoxic soils and sediments, such as ferrihydrite and other forms of iron oxides (31). These biogenic iron oxides have the potential to adsorb arsenic.
The objective of this study was to evaluate the anoxic oxidation of Fe(II) and As(III) linked to denitrification as a means of immobilizing As in the form of As(V) adsorbed on biogenic Fe(III) (hydr)oxides. This study was performed in continuous flow columns packed with sand with the goal of demonstrating the potential of utilizing nitrate injection as a bioremediation approach for removing aqueous As from anoxic groundwater contaminated with co-occurring Fe(II) and As(III).
Materials and Methods
Attenuation of As(III) and Fe(II) in denitrifying sand packed bed columns
Anoxic As(III) and Fe(II) oxidation under denitrifying conditions was investigated in two glass sand packed bed columns (each 420 ml) continuously fed with synthetic basal medium. The columns were placed in a climate controlled room at 30 ± 2°C and covered with aluminum foil to avoid growth of phototrophic microorganisms. Each reactor was packed with 600 g dry weight of white quartz sand (SiO2; 50–70 mesh) and inoculated with 2.94 g VSS l−1 anaerobic sludge obtained from the laboratory-scale As(III)-oxidizing denitrifying bioreactor. The columns were packed with increments of dry sand and after each increment, the sludge was mixed in. The treatment “sand filled” column (SF1) was the biologically active column inoculated with chemolithotrophic As(III)-oxidizing denitrifying bacteria and fed with basal medium, As(III) (0.5 mg l−1) and Fe(II) (20 mg l−1 Fe supplied as FeCl2) as electron donating substrates, nitrate (155 mg l−1 NO3− supplied as KNO3) as the electron acceptor and bicarbonate as the major carbon source, except that 18.8 mg l−1 acetate was added to support microbial consumption of any traces of dissolved oxygen. The control “sand filled” column (SF2) was the same as SF1, but lacked nitrate in the medium. The influent of both reactors was maintained at all times under a N2 atmosphere supplied via a gas bag (SKC-West Inc, Fullerton, CA) to prevent any exposure to O2.
The columns were operated with hydraulic retention times averaging 24 h for the whole experiment. Fresh liquid samples were collected periodically from the influent and effluent lines and prepared immediately for analysis to minimize possible changes in As speciation upon exposure to the atmosphere. The pH value was determined immediately after sampling.
Iron and arsenic extraction from iron coated sands
Iron coated sand (ICS) samples collected at the end of the column experiments were analyzed for Fe and As. The wet ICS samples were flushed under N2 in anaerobic bottles, and dried overnight at room temperature. Dry ICS samples for analysis of Fe were extracted in 6.0 N HCl under an N2 atmosphere in 30-ml anaerobic glass tubes with butyl rubber stoppers. All tubes were incubated overnight at 30 ± 2°C in a shaking water bath (100 rpm). The Fe(II) and total Fe in extracts were analyzed using the phenanthroline method described in the supplemental information. Dry ICS samples collected for analysis of As were extracted in 20 ml 6.0 N HNO3 under an N2 atmosphere in 30-ml anaerobic glass tubes with butyl rubber stoppers. All tubes were incubated at 30 ± 2°C in a shaking water bath (100 rpm) for overnight. The tubes were allowed to settle, samples of the supernatants were collected and they were adjusted immediately to pH 6.0–6.5 with NaOH (8.0 N). Subsequently, the samples were membrane filtered (0.45 μm) and stored in polypropylene vials at −20°C until analysis. A simple assay was set up to confirm the preservation of As speciation during the extraction process. The results (Table S1) demonstrated that the HNO3 did not cause major changes in the As speciation during the overnight incubation.
Results
Microbial nitrate-dependent oxidation of Fe(II) in the denitrifying sand packed bed columns
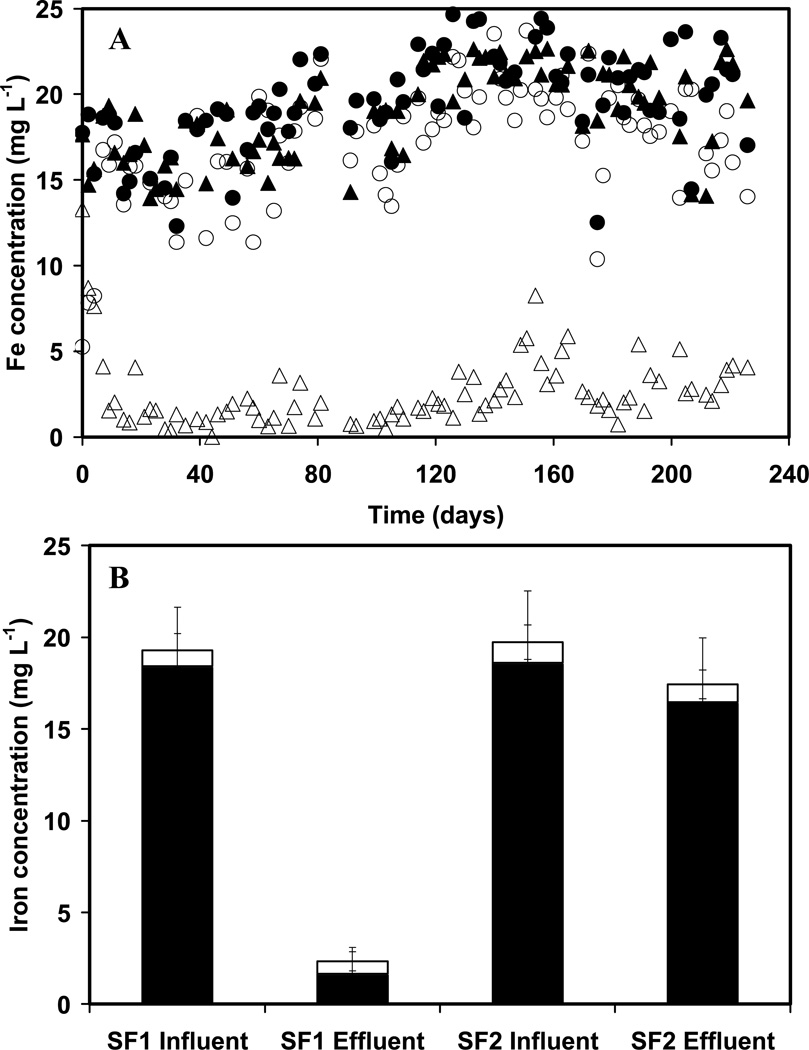
The time-course of the influent and effluent total Fe concentrations is illustrated in Figure 1A for columns SF1 and SF2. Initially in the SF1 column, Fe removal improved over the first 12 days. However, after day 12, steady state conditions were achieved and the removal averaged 91.3 (± 6.9)% for the remainder of the experiment. In contrast, the removal of Fe averaged only 14.1 (± 8.3)% from day 2 onwards in the control column lacking nitrate (SF2).The results indicate that the removal of soluble iron was 6.5-fold greater in SF1 compared to SF2, which is in accordance with the expectation that Fe(II) was oxidized and retained as Fe(III) (hydr)oxides in the SF1 column due to the presence of nitrate. The results of Fe in acidified samples were similar to those of soluble Fe and therefore they are not shown.
Figure 1.
Panel A, concentrations of soluble total Fe in the influent and effluent of biological column SF1 as a function of time: Column SF1 (fed with 360 μM Fe(II), 6.67 μM As(III) and 2.5 mM nitrate): (▲) influent, (Δ) effluent; Column SF2 (fed with 360 μM Fe(II) and 6.67 μM As(III) without nitrate): (●) influent, (○) effluent; panel B, iron speciation in the influent and effluent of sand packed columns SFF1 or SF2: Fe(II) (solid bars) and Fe(III) (empty bars).
Figure 1B illustrates the average soluble speciation of Fe in the influent and effluent of columns SF1 and SF2 during the steady state period of operation. In SF1, the soluble Fe(III) concentration is low in both the influent and effluent, thus the Fe(III) formed most likely accumulated as insoluble Fe(III) (hydr)oxides inside the column. However, in column SF2, the influent and effluent were dominated by Fe(II), with more than 94.4 (± 4.4)% of Fe(II) entering SF2 passing through the column.
In order to demonstrate the capacity of microorganisms in SF1 to couple the oxidation of Fe(II) to denitrification, an assay was set up using column effluent as inoculum. The results shown in Figure S2A confirm that SF1 effluent has microbial activity linking Fe(II) oxidation to denitrification. The biological removal of Fe(II) was associated with the formation of Fe(III) as detected in suspended acidified samples (Figure S2B). There was no obvious Fe(II) oxidation in the abiotic control, nor in the biologically active control lacking NO3−.
Microbial nitrate-dependent oxidation of As(III) in the denitrifying sand packed bed columns
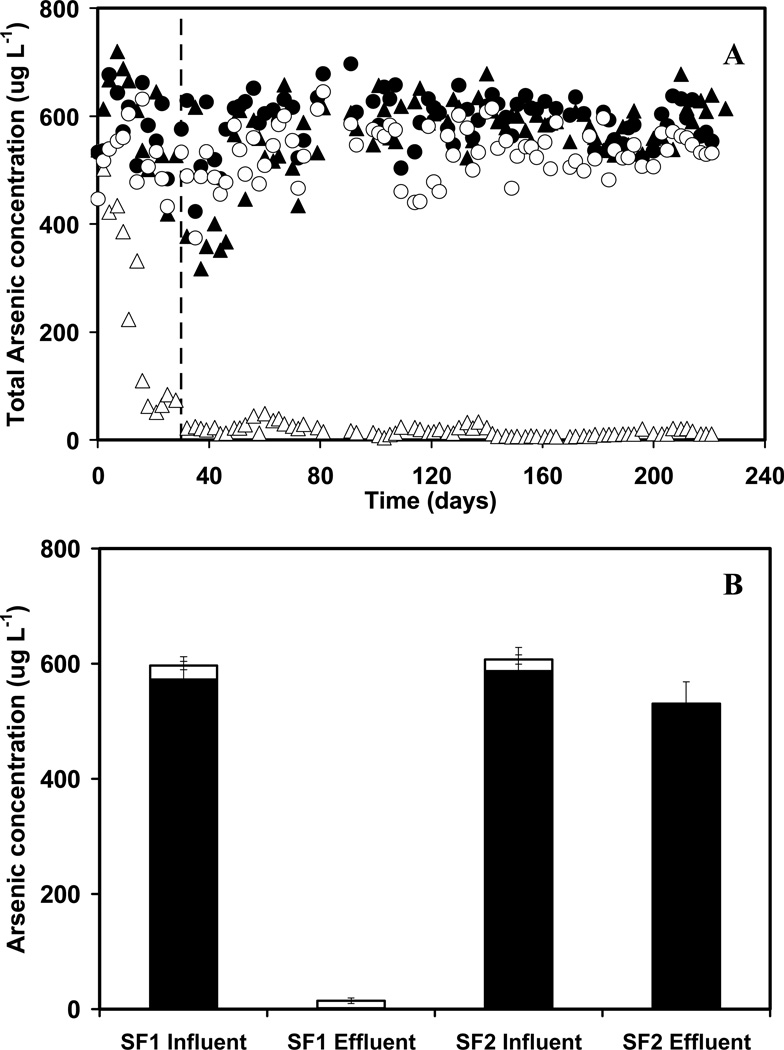
The time-course of the influent and effluent total soluble As concentrations from the two columns is illustrated in Figure 2A. The result shows that the release of soluble As was greater in SF2 compared to SF1, which is in accordance with the expected adsorption of As on the Fe(III) (hydr)oxides formed from anoxic Fe(II) oxidation. From day 16 to 30, the total As concentration passing through column SF1 started to gradually decrease. After 30 days, the column stabilized with a high removal of As. From day 30 onwards, the effluent total As concentration averaged 10.6 (±9.6) µg l−1, which corresponded to an average removal efficiency of 97.2 (±3.3)%. In SF2, only 10.2 (±6.1)% of the As was removed with the majority of the As passing through column unabsorbed.
Figure 2.
Panel A, removal of soluble total As in two sand packed columns fed with a mineral medium containing 6.67 μM As(III) and 360 μM Fe(II). Column SF1 (fed with 2.5 mM nitrate): (▲) influent, (Δ) effluent; Column SF2 (without nitrate): (●) influent, (○) effluent. The dashed line indicates the day when the steady state operation was achieved; panel B, arsenic speciation in the influent and effluent of sand packed columns starting from day 30 when steady state was achieved: As(III) (solid bars) and As(V) (empty bars).
Figure 2B illustrates the average soluble As species in the influent and effluent of columns SF1 and SF2 during the steady state period of operation from day 30 onwards. The results show that 99.7% As(III) was eliminated from column SF1, and it was not recovered as soluble As(V) in the effluent. Therefore, the As removed was most likely adsorbed inside the column. As(V) was the dominant species (89.5% of As) of the small fraction of As that was discharged in the effluent of SF1. In contrast, As(III) removal was marginal (9.7%) in the control column lacking nitrate (SF2). The results suggest that no adsorption had occurred, which was consistent with the low retention of Fe in the column. As(III) was the prevalent form of As in the effluent of SF2 (99.8% of As) (Figure 2B). These findings indicate that no significant oxidation of As(III) had occurred in SF2.
Residual iron and arsenic in the sand bed at the end of reactor operation
At the end of the column experiment, iron and As retained on the ICS were extracted to determine the solid-phase iron and As retained in the column packing. The extracted As and iron from the column packing were compared with the quantity of these elements cumulatively retained in the column as estimated from the differences between the influent and effluent As (As species and total As) and iron (total Fe) to make the mass balances. The recovery, calculated as the ratio of extracted to retained As or Fe, for both columns is shown in Table 1.
Table 1.
Mass balance of arsenic and iron at the end of the experiment.
| Parameter | Column* | Retained in the column‡ Influent-Effluent (mg) |
Extracted from column packing (mg) |
Recovery† (%) |
|---|---|---|---|---|
| Sum of species of As | SF1 | 45.16 | 49.65 | 109.9 |
| SF2 | 6.4 | 6.24 | 97.5 | |
| Total iron | SF1 | 1290.3 | 1501.5 | 116.4 |
| SF2 | 181.4 | 210.3 | 115.9 | |
SF1: Column fed with nitrate (2.5 mM), Fe(II) (0.36 mM) and As(III) (6.67 μM). SF2: Column fed with Fe(II) (0.36 mM) and As(III) (6.67 μM) only.
Recovery was calculated from the mass ratio of extracted to retained.
Calculated from cumulative difference between influent and effluent over 220 d of column operation.
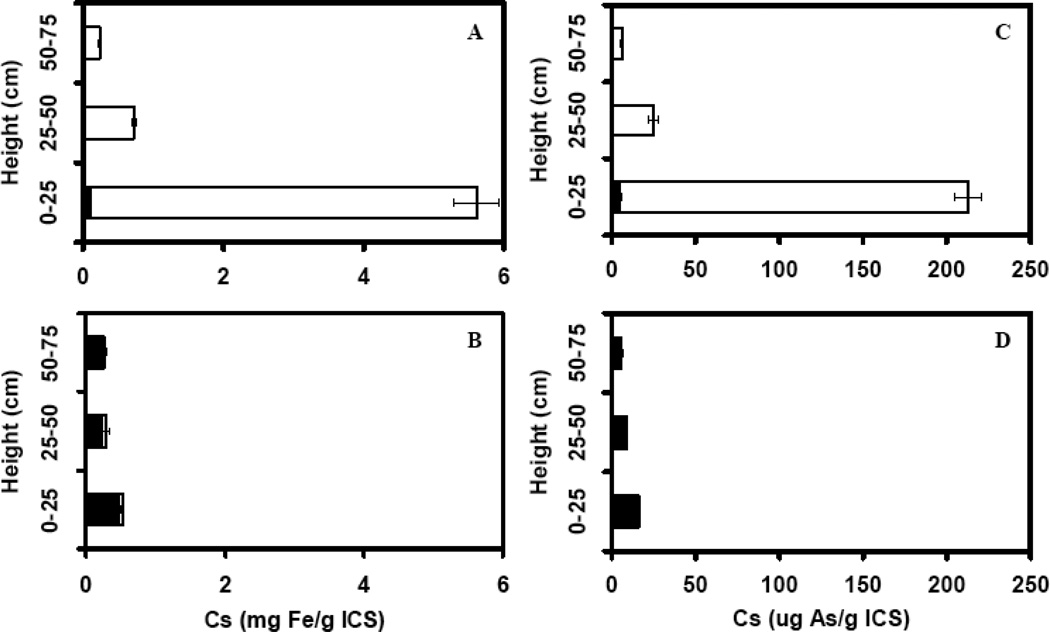
The total iron extracted from the sand bed was approximately the same as the amount of iron retained in column SF1 and SF2. The results indicate that the iron removed could be accounted for by extractable iron in the sand bed. The small discrepancy between the values can be attributed to experimental errors. The quantity of Fe extracted from the sand bed of the SF1 column was 7-fold greater than that from the SF2 column, corresponding to a greater retention of Fe in SF1. The speciation and profile of the extracted iron in the two columns is shown in Figure 3. The iron in column SF1 was predominantly present in the Fe(III) form indicating precipitation of Fe(III) (hydr)oxides in the sand bed. The Fe(III) was more concentrated at the base of the column where Fe(II) entered the column and became oxidized. In contrast, Fe(II) was the dominant species in the SF2 column. The iron was more uniformly immobilized at all heights inside the column SF2.
Figure 3.
The profile of sorbed iron and As on the ICS in the two sands packed up-flow columns at the end of the continuous experiment. Panel A and C: column SF1 fed with with 6.67 μM As(III), 360 μM Fe(II) and 2.5 mM nitrate (column SF1), or panel B and D: only 6.67 μM As(III) and 360 μM Fe(II) (column SF2). Panel A and B, Fe(II): (solid bars); Fe(III): (empty bars). Panel C and D, As(III): (solid bars); As(V): (empty bars). The dry wt. mass of the sand bed at bottom, middle and top of columns SF1 and SF2 were 199.9, 195.1 and 211.7 g for SF1, and 197.6, 203.7 and 202.2 g for SF2, respectively.
The extracted As at the end of the experiment was accounted for by the As retained in columns SF1 and SF2, respectively (Table 1). The As extracted from the sand bed of SF1 was largely in the form of As(V) (Figure 3), indicating that it had been oxidized. The As(V) was more concentrated at the base corresponding to the iron profile. On the other hand, the As extracted from the sand bed of SF2 was predominantly in the As(III) form, indicating it was not oxidized. Also the As(III) was more uniformly distributed over the profile of column SF2.
Mineralogy of iron and arsenic on the surface of sand
The SEM was used to observe the surface characteristics of the sand before and after the column studies. The images of original sand (Figure S3A) showed very ordered silica crystals at the surface. The natural sand had a relatively uniform and smooth surface, with small cracks, microspores and light roughness on the sand surface. The surface of the sand in the control column, SF2, had a morphology similar to the original sand (Figure S3E). In contrast, the image obtained for ICS from SF1 (Figure S3C) has significantly rougher surfaces with more porous structure, and the original sand surfaces are not visible. Instead, bacteria and fresh precipitates are evident from the image. The precipitates appeared to be a mixture of amorphous and crystalline forms. The bacteria immobilized on the surface are rods and roughly 1.0–2.5 µm.
The EDS of the original sand reveals the presence of silica, oxygen and aluminum (Figure S3B). The EDS of the sand from SF2 is very similar but it additionally contains traces of sulfur and iron (Figure S3F). The EDS from the SF1 is radically different. It contains a clear peak for arsenic and bigger iron peak compared to SF2 as well as a defined phosphorus peak (Figure S3D). The silica peak is no longer dominant since it is diluted by the coating of iron (hydr)oxides.
The XRD analysis (Figure S4) of precipitates recovered at the end of the SF1 experiment revealed the presence of SiO2 (from the original sand), and a mixture of Fe(III) oxides dominated by crystalline hematite suggesting the formation of Fe(III) oxides due to nitrate-dependent oxidation of soluble Fe(II). On the other hand, the spectra demonstrated that the surface of SF2 was predominantly composed of SiO2 and a limited amount of Fe(II)-containing minerals such as siderite (FeCO3).
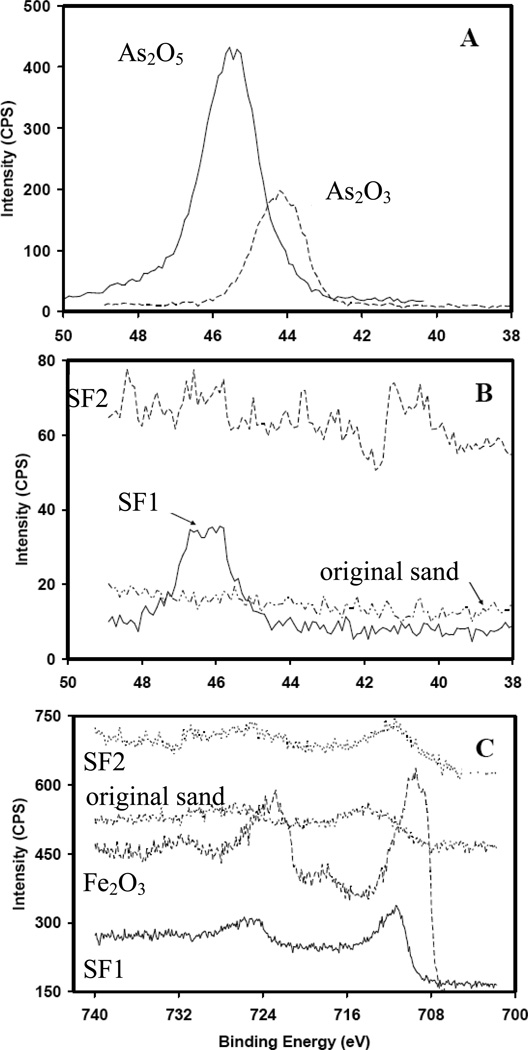
The XPS results of samples from the original sand, SF1 and SF2 are compared with the spectrum of As(III) (As2O3) and As(V) (As2O5) and hematite(Fe2O3) standards in Figure 4. In the panel, depicting the range of 700 to 740 ev (Figure 4C), the spectra of the original sand surface was similar to the sample from the SF2 control column lacking nitrate. The surface of the SF1 sand clearly contained Fe(III) with a spectrum matching the hematite standard; whereas the spectrum for the SF2 sample did not have any signature for hematite. In the panel depicting the range of 38 to 50 ev (Figure 4B), the oxidation state of As cannot be clearly identified on the surface of SF2 sand due to very low As content. The As spectra in the SF1 sample indicates the presence of As(V) as evidenced by the close match with the As2O5 standard.
Figure 4.
XPS for original sand, SF1 and SF2 column profile: arsenic standards (panel A), arsenic samples (panel B), and iron standard with samples (panel C).
Discussion
Bioremediation of As in the presence of NO3−
In this study, two continuous flow sand-filled columns were operated to simulate a natural anaerobic groundwater-sediment system with co-occurring As(III) and Fe(II) in the presence or absence of nitrate. The results provide evidence that the microbial nitrate-dependent oxidation of Fe(II) and As(III) enhanced the adsorption of As on the freshly formed solid-phase Fe(III) (hydr)oxides coating sand surfaces. During the operation period from day 30 to 225, 94.4 (±4.4) and 99.7 (±0.1)%, respectively of the influent Fe(II) and As(III) entering the column were retained in the column with added nitrate (SF1), compared with only 8.9 (±7.8) and 3.6 (±3.3)% in the control column lacking nitrate (SF2). Direct extraction of the retained minerals at the end of the experiment revealed that the sand in SF1 contained 7–8 fold higher quantities of Fe and As than SF2. These results indicate that the biological denitrification activity in column played an important role in the immobilization process of Fe and As since the only difference in the set up of SF1 and SF2 was the lack of nitrate in the feed of the latter column.
The mechanisms of immobilization of As on iron (hydr)oxides in anoxic environments
The main mechanism of As removal is hypothesized to occur through a two-step process: firstly, formation of Fe(III) (hydr)oxides due to nitrate-dependent Fe(II) oxidation, and secondly, subsequent adsorption or co-precipitation of arsenic. In this study, there are multiple evidences for the first step. Firstly, more than 91% of Fe(II) entering the column SF1 was retained in the sand bed in the presence of nitrate. Secondly, the results of solid-phase extraction revealed that the amount of iron extracted at the end of experiment was close to that of soluble Fe(II) eliminated during column operation. Likewise the speciation of the extract indicated that the iron retained on the sand surface was Fe(III). Thirdly, the EDS results showed a higher iron content on the sand surface of column SF1 than that from column SF2 or the original sand. Lastly, the mineralogy analysis of XRD and XPS illustrated that the mixture of Fe(III) (hydr)oxides contained crystalline hematite, suggesting the formation of Fe(III) (hydr)oxides due to nitrate-dependent oxidation of soluble Fe(II).
The microbial nitrate-dependent Fe(II) oxidation to Fe(III) (hydr)oxides is a well established process among prokaryotes in diverse ecosystems (26, 32). Organisms with this activity have been observed in activated sewage sludge, anoxic aquifer sediments, and marine sediments (27, 33). In this study, it was also demonstrated that the effluent from the SF1 column had microbial activity linking the anoxic oxidation of Fe(II) to denitrification. Several previous studies have identified nitrate-dependent biogenic formation of minerals such as amorphous Fe(III) (hydr)oxide, goethite, hematite, and magnetite in anoxic environments (27, 30, 34).
Once Fe(III) (hydr)oxides are formed, the second step involves the sorption of arsenic by the newly formed secondary minerals. Amorphous (ferrihydrite) and mineral forms of Fe(III) (hydr)oxides (goethite and magnetite) are well-established adsorbents of both As(V) and As(III) (4, 35–37). Arsenic is adsorbed by electrostatic interactions and direct covalent bonds with Fe(III) (hydr)oxides (4, 38). Electrostatic interactions are highly pH dependent since pH effects the dissociation of As(III) and As(V) as well as the protonation of metal hydroxide surface groups. Both forms of arsenic also undergo chemisportion via inner sphere complexation with Fe(III) (hydr)oxides (4, 38–40). In many studies at circumneutral pH, As(III) and As(V) are adsorbed by common-occurring Fe(III) (hydr)oxides at capacities of similar orders of magnitude (4, 5, 39, 41, 42). However, the desorption kinetics are more rapid for As(III) (35, 37). In this study, amorphous Fe(III) (hydr)oxides were formed biologically by nitrate-dependent oxidation of Fe(II) and subsequently precipitated on sand by alkaline pH treatment. The resulting ICS was shown to adsorb both As(V) and As(III) in isotherm studies. As(V)was adsorbed to a much greater capacity compared to As(III) at environmentally relevant equilibrium concentrations. Thus the conversion of As(III) to As(V) would contribute to a decrease in arsenic mobility.
Evidence of nitrate-dependent oxidation of As(III) to As(V)
The evidence from this study clearly indicates that As(V) was formed from the nitrate-dependent biological oxidation of As(III). As(V) was the dominant arsenic species in the effluent of column SF1 while As(III) was the prevalent form in the influent of the column. The extract of the As retained on the sand surfaces of column SF1 was predominately in the As(V) form. Furthermore, the results obtained by XPS show a strong indication that As(V) was the dominant As species speciation retained on the ICS of column SF1. In contrast, the As extracted from column SF2 was present in the form of As(III), and the main As species in the effluent was also As(III), suggesting no oxidation of As(III) in column SF2 without nitrate.
In our previous studies, we reported that several anaerobic natural mixed cultures were involved in the anoxic oxidation of As(III) linked to denitrification (25). The biological nature of the reaction is inferred from the lack of any conversion in non-inoculated samples and heat-killed samples (25, 29). An enrichment culture derived from As(III)-denitrifying biofilm inoculum used in this study abundantly contained microorganisms from the genus Azoarcus and the family Comamonadaceae (29). These are microorganisms known to be responsible for the anoxic nitrate-dependent oxidation of As(III) (24, 29).
Fe(III) (hydr)oxides adsorb both As(V) and As(III) and, thus, offer significant potential in controlling the dissolved As concentrations in natural environments. Anaerobic microbial reduction and dissolution of Fe(III) (hydr)oxides, as well as dissimilitary reduction of As(V) to As(III) are major mechanisms of mobilizing As in soil and sediments (7, 43). Fe(II) and As(III) commonly co-occur in contaminated groundwater and surface water under anaerobic conditions. The reversal of the process by the oxidation of Fe(II) and As(III) could be an important bioremediation strategy to generate Fe(III) (hydr)oxides that immobilize As(V) on the solid phases. Although dissolved oxygen can readily oxidize Fe(II) and As(III) abiotically and biologically, respectively, it is difficult to diffuse dissolved oxygen into anoxic zones of the submerged subsurface due to its low solubility and high reactivity. However, nitrate could be utilized as an alternative electron acceptor with advantages of having a high solubility, and lower reactivity, which will enable it to disperse in the saturated subsurface. The study presented here validates that microbial nitrate-dependent oxidation of Fe(II) and As(III) enhances the immobilization of As in the anoxic environments. In the model column used here, high levels of As(III) (500 µg l−1) were decreased to around the U.S. environmental protection agency maximum contaminant level of 10 µg l−1 for a prolonged period of time by promoting chemolithotrophic denitrification linked to As(III) oxidation.
Supplementary Material
Acknowledgments
The work presented here was funded by a USGS, National Institute for Water Resources 104G grant (2005AZ114G), and by a grant of the NIEHS-supported Superfund Basic Research Program (NIH ES-04940). The use of trade, product, or firm names in this report is for descriptive purposes only and does not constitute endorsement by the U.S.Geological Survey. Authors are grateful to Estefania Marcos for some of the experimental work.
Footnotes
Supporting Information Available
Additional details on the material and methods on the inoculum and basal medium of the continuous column experiment, as well as for the preparation of ICS, adsorption isotherms of arsenic on ICS, batch assay for confirming microbial oxidation of Fe(II), methods for XRD, SEM/EDS, XPS and analytical methods utilized. Results are presented concerning the adsorption isotherm of arsenic on ICS as well as the microbial oxidation of Fe(II). Figures are also shown with SEMS/EDS and XRD data.
Literature Cited
- 1.ATSDR. Toxicological profile for arsenic. Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services; Atlanta: 2007. p. 500. [PubMed] [Google Scholar]
- 2.Oremland RS, Stolz JF. The ecology of arsenic. Science. 2003;300(5621):939–944. doi: 10.1126/science.1081903. [DOI] [PubMed] [Google Scholar]
- 3.Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002;17(5):517–568. [Google Scholar]
- 4.Dixit S, Hering JG. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environ. Sci. Technol. 2003;37(18):4182–4189. doi: 10.1021/es030309t. [DOI] [PubMed] [Google Scholar]
- 5.Raven KP, Jain A, Loeppert RH. Arsenite and arsenate adsorption on ferrihydrite: Kinetics, equilibrium, and adsorption envelopes. Environ. Sci. Technol. 1998;32(3):344–349. [Google Scholar]
- 6.Oremland RS, Kulp TR, Blum JS, Hoeft SE, Baesman S, Miller LG, Stolz JF. A microbial arsenic cycle in a salt-saturated, extreme environment. Science. 2005;308(5726):1305–1308. doi: 10.1126/science.1110832. [DOI] [PubMed] [Google Scholar]
- 7.Anawar HM, Akai J, Yoshioka T, Konohira E, Lee JY, Fukuhara H, Alam MTK, Garcia-Sanchez A. Mobilization of arsenic in groundwater of Bangladesh: evidence from an incubation study. Environ. Geochem. Health. 2006;28(6):553–565. doi: 10.1007/s10653-006-9054-0. [DOI] [PubMed] [Google Scholar]
- 8.Cummings DE, Caccavo F, Fendorf S, Rosenzweig RF. Arsenic mobilization by the dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY. Environ. Sci. Technol. 1999;33(5):723–729. [Google Scholar]
- 9.Sierra-Alvarez R, Field JA, Cortinas I, Feijoo G, Moreira MT, Kopplin M, Gandolfi AJ. Anaerobic microbial mobilization and biotransformation of arsenate adsorbed onto activated alumina. Water Res. 2005;39(1):199–209. doi: 10.1016/j.watres.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Arai Y, Elzinga EJ, Sparks DL. X-ray absorption spectroscopic investigation of arsenite and arsenate adsorption at the aluminum oxide-water interface. J. Colloid Interface Sci. 2001;235(1):80–88. doi: 10.1006/jcis.2000.7249. [DOI] [PubMed] [Google Scholar]
- 11.Lin TF, Wu JK. Adsorption of arsenite and arsenate within activated alumina grains: Equilibrium and kinetics. Water Res. 2001;35(8):2049–2057. doi: 10.1016/s0043-1354(00)00467-x. [DOI] [PubMed] [Google Scholar]
- 12.Lytle DA, Magnuson ML, Snoeyink VL. Effect of oxidants on the properties of Fe(III) particles and suspensions formed from the oxidation of Fe(II) J. Am. Water Works Assn. 2004;96(8):112–124. [Google Scholar]
- 13.Roberts LC, Hug SJ, Ruettimann T, Billah M, Khan AW, Rahman MT. Arsenic removal with iron(II) and iron(III) waters with high silicate and phosphate concentrations. Environ. Sci. Technol. 2004;38(1):307–315. doi: 10.1021/es0343205. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg S. Competitive adsorption of arsenate and arsenite on oxides and clay minerals. Soil Sci. Soc. Am. J. 2002;66(2):413–421. [Google Scholar]
- 15.Lin Z, Puls RW. Adsorption, desorption and oxidation of arsenic affected by clay minerals and aging process. Environ. Geol. 2000;39(7):753–759. [Google Scholar]
- 16.Park B, Dempsey BA. Heterogeneous oxidation of Fe(II) on ferric oxide at neutral pH and a low partial pressure of 0(2) Environ. Sci. Technol. 2005;39(17):6494–6500. doi: 10.1021/es0501058. [DOI] [PubMed] [Google Scholar]
- 17.James RE, Ferris FG. Evidence for microbial-mediated iron oxidation at a neutrophilic groundwater spring. Chem. Geol. 2004;212(3-4):301–311. [Google Scholar]
- 18.Bissen M, Frimmel FH. Arsenic - a review. Part II: Oxidation of arsenic and its removal in water treatment. Acta Hydroch. Hydrob. 2003;31(2):97–107. [Google Scholar]
- 19.Inskeep WP, Macur RE, Hamamura N, Warelow TP, Ward SA, Santini JM. Detection, diversity and expression of aerobic bacterial arsenite oxidase genes. Environ. Microbiol. 2007;9(4):934–943. doi: 10.1111/j.1462-2920.2006.01215.x. [DOI] [PubMed] [Google Scholar]
- 20.Rhine ED, Ni Chadhain SM, Zylstra GJ, Young LY. The arsenite oxidase genes (aroAB) in novel chemoautotrophic arsenite oxidizers. Biochem. Biophys. Res. Commun. 2007;354(3):662–667. doi: 10.1016/j.bbrc.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Battaglia-Brunet F, Itard Y, Garrido F, Delorme F, Crouzet C, Greffie C, Joulian C. A simple biogeochemical process removing arsenic from a mine drainage water. Geomicrobiol. J. 2006;23(3-4):201–211. [Google Scholar]
- 22.Harvey CF, Swartz CH, Badruzzaman ABM, Keon-Blute N, Yu W, Ali MA, Jay J, Beckie R, Niedan V, Brabander D, Oates PM, Ashfaque KN, Islam S, Hemond HF, Ahmed MF. Arsenic mobility and groundwater extraction in Bangladesh. Science. 2002;298(5598):1602–1606. doi: 10.1126/science.1076978. [DOI] [PubMed] [Google Scholar]
- 23.Oremland RS, Hoeft SE, Santini JA, Bano N, Hollibaugh RA, Hollibaugh JT. Anaerobic oxidation of arsenite in Mono Lake water and by facultative, arsenite-oxidizing chemoautotroph, strain MLHE-1. Appl. Environ. Microbiol. 2002;68(10):4795–4802. doi: 10.1128/AEM.68.10.4795-4802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhine ED, Phelps CD, Young LY. Anaerobic arsenite oxidation by novel denitrifying isolates. Environ. Microbiol. 2006;8(5):899–908. doi: 10.1111/j.1462-2920.2005.00977.x. [DOI] [PubMed] [Google Scholar]
- 25.Sun WJ, Sierra R, Field JA. Anoxic oxidation of arsenite linked to denitrification in sludges and sediments. Water Res. 2008;42(17):4569–4577. doi: 10.1016/j.watres.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Straub KL, Benz M, Schink B. Iron metabolism in anoxic environments at near neutral pH. FEMS Microbiol. Ecol. 2001;34(3):181–186. doi: 10.1111/j.1574-6941.2001.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 27.Weber KA, Urrutia MM, Churchill PF, Kukkadapu RK, Roden EE. Anaerobic redox cycling of iron by freshwater sediment microorganisms. Environ. Microbiol. 2006;8(1):100–113. doi: 10.1111/j.1462-2920.2005.00873.x. [DOI] [PubMed] [Google Scholar]
- 28.Hoeft SE, Blum JS, Stolz JF, Tabita FR, Witte B, King GM, Santini JM, Oremland RS. Alkalilimnicola ehrlichii sp nov., a novel, arsenite-oxidizing haloalkaliphilic gammaproteobacterium capable of chemoautotrophic or heterotrophic growth with nitrate or oxygen as the electron acceptor. Int J Syst Evol Microbiol. 2007;57:504–512. doi: 10.1099/ijs.0.64576-0. [DOI] [PubMed] [Google Scholar]
- 29.Sun WJ, Sierra-Alvarez R, Fernandez N, Sanz JL, Amils R, Legatzki A, Maier RM, Field JA. Molecular characterization and in situ quantification of anoxic arsenite-oxidizing denitrifying enrichment cultures. FEMS Microbiol. Ecol. 2009;68(1):72–85. doi: 10.1111/j.1574-6941.2009.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Straub KL, Schonhuber WA, Buchholz-Cleven BEE, Schink B. Diversity of ferrous iron-oxidizing, nitrate-reducing bacteria and their involvement in oxygen-independent iron cycling. Geomicrobiol. J. 2004;21(6):371–378. [Google Scholar]
- 31.Weber KA, Achenbach LA, Coates JD. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 2006;4(10):752–764. doi: 10.1038/nrmicro1490. [DOI] [PubMed] [Google Scholar]
- 32.Weber KA, Pollock J, Cole KA, O'Connor SM, Achenbach LA, Coates JD. Anaerobic nitrate-dependent iron(II) bio-oxidation by a novel lithoautotrophic betaproteobacterium, strain 2002. Appl. Environ. Microbiol. 2006;72(1):686–694. doi: 10.1128/AEM.72.1.686-694.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senko JM, Dewers TA, Krumholz LR. Effect of oxidation rate and Fe(II) state on microbial nitrate-dependent Fe(III) mineral formation. Appl. Environ. Microbiol. 2005;71(11):7172–7177. doi: 10.1128/AEM.71.11.7172-7177.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhuri SK, Lack JG, Coates JD. Biogenic magnetite formation through anaerobic biooxidation of Fe(II) Appl. Environ. Microbiol. 2001;67(6):2844–2848. doi: 10.1128/AEM.67.6.2844-2848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herbel M, Fendorf S. Biogeochemical processes controlling the speciation and transport of arsenic within iron coated sands. Chem. Geol. 2006;228(1-3):16–32. [Google Scholar]
- 36.Su CM, Puls RW. Arsenate and arsenite sorption on magnetite: Relations to groundwater arsenic treatment using zerovalent iron and natural attenuation. Water Air Soil Pollut. 2008;193(1-4):65–78. [Google Scholar]
- 37.Tufano KJ, Reyes C, Saltikov CW, Fendorf S. Reductive Processes Controlling Arsenic Retention: Revealing the Relative Importance of Iron and Arsenic Reduction. Environ. Sci. Technol. 2008;42(22):8283–8289. doi: 10.1021/es801059s. [DOI] [PubMed] [Google Scholar]
- 38.Sun XH, Doner HE. An investigation of arsenate and arsenite bonding structures on goethite by FTIR. Soil Sci. Soc. Am. J. 1996;161(12):865–872. [Google Scholar]
- 39.Gimenez J, Martinez M, de Pablo J, Rovira M, Duro L. Arsenic sorption onto natural hematite, magnetite, and goethite. J. Hazard. Mater. 2007;141(3):575–580. doi: 10.1016/j.jhazmat.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 40.Ona-Nguema G, Morin G, Juillot F, Calas G, Brown GE. EXAFS analysis of arsenite adsorption onto two-line ferrihydrite, hematite, goethite, and lepidocrocite. Environ. Sci. Technol. 2005;39(23):9147–9155. doi: 10.1021/es050889p. [DOI] [PubMed] [Google Scholar]
- 41.Bowell RJ. Sorption of Arsenic by Iron-Oxides and Oxyhydroxides in Soils. Appl. Geochem. 1994;9(3):279–286. [Google Scholar]
- 42.Goldberg S, Johnston CT. Mechanisms of arsenic adsorption on amorphous oxides evaluated using macroscopic measurements, vibrational spectroscopy, and surface complexation modeling. J. Colloid Interface Sci. 2001;234(1):204–216. doi: 10.1006/jcis.2000.7295. [DOI] [PubMed] [Google Scholar]
- 43.Oremland RS, Stolz JF. Arsenic, microbes and contaminated aquifers. Trends in Microbiology. 2005;13(2):45–49. doi: 10.1016/j.tim.2004.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.