Abstract
A new study entitled “Normal gut microbiota modulates brain development and behavior”, published in the Proceedings of the National Academy of Sciences, requires that we reconsider the notion that the brain is an immune-privileged site. The authors demonstrate that intestinal microbiota must be present within a set time-frame for normal synaptogenesis to occur in the brain. In the absence of intestinal microbiota, histopathological and behavioral abnormalities arise. These observations necessitate a new look at the many interconnections of the immune system and the brain, suggesting new frontiers for research and new therapeutic strategies for neurodevelopmental diseases.
Keywords: brain development, gut microbiota, immune signaling
Commensal bacteria are essential for immune and neural development
During fetal development, neurons are added to the brain at astonishing rates. As a result, the human brain has achieved its nearly complete neuronal capacity by birth [1]. However, brain development does not cease at birth. Rather, during infancy the brain establishes the myriad synaptic connections that provide the essential substrate for functional brain networks that underlie perception, cognition, and action. There is significant interest in understanding the factors and mechanisms that regulate these developmental programs and pathways.
A recent study [2] surprisingly reveals that the bacterial content of the gut, which changes rapidly after birth as food ingestion begins, can modulate brain developmental pathways. It had long been known that commensal bacteria are critical to intestinal function, and a number of studies have shown that intestinal microorganisms are also critical for activation of systemic immunity [3]. For instance, only limited generation of the TH17 subset of effector T helper cells occurs in gut or spleen in the absence of intestinal microorganisms [4–6]. TH17 cells are the predominant T cell subset involved in the control of a variety of extracellular bacterial and fungal infections [4, 5, 7]. Germ-free mice, which lack normal numbers of TH17 cells, are immunocompromised with respect to infection, but relatively resistant to induction of autoimmunity including experimental autoimmune encephalomyelitis [7, 8]. When segmented filamentous bacteria are inoculated into the intestine of germ-free mice, immunity to infectious organisms is enhanced, but susceptibility to autoimmune disease is likewise enhanced [6]. Intestinal microbes also regulate the function of macrophages that reside in the lamina propria of the intestine [9]. Normally, these macrophages produce interleukin (IL)-10 and dampen the production of pro-inflammatory cytokines by resident dendritic cells; in the absence of commensal bacteria, the production of IL-10 is significantly diminished [9].
The study by Heijtz et al. [2] shows that commensal microbiota are necessary not only for the development of the immune system, but also for brain development, and that this regulation has explicit time constraints. They observed that mice raised in germ-free conditions have significantly increased motor activity and decreased anxiety as compared to mice bearing normally colonized intestines. In addition, strikingly, these developmental deficits in the germ-free pups are completely normalized by re-colonization of gastrointestinal flora. This reversal is dependent upon the stage of brain development, because microbial colonization of germ-free adult mice fails to reverse the behavioral phenotype. Thus, there is a critical developmental window during which intestinal microbiota are critical to normal brain development.
The observations that both brain development and immunity are modulated by gut flora raise important questions in developmental physiology, neuroscience, and immunology. Here we suggest that understanding the connections linking these mechanisms will reveal new avenues for improving the therapy of neurodevelopmental, autoimmune, and inflammatory diseases.
In their seminal paper, Heijtz et al. [2] further demonstrate that changes in gene expression accompany the behavioral phenotype present in the germ-free mice. Of particular interest, they show reduced expression of the synaptic proteins synaptophysin and PSD-95 in the striatum. Given that both of these proteins are involved in synapse formation, the authors hypothesize that gut microbiota might modulate synaptogenesis during a critical developmental window. Moreover, in the adult brain these synaptic proteins are intimately involved in the processes of excitatory synaptic function and synaptic plasticity [10, 11], which leads to the possibility that early exposure to gut microbiota, necessary for integrated brain function in the mature individual, helps establish the program of synaptic connectivity (Fig. 1).
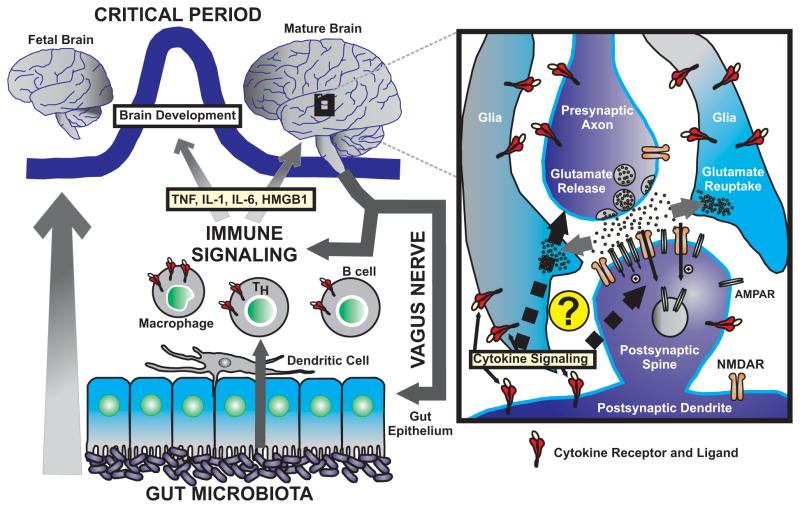
Figure 1.
Novel evidence shows that the gut microbiota exert a strong influence over brain development, most likely via immune signaling, which might include soluble signals such as cytokines (TNF, IL-1, IL-6, and HMGB1 are highlighted) and chemokines as well as immune cells (dendritic cells, macrophages, T cells, and B cells are pictured). Notably, there is a “critical period” that is a developmental window during which the gut flora can influence the developing brain. Additionally, the mature brain exerts a strong influence on the immune system via the efferent arc of the vagus nerve. The right panel depicts the excitatory brain synapse as the most likely site of action for intrinsic immune-related mechanisms. The presynaptic axon releases glutamate into the synaptic cleft, which binds to glutamatergic receptors (AMPAR and NMDAR are pictured). The neighboring glia cells are responsible for glutamate reuptake and other processes. Studies have revealed the presence of receptors for cytokines in both glia and the neural elements of the synapse. It is, thus, hypothesized that cytokine levels induced by gut microbiota might influence the genesis and function of central synapses.
Immune influences on brain development and function
Although not addressed experimentally in the work to date, it is likely that immune cells and their soluble products mediate the molecular mechanisms by which commensal bacteria affect the developing brain. This possibility is directly supported by a growing literature showing that the immune system is capable of modulating brain function during development and adulthood [12–14]. These observations also negate the traditional, dogmatic view that the brain is a privileged environment inaccessible to immunological influences. Abundant data indicate that neurons, astrocytes, and microglial cells express membrane surface receptors that are specific to the molecular products of immune cells, which underlie brain cellular responses to immunological signals [12–14]. Indeed, brain cells make, and respond to, principal molecules of the innate immune system, including pro-inflammatory cytokines, components of the complement cascade, and chemokines [14–17]. They also respond to other immunological molecules with roles best characterized within the adaptive immune system, including the major histocompatibility class I (MHCI) family [18]. Although still in its infancy, there is a rapidly expanding field focused on identifying and mapping the expression of immune-based molecules in brain cells. It has already become abundantly clear, however, that immune-related signaling occurs not only in glia, such as astrocytes and microglia, which are intimately related to monocytes and macrophages, but also in neurons and endothelial cells present in the brain vasculature [19]. Science is, therefore, at the verge of uncovering a novel set of molecular interactions between the immune system and the brain.
A new paradigm of how immune signals shape brain development comes from a series of studies of the developing visual system of mammals. Researchers have shown that MHCI molecules, which govern the selection and activation of the CD8+ subset of effector T cells, are involved in the assembly of the appropriate connections between the retina and the dorsal lateral geniculate nucleus (LGN) [20]. Specifically, knockout mice lacking the classical MHCI molecules H2-K and H2-D fail to achieve the correct developmental program, and instead generate supernumerary connections between the retina and the LGN [21]. Double knockout β2m−/− TAP−/− mice, which do not express functional MHCI receptors, exhibit a comparable phenotype [22]. Similarly, recent studies of the C1q and C3 components of the complement cascade have demonstrated that C1q- and C3-deficient mice also show impaired retina-LGN connectivity [15]. Therefore, components of both the innate and the adaptive immune system influence sensory pathways and affect perception.
Immune modulation of brain physiology
Immune molecules also influence cognition. When added to hippocampal slices, IL-6 and IL-1β significantly impede long-term potentiation (LTP) in the synapses connecting the CA3 with the CA1 neurons of the hippocampus, a neural signaling mechanism that is critical to learning and memory [23, 24]. The sequence of events underlying LTP is well understood. Glutamate binding to the α-amino-3-hydroxy-5-methyl-4-iso-xazolepropionic acid receptor (AMPAR) results in the brief opening of an ion channel, which allows Na+ to enter into the dendritic spine causing a small degree of excitation. The N-methyl-D-aspartate receptor (NMDAR) does not open immediately because its pore is blocked by Mg2+ ions. High-frequency stimulation removes the Mg2+ block of the NMDAR. When the NMDAR opens, it permeates Na+ and Ca2+ ions, leading to the activation of several kinases, particularly calcium-calmodulin kinase II, and to the up-regulation of AMPARs and to their trafficking into the synapse. Interestingly in the context of the observed effects of IL-1 and IL-6 on LTP, tumor necrosis factor (TNF) fails to block LTP [25]. Rather, it induces a dose-dependent increase in synaptic expression of AMPARs that occurs over a period of several hours. The current model, therefore, indicates that when neurons undergo a diminished level of activity, the neighboring glia cells secrete TNF. This facilitates the expression of newly synthesized AMPARs in the synapses of neurons, restoring normal levels of activity. This model places TNF at a crucial position in mediating the homeostatic regulation of excitatory synapses that normally maintain a constant level of overall activity in a neuron, a process termed synaptic scaling.
A critical time for brain development
One important aspect of the paper by Heijtz et al. [2] is that the influence of microbiota on brain development occurs only during a fixed developmental window. Understanding the window of vulnerability when alterations in immune function can lead to brain impairment and the therapeutic window when normal brain function can be restored is of clear importance. Patterson and colleagues [26] have shown that exposure to high levels of IL-6 in utero alters brain development and leads to permanent behavioral impairments. These impairments occur only in the fetus and not in the gestating dam that is exposed to the same concentration of IL-6. Perhaps, this reflects a stage-specific response to IL-6, or the development of homeostatic compensatory mechanisms in the adult organism.
Cytokines are not the only immune cell-derived molecules that have been implicated in modulating brain development during a critical development window; recent data reveal that brain-reactive antibodies are significantly more toxic to the developing fetus than to the adult animal. This differential vulnerability may be due in part to a porous blood-brain barrier in utero and a competent barrier in the adult animal, so that only the fetal brain is exposed to antibody [27]. While these examples address vulnerability to developmental impairments, it is perhaps more intriguing to consider that there may also be a window of time when sensory input is required for normal brain development. Seminal studies of visual physiology have shown that appropriate connectivity in the visual system is established only with afferent perceptual processing of visual stimuli through the retina [18, 21]. The study of Heijtz et al. [2] suggests that the immune system also initiates afferent processes that are necessary for appropriate connectivity, perhaps through synaptic pruning.
The brain can modulate immune function
While the influence of the immune system on the brain has been studied for many years, dating back to early work in fever and weight loss associated with infection, a more recent body of work has revealed that, reciprocally, the nervous system exerts significant influences over the immune system [28]. It has been clearly established that action potentials originating in the brain modulate the output of the immune system through a reflex control set point [29]. Tracey [28, 30] has demonstrated the existence of a cholinergic anti-inflammatory pathway, originating in the brain, transduced through the vagus nerve, and culminating in the down-regulation of monocytes in the spleen and other tissues. This neural circuit provides a mechanism through which the brain exerts a tonic inhibitory influence that prevents the overexpression of cytokines that can be harmful to the host. The afferent pathways that sense inflammation and activate the efferent responses in brain are under study, but extensive data indicate that cytokine receptors in both the peripheral and the central nervous system activate sensory pathways in the vagus nerve [31].
Increasingly, it will be possible to map the neural pathways, whereby the immune system alerts the brain to the presence of sterile or infectious tissue damage, which in turn activate efferent pathways that underlie brain modulation of immune function. The new concepts of “the inflammatory reflex” and “the immunological homunculus” reflect advances in physiology, anatomy, and immunology. Shared signaling molecules and receptors between the immune system and the central nervous system provide for linkages that, in view of the current work by Heitjtz et al. [2], demonstrate an early and critical temporal window for immune-mediated influences on adult behaviors. This critical period remains operational during early postnatal life when appropriate brain development can be initiated by challenge with organizing gut microbiota.
Conclusions
Taken together, previous work and the new article by Heijtz et al. [2] extend the complex set of interactions of the immune system with the central nervous system from a modulatory function to the synaptogenesis necessary for normal cognitive function. The immune system must be understood, not just as a defense against microbial invasion, but also as a sensory organ that informs the brain about the status of infectious or sterile immune activation throughout the body. The activation of this afferent pathway results in targeted synaptogenesis. The brain, in turn, modulates the strength of the immune response. Together, these studies reveal a model for understanding how the brain establishes a set point for systemic inflammatory processes, and how the immune system maintains a homeostatic regulation of neurotransmitter-mediated synaptic activation. The implications are legion, and it is clear that future studies of immune-mediated developmental pathways in the brain may provide clues to multiple neurodevelopment impairments, most notably autism spectrum disorders.
Abbreviations
- IL
interleukin
- LGN
lateral geniculate nucleus
- LTP
long-term potentiation
- MHCI
major histocompatibility class I
- TNF
tumor necrosis factor
References
- 1.Marín-Padilla M. The Human Brain: Prenatal Development and Structure. New York: Springer; 2010. [Google Scholar]
- 2.Heijtz RD, Wang S, Anuar F, Qian Y, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–52. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ivanov II, Littman DR. Modulation of immune homeostasis by commensal bacteria. Curr Opin Microbiol. 2011;14:106–14. doi: 10.1016/j.mib.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivanov II, Atarashi K, Manel N, Brodie EL, et al. Induction of intestinal Th17cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atarashi K, Nishimura J, Shima T, Umesaki Y, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–12. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 6.Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, et al. The key role of segmented filamentous bactria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–89. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Wu HJ, Ivanov II, Darce J, Hattori K, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17cells. Immunity. 2010;32:815–27. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2011;108:4615–22. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueda Y, Kayama H, Jeon SG, Kusu T, et al. Commensal micro-biota induce LPS hyporesponsiveness in colonic macrophages via the production of IL-10. Int Immunol. 2010;22:953–62. doi: 10.1093/intimm/dxq449. [DOI] [PubMed] [Google Scholar]
- 10.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–81. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 11.Valtorta F, Pennuto M, Bonanomi D, Benfenati F. Synaptophysin: Leading actor or walk-on role in synaptic vesicle exocytosis? BioEssays. 2004;26:445–53. doi: 10.1002/bies.20012. [DOI] [PubMed] [Google Scholar]
- 12.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Bhat R, Steinman L. Innate and adaptive autoimmunity directed to the central nervous system. Neuron. 2009;64:123–32. doi: 10.1016/j.neuron.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Stevens B, Allen NJ, Vazquez LE, Howell GR, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Haynes RL, Sidman RL, Vartanian T. TLR8: an innate immune receptor in brain, neurons and axons. Cell Cycle. 2007;6:2859–68. doi: 10.4161/cc.6.23.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyon A, Massa F, Rovere C, Nahon JL. How cytokines can influence the brain: A role for chemokines? J Neuroimmunol. 2008;198:46–55. doi: 10.1016/j.jneuroim.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Shatz CJ. MHC class I: an unexpected role in neuronal plasticity. Neuron. 2009;64:40–5. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenwood J, Heasman SJ, Alvarez JI, Prat A, et al. Review: leucocyte-endothelial cell crosstalk at the blood-brain barrier: a prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 20.Fourgeaud L, Boulanger LM. Role of immune molecules in the establishment and plasticity of glutamatergic synapses. Eur J Neurosci. 2010;32:207–17. doi: 10.1111/j.1460-9568.2010.07342.x. [DOI] [PubMed] [Google Scholar]
- 21.Datwani A, McConnell MJ, Kanold PO, Micheva KD, et al. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–70. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huh GS, Boulanger LM, Du H, Riquelme PA, et al. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–9. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellinger FP, Madamba S, Siggins GR. Interleukin 1β inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628:227–34. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- 24.Huerta PT, Volpe BT. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J Neuroeng Rehabil. 2009;6:7. doi: 10.1186/1743-0003-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–9. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 26.Smith SE, Li J, Garbett K, Mirnics K, et al. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JY, Huerta PT, Zhang J, Kowal C, et al. Neurotoxic auto-antibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat Med. 2009;15:91–6. doi: 10.1038/nm.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 29.Rosas-Ballina M, Tracey KJ. The neurology of the immune system: neural reflexes regulate immunity. Neuron. 2009;64:28–32. doi: 10.1016/j.neuron.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tracey KJ. Understanding immunity requires more than immunology. Nat Immunol. 2010;11:561–4. doi: 10.1038/ni0710-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–28. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]