Abstract
Integration of vestibular and proprioceptive afferent information within the central nervous system is a critical component of postural regulation. We recently demonstrated that labyrinthine and hindlimb signals converge onto vestibular nucleus neurons, such that hindlimb movement modulates the activity of these cells. However, it is unclear whether similar convergence of hindlimb and vestibular signals also occurs upstream from the vestibular nuclei, particularly in the rostral fastigial nucleus (rFN). We tested the hypothesis that rFN neurons have similar responses to hindlimb movement as vestibular nucleus neurons. Recordings were obtained from 53 rFN neurons that responded to hindlimb movement in decerebrate cats. In contrast to vestibular nucleus neurons, which commonly encoded the direction of hindlimb movement (81% of neurons), few rFN neurons (21%) that responded to leg movement encoded such information. Instead, most rFN neurons responded to both limb flexion and extension. Half of the rFN neurons whose activity was modulated by hindlimb movement received convergent vestibular inputs. These results show that rFN neurons receive somatosensory inputs from the hindlimb, and that a subset of rFN neurons integrates vestibular and hindlimb signals. Such rFN neurons likely perform computations that participate in maintenance of balance during upright stance and movement. Although vestibular nucleus neurons are interconnected with the rFN, the dissimilarity of responses of neurons sensitive to hindlimb movement in the two regions suggest that they play different roles in coordinating postural responses during locomotion and other movements which entail changes in limb position.
Keywords: Multisensory convergence, Limb, Balance, Rostral fastigial nucleus, vestibular nucleus
Introduction
Maintenance of balance is inherently multimodal: vestibular, proprioceptive, and visual information is processed by the central nervous system to provide an estimate of body position and movement in space, thereby creating a framework that permits corrective responses (Peterka 2002). The movement of a limb needed to prevent a fall is dependent on the position of the limb in space when a postural perturbation occurs. Hence, postural responses (presumably mediated through vestibulospinal pathways) are modified in accordance with limb position (Welgampola and Colebatch 2001; Marsden et al. 2002; Grasso et al. 2011). Consequently, it has been hypothesized that vestibular nucleus (VN) neurons integrate somatosensory inputs from the limbs and labyrinthine signals in order to appropriately adjust vestibulospinal outflow (Arshian et al. 2014). Several physiologic studies provide evidence supporting this hypothesis. VN neurons respond to electrical stimulation of hindlimb nerves (Wilson et al. 1966; Jian et al. 2002; McCall et al. 2013) and the activity of VN neurons in decerebrate felines is modulated during hindlimb movement (Fredrickson et al. 1966; Orlovsky 1972; Arshian et al. 2014), with most VN neurons encoding the direction of hindlimb movement (Arshian et al. 2014).
It remains unclear how somatosensory information from the hindlimb is conveyed within the central nervous system to shape the responses of VN neurons during movement. One possibility is that direct spinovestibular projections convey hindlimb somatosensory inputs to the VN. However, the direct spinovestibular projection is relatively sparse, particularly from the lumbar spinal cord (McKelvey-Briggs et al. 1989). Furthermore, electrical stimulation of hindlimb nerves alters VN neural activity at latencies consistent with multisynaptic connections (Jian et al. 2002). Therefore, indirect pathways, including pathways through the cerebellum, represent the more likely means of conveyance of hindlimb somatosensory information to the VN.
In particular, we hypothesized that neurons in the cerebellar rostral fastigial nucleus (rFN) integrate somatosensory inputs from the limbs with vestibular inputs and subsequently influence vestibulospinal outflow. Several lines of evidence support this hypothesis. First, rFN neurons perform an analogous function utilizing somatosensory inputs from the neck: a population of rFN neurons integrates somatosensory signals from the neck with vestibular inputs in order to compute body position in space (Brooks and Cullen 2009). Second, the rFN is a principle outflow nucleus of the cerebellar vermis, and neurons in the vermal regions of the anterior and posterior cerebellum are responsive to hindlimb movement (Oscarsson 1969; Gray et al. 1993). Furthermore, the activity of rFN neurons is modulated during mechanical manipulations of the hindlimb (toe taps and pressure to foot pads) and electrical stimulation of hindlimb nerves (Eccles et al. 1974a; Eccles et al. 1974b; Wilson et al. 1978). Third, rFN neuronal activity is modulated by vestibular afferent activation (Ghelarducci 1973; Ghelarducci et al. 1974; Stanojevic et al. 1980; Stanojevic 1981; Siebold et al. 1997; Shaikh et al. 2005; Miller et al. 2008). Finally, rFN neurons project extensively to the vestibular nuclei (Oscarsson 1965; Batton et al. 1977; Asanuma et al. 1983; Homma et al. 1995; Matsushita 1999) and affect the activity of VN neurons, including vestibulospinal neurons (Ito et al. 1970; Matsuyama and Jankowska 2004).
In the present study, we examined the responses of rFN neurons to movement of the hindlimb. We hypothesized that the activity of rFN neurons is modulated during hindlimb movement in a fashion similar to that of VN neurons (Arshian et al. 2014), which would support the notion that somatosensory afferent signals from the hindlimb are conveyed through the rFN to VN neurons. In addition, we ascertained whether rFN neurons that respond to limb flexion or extension receive convergent vestibular inputs.
Methods
Most of the methods used in these experiments were detailed in a recent publication (Arshian et al. 2014), and thus will only be succinctly summarized below. The University of Pittsburgh’s Institutional Animal Care and Use Committee prospectively approved the experimental procedures on animals. These procedures conformed to the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academies Press, Washington D.C., 2011). Experiments were performed on 15 purpose-bred felines weighing between 2.8 and 4.7kg (Liberty Research, Waverly, NY).
Surgical Procedures
Felines were initially anesthetized with isoflurane vaporized in oxygen and a tracheotomy was performed. The left femoral vein was cannulated to permit intravenous injections. Blood pressure was monitored via a transducer (Millar instruments, Houston, TX) inserted into the left femoral artery and, if necessary, maintained > 90 mmHg with a saline infusion. The carotid arteries were ligated and a midcollicular decerebration was performed. A craniotomy was performed to expose the cerebellum, providing access for neural recordings.
After the decerebration was complete, isoflurane was discontinued and vecuronium bromide (0.1mg/kg IV, every 20 minutes) was administered to paralyze the animal thereby prohibiting spontaneous limb movement. Following paralysis, animals were artificially ventilated; end-tidal CO2 was monitored continuously and maintained near 4% by adjusting tidal volume and ventilation rate. Dexamethasone (1mg/kg IV) and atropine sulfate (0.5mg/kg IM) were administered every six hours to reduce brain edema and airway secretions, respectively. Temperature was monitored via a rectal probe and maintained between 37–38°C using a DC-powered heating pad and a heating lamp.
The head was secured in a stereotaxic frame mounted on a servo-controlled hydraulic tilt table (Neurokinetics, Pittsburgh, PA). Hip pins and a clamp on the T1 spinous process were used to secure the body to the stereotaxic frame. A Velcro strap wrapped immediately proximal to the ankle joint connected the right hindlimb to a servo-controlled motor, which was attached to the tilt table.
Recording Procedures
Extracellular recordings from rFN were obtained using tungsten microelectrodes (4–6 MΩ; FHC, Bowdoin, ME). Recording sites were determined using stereotaxic coordinates (Berman 1968) and confirmed histologically (see histological procedures below). Potentiometers on the tilt table and servo-controlled motor provided for recordings of body and limb position, respectively. Unit activity was sampled at 25kHz, while blood pressure and potentiometer recordings were sampled at 100Hz using a Cambridge Electronic Design 1401 data collection system and Spike 2 software (Cambridge, UK).
Limb movement and vestibular stimulation protocols
A series of ramp-and-hold movements of the hindlimb were performed using the servo-controlled motor. The hindlimb was initially held in the neutral (midline) position, then extended 60°, returned to neutral, flexed 60°, and returned to neutral (Fig. 1). Following each ramp movement, the hindlimb was held in position for seven seconds before proceeding with the next ramp movement. Each hindlimb movement was tested at three different velocities: 60°/sec, 30°/sec, and 15°/sec. For each velocity, the ramp-and-hold paradigm was repeated five times.
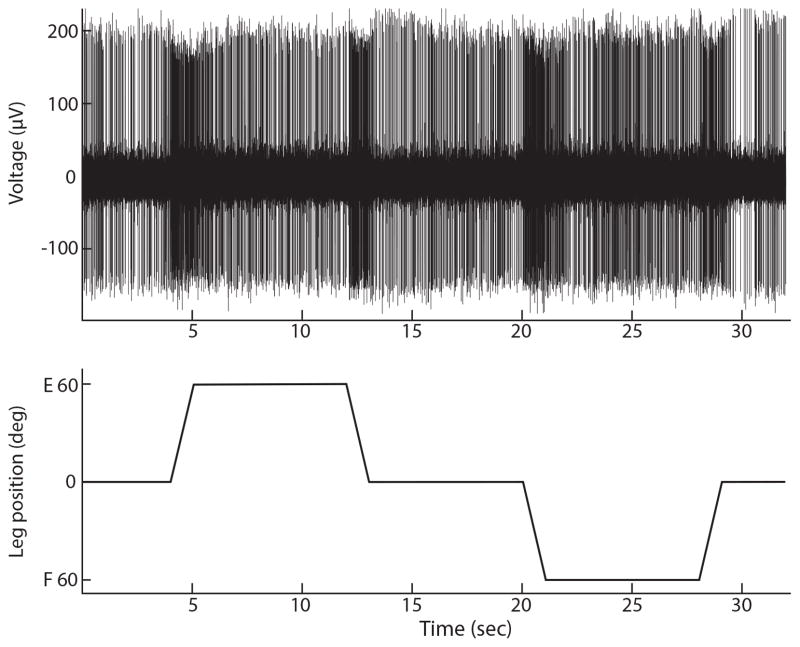
Figure 1.
rFN neuronal activity is modulated with hindlimb movement. A: activity of a single rFN neuron. B: recording from the potentiometer of the servomotor used to move the hindlimb, which reflects hindlimb position. Hindlimb position started at neutral, was then moved to extension, returned to neutral, moved to flexion, and returned to neutral. Movements in this example were at 60°/s. As illustrated in A, this rFN neuron exhibited an excitatory response with hindlimb movement, irrespective of the direction of movement.
To ascertain if a neuron received vestibular inputs, wobble stimuli (Schor et al. 1984) were delivered at 0.5Hz, 5°. The wobble stimulus is a fixed amplitude tilt whose direction moves at constant velocity around the animal, and is produced by delivering a sine wave to the pitch axis of the tilt table and a cosine wave to the roll axis. Clockwise (CW) wobble stimuli moved the animal successively through the nose down, right ear down, nose up, and left ear down positions. Counterclockwise (CCW) wobble stimuli were produced by inverting the sinusoidal command signal to the pitch axis. When a neuron was determined to have its activity modulated by both directions of wobble stimulation, it was then tested for response dynamics in the plane of tilt near its response vector orientation (tested from 0.05 to 2Hz; 2.5–10°). Limb movement was typically provided as a search stimulus during tracking, although a sub-population of rFN neurons was first identified by employing wobble stimuli and then tested for responses to hindlimb movement.
Data Analysis Procedures
The spike-sorting feature of the Spike 2 software was used to isolate the activity of single neurons within the recording field. Trials in which the activity of single units could not be discriminated were discarded.
Responses to ramp-and-hold hindlimb movements were analyzed as previously described (Arshian et al. 2014). Counts of unit firing were binned in 0.1-second increments. For each velocity, a composite response histogram for five trials was constructed. Counts from the last ten bins of the neutral position were used to represent the baseline firing rate because some neuronal responses slowly decayed over the hold phase, as previously described for vestibular nucleus neurons (Arshian et al. 2014). We first considered whether a change in neuronal firing rate from baseline was evident during the first 20 bins following onset of hindlimb extension. For units with an apparent excitatory (or inhibitory) response during extension, the bin representing the peak (or trough) of neural activity and the surrounding ten bins were selected for quantitative and statistical comparisons with baseline activity. This analysis was repeated for the flexion segment of the composite response histogram, as well as movements away from maximal flexion or extension and towards the neutral. Responses to each of the three velocities of movement tested were analyzed separately. Responses were considered significant if there was a 20% change in bin counts compared with baseline; significance was confirmed using the Wilcoxon-Mann-Whitney test (p < 0.05) performed with Prism 6 software (GraphPad software, San Diego, CA).
We additionally considered whether sustained changes in neuronal activity were present while the hindlimb was maintained in the position of maximal extension or flexion. The counts for the last 10 bins of each hold segment, corresponding to the last second the hindlimb was maintained in the extended or flexed positions, were compared with bin counts when the limb was in the neutral position. A similar analysis was completed as described above: responses were considered significant if there was a 20% change in bin counts from baseline, which was confirmed using the Wilcoxon-Mann-Whitney test.
The effect of hindlimb velocity on neuronal responses was determined by comparing the average bin counts during movement across the three velocities tested (Friedman test with Dunn’s multiple comparisons test). Comparisons across neurons grouped by response type were made using the Wilcoxon-Mann-Whitney test.
Responses to vertical vestibular stimulation
Responses during sinusoidal whole-body tilts were binned at 500 bins/cycle and averaged over the stimulus period. Sine waves were fit to neuronal responses using a least-squares minimization technique and a signal-to-noise ratio was calculated (Schor et al. 1984). Responses were considered significant if the signal-to-noise ratio was > 0.5, only the first harmonic was prominent, and the responses were consistent from trial to trial. Response gain and phase were calculated by comparing the stimulus sinusoid with the least-squares fit sinusoid.
Histological Procedures
Electrolytic lesions were created near recording sites by passing a 100μA negative current through the electrode for 60 seconds. After lesions were generated, animals were euthanized with Euthasol solution. The cerebellum and underlying brainstem were removed and fixed in 10% formalin solution. Specimens were then embedded in 2% agar, transversely sectioned (100μm thickness), mounted serially on slides, and stained with 1% thionine. Recording sites were reconstructed with reference to the electrolytic lesions, the relative positions of the recording tracks, and the relative depths of the units.
Results
Responses of rFN neurons to hindlimb movement
The activity of 53 neurons whose locations were histologically confirmed in the rFN were modulated by hindlimb movement. We previously formulated a categorization schema to describe neuronal responses to hindlimb movement (Arshian et al. 2014). There were three potential neuronal responses to each hindlimb movement: excitation response, inhibition response, and non-response. Neuronal activity elicited by each hindlimb movement (neutral to extension; extension to neutral; neutral to flexion; and flexion to neutral) was analyzed independently for a response. Responses to hindlimb movements away from the neutral position (to maximum flexion or extension) were the primary criteria utilized to segregate neurons into categories. Secondary criteria considered were responses to movements toward the neutral and sustained activity of a neuron when the neuron was held in the extension or flexion position. Neuronal responses to hindlimb movement could be segregated into the following categories, which are discussed below: omnidirectional, bidirectional, unidirectional, reciprocal, and complex.
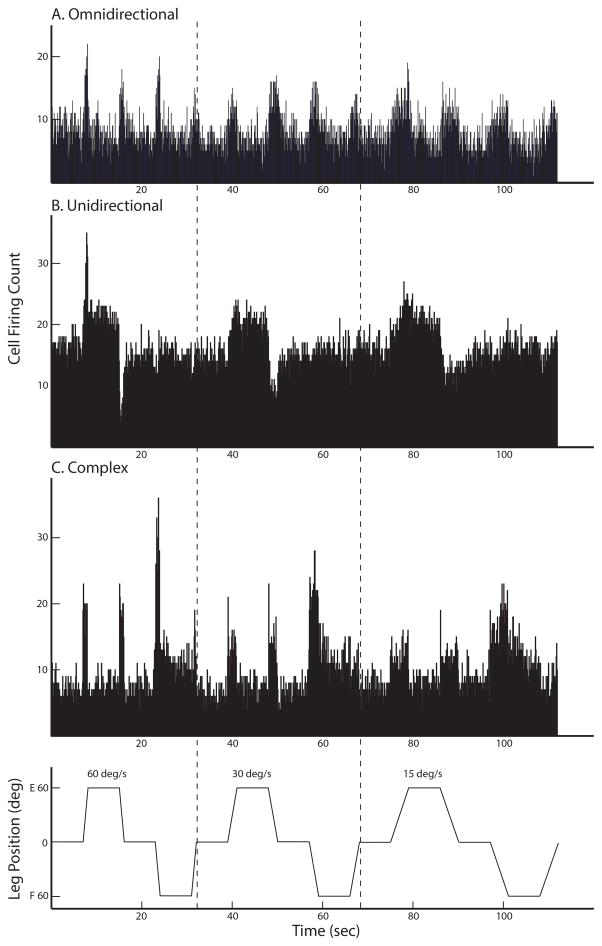
The activities of 41 rFN neurons (41/53, 77%) were modulated during all hindlimb movements (neutral to extension; extension to neutral; neutral to flexion; and flexion to neutral), and thus were classified as omnidirectional (Arshian et al. 2014). Thirty-four of these neurons exhibited an increase (Fig. 1 and 2A) and 7 neurons had a decrease in firing rate during all directions of hindlimb movement.
Figure 2.
Categorization schema for responses of rFN neurons to hindlimb movement. A: The omnidirectional response type. In this example, the neuron exhibited an increase in firing rate with every movement of the hindlimb, regardless of the direction of movement. B: The unidirectional response type. In this example, the neuron exhibited an increase in firing rate when the hindlimb was extended (E). The increased activity was maintained until the end of the seven-second long hold segment. Additionally, this neuron exhibited a much more robust response to hindlimb movement when the velocity of movement was increased to 60°/sec (left panel) than at 30 or 15°/sec (middle and right panels, respectively). C: Complex responses appeared to be a combination of the omnidirectional and unidirectional response types. This neuron had an excitation response during all directions of hindlimb movement, along with a sustained increase in firing when the limb was maximally flexed (F).
One rFN neuron was classified as bidirectional (Arshian et al. 2014). It exhibited a decrease in firing rate as the hindlimb was moved in both directions away from the neutral position (neutral to extension and neutral to flexion), but its firing rate was not modulated by movement toward the neutral position.
Omnidirectional and bidirectional neurons do not overtly encode the direction in which the hindlimb is being moved from the neutral position (Arshian et al. 2014). Thus, the majority of rFN neurons (42/53, 79%) did not encode the direction of limb movement.
In contrast, eleven rFN neurons (11/53, 21%) exhibited responses that did encode the direction of limb movement. Six of these neurons had unidirectional responses (Fig. 2B): their firing rate was increased (or decreased) during one direction of hindlimb movement away from neutral (neutral to extension or neutral to flexion), but was not altered by movement in the other direction away from neutral (Arshian et al. 2014). The firing rate of most of the rFN neurons with unidirectional responses (5 neurons) was altered by hindlimb extension; two of these units had excitation responses and three had inhibition responses. One rFN neuron with a unidirectional response responded to hindlimb flexion; it exhibited an excitation response. Two rFN neurons had reciprocal responses to hindlimb movement; reciprocal neurons exhibit an excitation response to one direction of hindlimb movement away from neutral and an inhibition response to the other direction of movement away from neutral (Arshian et al. 2014). The excitation response was with hindlimb extension in one neuron and with hindlimb flexion in the other neuron.
In addition to analyzing neural responses to hindlimb movement, as described above, we ascertained if responses were sustained while the hindlimb was held in extension or flexion. Five of six neurons with unidirectional responses, both neurons with reciprocal responses, and the neuron with bidirectional responses to hindlimb movement also had sustained alterations in firing rate through the hold period following movements that elicited responses, suggesting that these neurons additionally encoded limb position (e.g., Fig. 2B). In contrast, the 41 neurons with omnidirectional responses did not have sustained changes in firing rate through the hold period.
Three rFN neurons had responses that did not fit well into the above schema because they appeared to represent a combination of responses. In all three cases, the units exhibited omnidirectional responses combined with a sustained increase in firing when the limb was held in an eccentric position, much like a unidirectional position-related response (e.g. Fig. 2C). We classified these neurons as having complex responses to hindlimb movement. All three of these neurons exhibited excitatory responses to all directions of limb movement. Two of these neurons exhibited sustained increases in activity as the limb was held in extension, but one had sustained responses to limb flexion. Neurons with complex responses, because of their unidirectional position-related response, encode directional hindlimb position information.
The responses of rFN neurons to hindlimb movement were different from those previously noted for VN neurons, as summarized in Table 1. For example, the omnidirectional categorization was the most common response among rFN neurons (77%) and the least common response pattern among VN neurons (4%). Furthermore, most VN neurons overtly encoded information that conveyed the direction of hindlimb movement (and/or position) (81%), whereas fewer rFN neurons (21%) encoded such information (p < 0.0001, Fisher’s exact test).
Table 1.
Frequency of response types to hindlimb movement among rostral fastigial nucleus and vestibular nucleus neurons
| Rostral Fastigial Nucleus | Vestibular Nucleus* | |
|---|---|---|
| Response types: | ||
| Omnidirectional | 77% (41/53) | 4% (3/70) |
| Bidirectional | 2% (1/53) | 14% (10/70) |
| Unidirectional | 11% (6/53) | 29% (20/70) |
| Reciprocal | 4% (2/53) | 53% (37/70) |
| Complex | 6% (3/53) | 0% (0/70) |
| Neuron encodes direction of hindlimb movement/position: | ||
| Not directionally encoded | 79% (42/53) | 19% (13/70) |
| Directionally encoded | 21% (11/53) | 81% (57/70) |
Data for vestibular nucleus neurons are from (Arshian et al. 2014).
The effect of the velocity of hindlimb movements on rFN neuronal responses was ascertained by comparing peak responses to the three velocities delivered (15, 30, and 60°/sec). We restricted our analysis to excitatory responses because inhibitory responses in nearly all cases became saturated (i.e. the firing rate became zero during movement). Overall, there was a modest velocity dependence of responses (Fig. 3). Doubling limb movement velocity from 15°/sec to 30°/sec increased the magnitude of responses by 15%; quadrupling limb movement velocity to 60°/sec increased responses by 33% (p < 0.05, Friedman’s test with Dunn’s multiple comparisons test).
Figure 3.
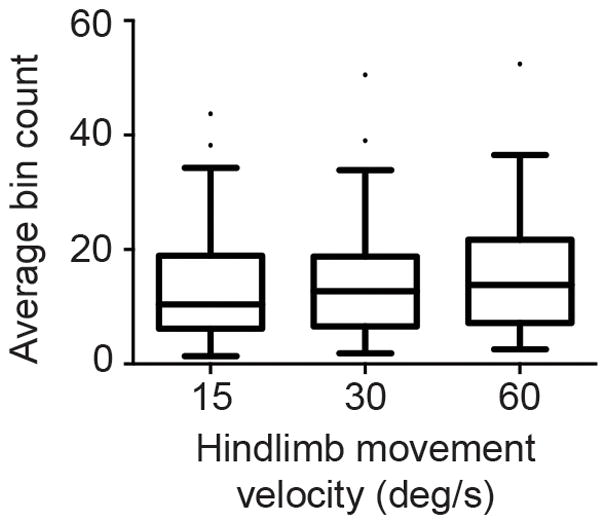
rFN neurons responsive to hindlimb movement are modestly sensitive to the velocity of movement. Data are presented as box-and-whisker Tukey plots: horizontal bars indicate the median; boxes indicate interquartile ranges (IQR); upper whiskers indicate the 75% percentile plus 1.5 IQR (or max data point if no outliers); lower whiskers indicate the 25% percentile minus 1.5 IQR (or min data point if no outliers); dots indicate outlier data points.
Responses of hindlimb movement sensitive rFN neurons to rotations in vertical planes
Thirty-four rFN neurons whose activity was modulated by hindlimb movement were also tested for responsiveness to rotations of the body in vertical planes (wobble stimuli) that activate labyrinthine receptors; the remaining units were lost before vestibular stimuli were delivered. The firing rate of half of these rFN neurons (17/34, 50%) was modulated by wobble stimuli. Over half of those units (9/17, 53%) responded robustly to only one direction of rotation (gain of responses to CW and CCW rotations differed > 2-fold, as illustrated in Fig. 4). Such responses are due to convergence of vestibular inputs with different spatial and temporal characteristics, and are thus referred to as spatiotemporal convergence (STC) responses (Schor and Angelaki 1992; Moy et al. 2012). STC responses have previously been reported to be common amongst rFN neurons in the decerebrate cat (Catanzaro et al. 2014). Twenty percent of rFN neurons (1/5) with directional encoding of hindlimb movement had STC responses, whereas 67% of rFN neurons (8/12) without directional encoding had STC responses. Of the 8 neurons whose activity was modulated during both directions of wobble rotation, only 3 were held long enough to test for response dynamics at multiple frequencies near the plane of maximum stimulation (data not shown).
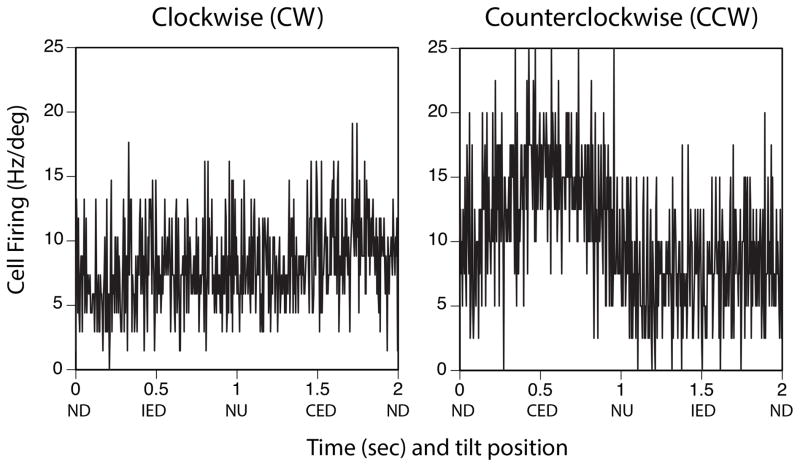
Figure 4.
Responses of a rFN neuron whose activity was modulated by one direction (CCW, average of 34 sweeps) of wobble stimulation but not the other (CW, average of 20 sweeps) and thus exhibited spatiotemporal convergence. Rotations were delivered at 0.5Hz with an amplitude of 5°. Signal-to-noise ratios were 0.93 for responses to CCW rotations and 0.26 for responses to CW rotation. CED contralateral ear down; CCW counterclockwise; CW clockwise; IED ipsilateral ear down; ND nose down; NU nose up.
Most rFN neurons were identified using hindlimb movement as the search stimulus. However, a subset of neurons was first identified using wobble stimuli that activated vestibular receptors, and then subsequently tested for responses to hindlimb movement. Of 23 rFN neurons that were selected for recording based on the presence of a response to vestibular stimulation, 2 (9%) exhibited responses to hindlimb movement.
Discussion
Our results demonstrate that the activity of a population of rFN neurons is modulated by both hindlimb movement and rotations of the body in vertical planes. These results build on prior work showing that rFN neurons receive convergent somatosensory inputs from the neck and vestibular inputs (Kleine et al. 2004; Brooks and Cullen 2009). Taken together, these results suggest that a group of rFN neurons utilize somatosensory information from all spinal levels to perform computations relevant to the maintenance of postural control.
Our central hypothesis for the present work was that hindlimb somatosensory inputs responsible for modulation of activity of VN neurons during hindlimb movement are routed through neurons within the rFN. While the data generated in this study do not completely exclude this possibility (discussed later), the results suggest the hypothesis be rejected. The responses of rFN neurons to hindlimb movement differed dramatically from those previously reported for VN neurons (Arshian et al. 2014). The most frequent response type among rFN neurons (omnidirectional, 77%) was infrequent amongst VN neurons (4%), and the most frequent response type amongst VN neurons (reciprocal, 53%) was infrequent amongst rFN neurons (4%). While the majority of VN neurons encoded the direction of hindlimb movement (81%), few rFN neurons did so (21%). These substantial differences in response types of rFN and VN neurons to hindlimb movement cannot be explained by differences in experimental approach, since all experiments were performed in decerebrate cats utilizing the same equipment, hindlimb movement paradigm, and investigators (Arshian et al. 2014). Consequently, these results suggest that somatosensory hindlimb inputs are not simply relayed to VN neurons through rFN neurons (or vice versa), excepting the possibility that a subgroup of rFN neurons (consisting predominantly of rFN neurons with directionally-encoded responses to limb movement) projects to the VN. Therefore the central question remains: How is somatosensory information conveyed from the hindlimb to VN neurons? It has been hypothesized that somatosensory afferent inputs to the cerebellum are carried via a parallel-distributed neural network (Osborn and Poppele 1992); perhaps somatosensory limb inputs are routed to the VN through a similar network, of which the rFN represents one portion of a single parallel route. Alternative indirect spinovestibular pathways, such as spinoreticular networks, and/or direct spinovestibular pathways could represent other portions of a parallel-distributed network, or alternatively may represent the dominant pathway conveying somatosensory hindlimb input to the VN (Maunz et al. 1978; McKelvey-Briggs et al. 1989; Huber et al. 1999). Ultimately, multiple parallel pathways may convey hindlimb somatosensory signals to VN neurons, which integrate the signals to subsequently alter vestibulospinal outflow. Further studies are needed to distinguish between these possibilities.
Another open question is how the responses of rFN neurons to hindlimb movement promotes postural stability. As previously indicated, the most common response pattern observed among rFN neurons during hindlimb movement was the omnidirectional type (77%), in which movement of the hindlimb is encoded with all directions of movement. This response type likely reflects the complex integration of proprioceptive signals that occurs at lower spinal cord levels. For example, some neurons in the dorsal spinocerebellar tract (DSCT) respond with excitation (or inhibition) responses to both flexion and extension movements of the hindlimb (Osborn and Poppele 1992; Bosco and Poppele 1993); such responses are strikingly similar to the omnidirectional responses to hindlimb movement that we observed so commonly among rFN neurons. We speculate that such responses may render rFN neurons more sensitive (in the case of excitatory omnidirectional responses) to vestibular inputs during limb movement, sending downstream signals to correct or adjust postural responses. Further studies designed to simultaneously provide hindlimb and body movement (vestibular stimulation) will be required to address this hypothesis. Moreover, the responses of DSCT neurons to hindlimb movement is highly dependent upon the characteristics of the movement strategy employed, such that neural responses can vary dramatically even when the resultant leg or muscle movements are well matched (Bosco and Poppele 1993; Bosco and Poppele 2001). Responses to hindlimb movement in the rFN may also vary with different hindlimb movement paradigms. For example, responses could be different if the limb movement was self-generated instead of passive. Furthermore, differences in preparation sometimes have significant effects on the behavior of neural networks, such that the same stimulus will elicit different responses if the animal is decerebrate or conscious (Destefino et al. 2011; Catanzaro et al. 2014). Therefore, follow-up studies in conscious animals, which incorporate both active and passive hindlimb movements, should shed light on how hindlimb movements are processed by rFN neurons, and may provide further insights regarding the relevance of these signals in postural control.
Acknowledgments
The authors thank Danielle Akinsanmi, George Bourdages, Alex Carter, Valerie Casuccio, and Tom Cooper for their contributions to the project. Funding was provided by a Triological Society Research Career Development Award and a Hearing Health Foundation Emerging Research Grant (to A. A. McCall) and by National Institutes of Health grant R01-DC003732 (to B.J. Yates).
References
- Arshian MS, Hobson CE, Catanzaro MF, et al. Vestibular nucleus neurons respond to hindlimb movement in the decerebrate cat. J Neurophysiol. 2014;111:2423–2432. doi: 10.1152/jn.00855.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma C, Thach WT, Jones EG. Brainstem and spinal projections of the deep cerebellar nuclei in the monkey, with observations on the brainstem projections of the dorsal column nuclei. Brain Res. 1983;286:299–322. doi: 10.1016/0165-0173(83)90017-6. [DOI] [PubMed] [Google Scholar]
- Batton RR, 3rd, Jayaraman A, Ruggiero D, Carpenter MB. Fastigial efferent projections in the monkey: an autoradiographic study. J Comp Neurol. 1977;174:281–305. doi: 10.1002/cne.901740206. [DOI] [PubMed] [Google Scholar]
- Berman AI. The Brain Stem of the Cat. University of Wisconsin Press; Madison: 1968. [Google Scholar]
- Bosco G, Poppele RE. Broad directional tuning in spinal projections to the cerebellum. J Neurophysiol. 1993;70:863–866. doi: 10.1152/jn.1993.70.2.863. [DOI] [PubMed] [Google Scholar]
- Bosco G, Poppele RE. Proprioception from a spinocerebellar perspective. Physiol Rev. 2001;81:539–568. doi: 10.1152/physrev.2001.81.2.539. [DOI] [PubMed] [Google Scholar]
- Brooks JX, Cullen KE. Multimodal integration in rostral fastigial nucleus provides an estimate of body movement. J Neurosci. 2009;29:10499–10511. doi: 10.1523/JNEUROSCI.1937-09.2009. 29/34/10499 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro MF, Miller DJ, Cotter LA, McCall AA, Yates BJ. Integration of vestibular and gastrointestinal inputs by cerebellar fastigial nucleus neurons: multisensory influences on motion sickness. Exp Brain Res. 2014;232:2581–2589. doi: 10.1007/s00221-014-3898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destefino VJ, Reighard DA, Sugiyama Y, et al. Responses of neurons in the rostral ventrolateral medulla to whole body rotations: comparisons in decerebrate and conscious cats. J Appl Physiol. 2011;110:1699–1707. doi: 10.1152/japplphysiol.00180.2011. japplphysiol.00180.2011 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Rantucci T, Sabah NH, Taborikova H. Somatotopic studies on cerebellar fastigial cells. Exp Brain Res. 1974a;19:100–118. doi: 10.1007/BF00233397. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Sabah NH, Taborikova H. Excitatory and inhibitory responses of neurones of the cerebellar fastigial nucleus. Exp Brain Res. 1974b;19:61–77. doi: 10.1007/BF00233395. [DOI] [PubMed] [Google Scholar]
- Fredrickson JM, Schwarz D, Kornhuber HH. Convergence and interaction of vestibular and deep somatic afferents upon neurons in the vestibular nuclei of the cat. Acta-Otolaryngol. 1966;61:168–188. doi: 10.3109/00016486609127054. [DOI] [PubMed] [Google Scholar]
- Ghelarducci B. Responses of the cerebellar fastigial neurones to tilt. Pflugers Arch. 1973;344:195–206. doi: 10.1007/BF00588460. [DOI] [PubMed] [Google Scholar]
- Ghelarducci B, Pompeiano O, Spyer KM. Distribution of the neuronal responses to static tilts within the cerebellar fastigial nucleus. Archives Italiennes de Biologie. 1974;112:126–141. [PubMed] [Google Scholar]
- Grasso C, Barresi M, Scattina E, Orsini P, Vignali E, Bruschini L, Manzoni D. Tuning of human vestibulospinal reflexes by leg rotation. Hum Mov Sci. 2011;30:296–313. doi: 10.1016/j.humov.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Gray C, Perciavalle V, Poppele R. Sensory responses to passive hindlimb joint rotation in the cerebellar cortex of the cat. Brain Res. 1993;622:280–284. doi: 10.1016/0006-8993(93)90829-c. [DOI] [PubMed] [Google Scholar]
- Homma Y, Nonaka S, Matsuyama K, Mori S. Fastigiofugal projection to the brainstem nuclei in the cat: an anterograde PHA-L tracing study. Neurosci Res. 1995;23:89–102. [PubMed] [Google Scholar]
- Huber J, Grottel K, Mrowczynski W, Krutki P. Spinoreticular neurons in the second sacral segment of the feline spinal cord. Neurosci Res. 1999;34:59–65. doi: 10.1016/s0168-0102(99)00034-6. [DOI] [PubMed] [Google Scholar]
- Ito M, Udo M, Mano N, Kawai N. Synaptic action of the fastigiobulbar impulses upon neurones in the medullary reticular formation and vestibular nuclei. Exp Brain Res. 1970;11:29–47. doi: 10.1007/BF00234200. [DOI] [PubMed] [Google Scholar]
- Jian BJ, Shintani T, Emanuel BA, Yates BJ. Convergence of limb, visceral, and vertical semicircular canal or otolith inputs onto vestibular nucleus neurons. Exp Brain Res. 2002;144:247–257. doi: 10.1007/s00221-002-1042-8. [DOI] [PubMed] [Google Scholar]
- Kleine JF, Guan Y, Kipiani E, Glonti L, Hoshi M, Buttner U. Trunk position influences vestibular responses of fastigial nucleus neurons in the alert monkey. J Neurophysiol. 2004;91:2090–2100. doi: 10.1152/jn.00849.2003. [DOI] [PubMed] [Google Scholar]
- Marsden JF, Castellote J, Day BL. Bipedal distribution of human vestibular-evoked postural responses during asymmetrical standing. J Physiol. 2002;542:323–331. doi: 10.1113/jphysiol.2002.019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M. Projections from the lowest lumbar and sacral-caudal segments to the cerebellar nuclei in the rat, studied by anterograde axonal tracing. J Comp Neurol. 1999;404:21–32. [PubMed] [Google Scholar]
- Matsuyama K, Jankowska E. Coupling between feline cerebellum (fastigial neurons) and motoneurons innervating hindlimb muscles. J Neurophysiol. 2004;91:1183–1192. doi: 10.1152/jn.00896.2003. [DOI] [PubMed] [Google Scholar]
- Maunz RA, Pitts NG, Peterson BW. Cat spinoreticular neurons: locations, responses and changes in responses during repetitive stimulation. Brain Res. 1978;148:365–379. doi: 10.1016/0006-8993(78)90725-4. [DOI] [PubMed] [Google Scholar]
- McCall AA, Moy JD, Puterbaugh SR, DeMayo WM, Yates BJ. Responses of vestibular nucleus neurons to inputs from the hindlimb are enhanced following a bilateral labyrinthectomy. J Appl Physiol (1985) 2013;114:742–751. doi: 10.1152/japplphysiol.01389.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey-Briggs DK, Saint-Cyr JA, Spence SJ, Partlow GD. A reinvestigation of the spinovestibular projection in the cat using axonal transport techniques. Anat Embryol (Berl) 1989;180:281–291. doi: 10.1007/BF00315886. [DOI] [PubMed] [Google Scholar]
- Miller DM, Cotter LA, Gandhi NJ, et al. Responses of rostral fastigial nucleus neurons of conscious cats to rotations in vertical planes. Neuroscience. 2008;155:317–325. doi: 10.1016/j.neuroscience.2008.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy JD, Miller DJ, Catanzaro MF, et al. Responses of neurons in the caudal medullary lateral tegmental field to visceral inputs and vestibular stimulation in vertical planes. Am J Physiol Regul Integr Comp Physiol. 2012;303:R929–940. doi: 10.1152/ajpregu.00356.2012. ajpregu.00356.2012 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovsky GN. Activity of vestibulospinal neurons during locomotion. Brain Res. 1972;46:85–98. doi: 10.1016/0006-8993(72)90007-8. [DOI] [PubMed] [Google Scholar]
- Osborn CE, Poppele RE. Parallel distributed network characteristics of the DSCT. J Neurophysiol. 1992;68:1100–1112. doi: 10.1152/jn.1992.68.4.1100. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Functional Organization of the Spino- and Cuneocerebellar Tracts. Physiol Rev. 1965;45:495–522. doi: 10.1152/physrev.1965.45.3.495. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of the dorsal spino-olivocerebellar path. J Physiol. 1969;200:129–149. doi: 10.1113/jphysiol.1969.sp008685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- Schor RH, Angelaki DE. The algebra of neural response vectors. Ann N Y Acad Sci. 1992;656:190–204. doi: 10.1111/j.1749-6632.1992.tb25209.x. [DOI] [PubMed] [Google Scholar]
- Schor RH, Miller AD, Tomko DL. Responses to head tilt in cat central vestibular neurons. I. Direction of maximum sensitivity. J Neurophysiol. 1984;51:136–146. doi: 10.1152/jn.1984.51.1.136. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Ghasia FF, Dickman JD, Angelaki DE. Properties of cerebellar fastigial neurons during translation, rotation, and eye movements. J Neurophysiol. 2005;93:853–863. doi: 10.1152/jn.00879.2004. [DOI] [PubMed] [Google Scholar]
- Siebold C, Glonti L, Glasauer S, Buttner U. Rostral fastigial nucleus activity in the alert monkey during three-dimensional passive head movements. J Neurophysiol. 1997;77:1432–1446. doi: 10.1152/jn.1997.77.3.1432. [DOI] [PubMed] [Google Scholar]
- Stanojevic M. Responses of cerebellar fastigial neurons to neck and macular vestibular inputs. Pflugers Arch. 1981;391:267–272. doi: 10.1007/BF00581505. [DOI] [PubMed] [Google Scholar]
- Stanojevic M, Erway L, Ghelarducci B, Pompeiano O, Willis WD., Jr A comparison of the response characteristics of cerebellar fastigial and vermal cortex neurons to sinusoidal stimulation of macular vestibular receptors. Pflugers Arch. 1980;385:95–104. doi: 10.1007/BF00588687. [DOI] [PubMed] [Google Scholar]
- Welgampola MS, Colebatch JG. Vestibulospinal reflexes: quantitative effects of sensory feedback and postural task. Exp Brain Res. 2001;139:345–353. doi: 10.1007/s002210100754. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Kato M, Thomas RC, Peterson BW. Excitation of lateral vestibular neurons by peripheral afferent fibers. J Neurophysiol. 1966;29:508–529. doi: 10.1152/jn.1966.29.3.508. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Uchino Y, Maunz RA, Susswein A, Fukushima K. Properties and connections of cat fastigiospinal neurons. Exp Brain Res. 1978;32:1–17. doi: 10.1007/BF00237385. [DOI] [PubMed] [Google Scholar]