Abstract
Objectives
Many novel strategies aimed at neuroprotection or neurorestoration involve surgical delivery of agents to deep nuclei along multiple trajectories. Using intracerebral hemorrhage (ICH) on a per trajectory basis as our primary endpoint, we quantified the level of surgical risk associated with agent delivery to deep nuclei. Secondarily, we quantified other event rates and examined relationships between ICH and eight variables related to patient and practice characteristics.
Methods
Meta-analytic techniques were used to pool complication rates reported in published articles involving deep brain stimulator electrode implantation or infusion of vectors, tissues, or trophic factors.
Results
109 studies were included in our analysis, comprising 6237 patients and 9890 trajectories to deep nuclei. The estimated per-trajectory ICH rate was 1.57% (95% confidence interval: 1.26%-1.95%). The proportion of trajectories leading to permanent or serious neurological deficits was 0.41% (0.28%- 0.60%). The estimated mortality rate per trajectory was 0.14% (0.07%-0.29%). No relationship between ICH and sex, age, duration of disease, or exclusion of patients with surgical complications was observed; a significant positive relationship was observed with use of microelectrode recording, and a significant negative relationship with putamenal delivery. Our results show significant differences in ICH rates between inoculations and electrode implantation.
Conclusions
Our findings suggest that studies involving multiple trajectories to deep nuclei involve a high level of risk. However, inoculations may be significantly safer than electrode implantation. Our analysis has implications for the ethics of preclinical research, independent review of risk, subject selection, and adverse event reporting.
Keywords: surgical delivery, Parkinson's disease, deep nuclei, gene transfer, cell transplantation, research ethics
Introduction
Over the past several decades, a variety of novel interventions have been tested in clinical trials for neurodegenerative disorders involving deep nuclei. Many such interventions, like tissue transplantation, involve surgical delivery to various structures in deep nuclei.1-3 While several issues on these trials have prompted ethical debates (e.g. surgical sham controls2; or fetal tissue4), in this paper, we address a less widely discussed ethical dimension of trials involving deep nuclei delivery: surgical risk. Each trajectory (that is, infusion or implantation of material using a needle or cannula) carries a baseline level of risk in addition to the experimental agents themselves. We have previously argued that the relatively high baseline risk associated with surgical procedures warrants careful ethical justification for initiating translational trials.5 However, reports on surgical risk vary widely, and generally pertain to electrode implantation and not inoculations.
In this study, we assume that surgical procedures used to implant electrodes are similar enough to those used for intracerebral delivery (growth factors, viral vectors, or cells) such that studies of the former can be used to estimate risk for the latter. We performed a systematic review to quantify and characterize the risk associated with trajectories to deep nuclei, and analyzed factors associated with higher complication rates.
Methods
Search
We searched the PubMed on May 1st, 2008 using the following terms: GDNF AND parkins*; fetal tissue AND parkinso*; (Deep OR subthala* OR pallid* OR striat* OR putam*) AND stimulat* AND parkinso*; Stereota* AND (Deep brain stimulat* OR electrod*) AND (complica* OR advers* OR hemorr* OR haemor* OR infection); trophic AND (factor OR factors) AND inject*; (cell therap* or gene) AND (parkins* OR movement disorder). Articles not reporting clinical trials or retrospective chart reviews, that were published before May 1998, or that were not in English were excluded. Article abstracts were then screened for whether they: involved surgical intervention targeting deep nuclei; actively reported adverse events; contained original data; and involved a movement disorder. Reports of lesioning (e.g. pallidotomy) were excluded because of inability to attribute cause to targeting versus lesion itself. We also added additional articles identified in bibliographies, reviews, and by performing “related article” searches.
Extraction
The extraction form was generated by JK and KD in consultation with co-authors. Information about study characteristics, patient characteristics, and surgical procedures was recorded. Certain items, like number of passes for each trajectory, were reported with insufficient frequency to justify recording in our extraction. Adverse events were classified as surgically related if they were explicitly attributed to cranial surgery or occurred within 30 days of cranial surgery. Events related to pulse generator implantation for deep brain stimulation (DBS) studies were not recorded, as this study was aimed at investigating events that might occur in studies involving inoculation. Adverse events (AEs) were classified according to presentation (e.g. psychiatric, neurological deficit, etc.), process (e.g. intracerebral hemorrhage (ICH)), reported severity (e.g. serious, minor, or asymptomatic) and duration (e.g. transient or permanent). We defaulted to negatives when items were not specified (e.g. if microelectrode recording was not mentioned in the methods, we recorded it as not performed). When minor operative complications were reported without specifically excluding major complications (e.g. transient post-operative confusion but no mention of any ICHs), we defaulted to a negative (e.g. no ICH occurred). After piloting to establish criteria for classifying studies and AEs, JK and KD extracted each of the 109 articles independently. Extractions for each article were compared for differences, which were then resolved by discussion.
Analysis
Data were entered into an Excel spreadsheet. The number and proportion of surgically related AEs were calculated on a per patient and per trajectory basis. We prospectively identified seven variables (described in results) that we believed might relate to rates of ICH. After our initial analysis, we added an eighth variable—whether the procedure involved inoculation or electrode implantation. In what follows, we use the word “trajectory” to denote any electrode implantation or agent inoculation. For calculating event rates on a per trajectory basis, data were analyzed using negative binomial regression. This model is a generalization of the Poisson model which assumes that the rate of ICHs per trajectory in each study is some constant value λ and that the variance is equal to the expected value. Instead, the negative binomial model assumes that the rate in the ith study is some number λi, that the average rate over all studies is some number λ, and that the variance between studies is equal to λ2/θ. The additional parameter θ therefore allows for heterogeneity between rates in different studies. Furthermore, the expected value is also allowed to depend on one or more explanatory variables such as the average age in the study or the brain region receiving the implantation. The heterogeneity between studies is inevitable, and may be caused by a variety of factors such as differences in patient population, surgical procedure, or surgeons' expertise. The statistical significance (p-value) of the explanatory variable was calculated using the likelihood ratio test. Data analysis was carried out in R, version 2.8.0.6 A limitation of this model is that it assumes each implantation to be independent and thus ignores between-patient variability. This limitation is imposed by the fact that the studies reviewed did not consistently provide any patient-level information.
Results
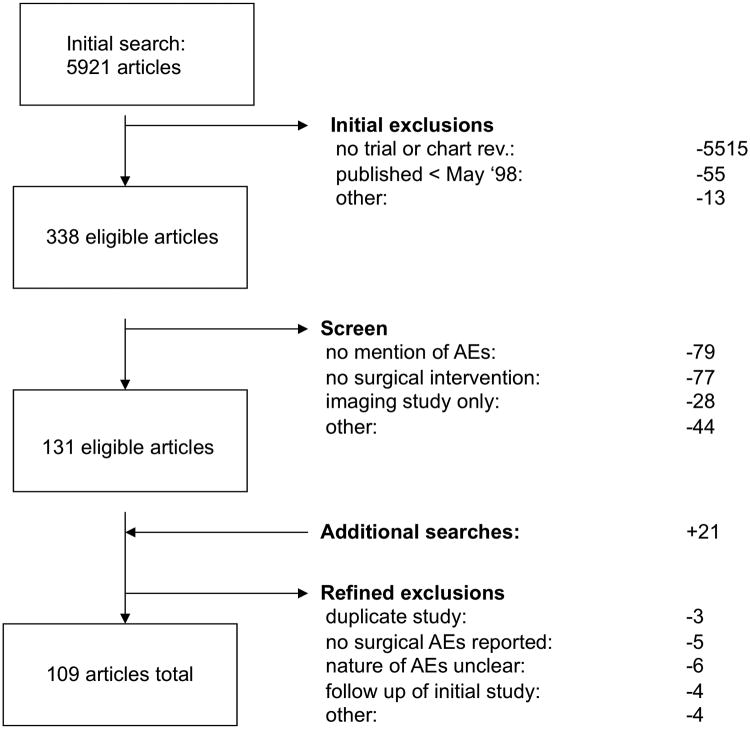
Our search identified 109 eligible articles (figure 1; see appendix for full listing). Basic characteristics of studies and procedures are shown in table 1; patient characteristics are shown in table 2.
Figure 1. Search Strategy for Meta-analysis.
Table 1. Characteristics of Studies Analyzed.
| Study Type | n (proportion) |
|---|---|
| Open label, prospective | 79 (.72) |
| Retrospective | 21 (.19) |
| Randomized Controlled | 9 (.083) |
| Location | |
| North America | 54 (.50) |
| Other | 55 (.50) |
| Year of Publication | |
| 1998- 2001 | 28 (.26) |
| 2002- 2005 | 40 (.37) |
| 2006-2008 | 41 (.38) |
| Procedure | |
| electrode implantation | 96 (.88) |
| trophic factor | 2 (.018) |
| gene transfer | 2 (.018) |
| cell transplantation | 9 (.082) |
| Microelectrode recording (DBS studies only) | |
| yes | 53 (.55) |
| no | 20 (.21) |
| variable or indeterminate | 23 (.24) |
| Exclusion of Sx-contraindications | |
| Yes | 52 (.48) |
| No | 57 (.52) |
| Total | 109 (1.0) |
Table 2. Patient and Procedure Characteristics.
| Sex | patients (proportion) | trajectories (proportion) |
|---|---|---|
| Males | 2919 (.47) | NA |
| Females | 1558 (.25) | NA |
| Indeterminate | 1760 (.28) | NA |
| Indication | ||
| Parkinson's | 3384 (.54) | 5803 (.59) |
| Dystonia | 50 (.0080) | 100 (.010) |
| Variable within study | 2803 (.45) | 3987 (.40) |
| Brain region targetted | ||
| STN | 1931 (.31) | 3584 (.36) |
| Thalamus | 323 (.052) | 314 (.032) |
| Gpi | 188 (.030) | 295 (.030) |
| Putamen | 188 (.030) | 1300 (.13) |
| Prelemniscal Rad. Pts | 20 (.0032) | 25 (.0025) |
| Variable deep nuclei | 3587 (.57) | 4372 (.44) |
| Timing of Procedure | ||
| Mean Age | 59.76 | NA |
| Age Range | 11 yrs - 88 yrs | NA |
| Mean Disease Duration | 13.39 | NA |
| Total | 6237 | 9890 |
With respect to our primary objective, the average risk of ICH per trajectory, adjusted for between-study heterogeneity, was 1.57% (95% CI, 1.26%-1.95%). For all events (including ICH), the average adjusted risk was 11.70% per trajectory (95% CI, 9.74% - 14.04%). Per trajectory adjusted risk of mortality was 0.14% (95% CI, 0.07%-0.29%); the rate for permanent or serious AEs was 0.41% (95% CI, 0.28%- 0.60%).
Psychiatric events were the most common adverse event reported (table 3); the majority of these were transient. The most common permanent or serious AEs involved neurological deficits—generally related to ICH. The clinical presentation of serious and/or permanent psychiatric and neurological AEs is provided in table 3C.
Table 3. Adverse Events on a Per Trajectory Basis.
| Total, n (%) | Permanent n (%) | Serious n (%) | Asympt n (%) | |
|---|---|---|---|---|
| A) Processes | ||||
| ICH | 193 (1.6) | 46 (.41) | 19 (.15) | 46 (.43) |
| Stroke | 5 (.05) | 1 (.01) | 0 (0.0) | 0 (0.0) |
| Total | 1072 (10) | 138 (1.3) | 73 (.61) | 48 (.49) |
| B) Clinical Presentation | ||||
| Total, n (%) | Permanent n (%) | Serious n (%) | ||
| Psychiatric | 377 (3.8) | 28 (.28) | 15 (.15) | |
| Neurological Deficit | 251(2.4) | 52 (.49) | 23 (.23) | |
| Other | 120 (1.2) | 4 (.04) | 2 (.020) | |
| Seizure | 47 (.48) | 1 (.01) | 0 (0.00) | |
| Infection | 30 (.29%) | 0 (0.0) | 0 (0.0) | |
| Pneumonia | 17 (.10) | 2 (.02) | 2 (.02) | |
| Death | 15 (.12) | NA | NA | |
| C) Serious or Permanent Adverse Events | ||||
| Total, n (%) | ||||
| Psychiatric | ||||
| Depression | 7 (.07) | |||
| Cognitive Impair/ Decline | 7 (.07) | |||
| Major Psychosis/ Psychiatric | 6 (.06) | |||
| Dementia | 4 (.04) | |||
| Other | 7 (.07) | |||
| Total | 31 (.31) | |||
| Neurological | ||||
| Not Specified: | 28 (.28) | |||
| Cognitive / Speech Deficits: | 22 (.22) | |||
| Hemiparesis: | 11 (.11) | |||
| Dysesthesia: | 2 (.02) | |||
| Hemiplegia: | 2 (.02) | |||
| Other | 6 (.06) | |||
| Total | 71 (.72) | |||
Note: all percentages reflect crude estimates (i.e. events / trajectories)
We sought to determine whether primary endpoint events could be related to seven prospectively selected variables: patient sex, age, disease duration, brain target, whether patients with surgical contraindications were excluded, date of publication, and use of microelectrode recording. Two variables showed significant relationships with ICH: brain region and microelectrode recording.
We identified six different brain region targets among the 80 manuscripts that listed brain region: Gpi (12), STN (49), putamen (10), thalamus (1), prelemniscal radiations (1) and variable(9) (note- many manuscripts had to be excluded from this analysis because they involved trajectories to more than one brain region but did not report per trajectory AEs according to targetting). Brain region entered into the binomial regression model as a categorical variable predicting ICH was highly significant (p<0.0001). Post-hoc comparisons indicated that implantations in the putamen were associated with a lower risk (p<0.0001) than other regions of the brain (RR=0.28, CI: 0.10-0.73) but no other comparisons were significant. Micro-electrode recording was associated with a higher risk of ICH (p=0.003) with an relative risk of 5.61 (95% CI, 1.29-24.4).
Because studies involving inoculations have often targeted the putamen and do not generally use microelectrode recording, we also performed a post-hoc analysis examining whether event rates were substantially different between studies involving electrode implantation and those involving inoculations. Rates of ICH were significantly lower in patients receiving inoculations than in electrodes (0.4% ICH/trajectory [95% CI: 0.2- .09] vs. 2.0%, [95% CI: 1.2-3.4]; p = 0.01).
Discussion
Our analysis represents the largest, most detailed study of surgical complication rates associated with delivery to deep nuclei. Given that the average number of trajectories per patient in our review was 1.92, our ICH rate of 1.57% per trajectory translates to an overall risk per patient of 3%. Recent meta-analyses of surgical risk for DBS reported slightly higher rates of per patient ICH (3.2%7 and 3.9%8), and found that mental status / behavioral changes were the most common adverse event. Another group recently reported lower event rates, with 1.31% ICH and.02% deaths on a per patient basis.9 Several factors might explain differences in our results: our literature search excluded all articles that did not actively report surgical events, we did not limit our analysis to interventions targeting the subthalamic nucleus, we included interventions other than DBS, we did not restrict our search to Parkinson's disease, and our time range was slightly different. Several teams have previously attempted to identify factors associated with elevated risk (table 4). Our study reproduced some, but not all, relationships previously observed between surgical risk factors and ICH.
Table 4. Studies Providing Statistical Analysis on Relationship of Risk Factors and Surgical Complications.
| Author | year | event | age | sex | disease duration | target | MER | hypertension | other |
|---|---|---|---|---|---|---|---|---|---|
| Binder | 2003 | ICH | NS | S | NS | previous cases in series (NS) | |||
| Binder et al | 2005 | ICH | NS | S | NS | ||||
| DBSPD group | 2001 | ICH | S | ||||||
| Goodmann | 2006 | confusion | NS | NS | length of operation (NS) | ||||
| Gorgulho | 2005 | ICH | NS | S | |||||
| Kenney | 2007 | AE | S* | NS | NS | previous cases in series (NS) | |||
| Oh | 2002 | AE | NS | NS | |||||
| Ory-Magne | 2007 | ICH | S | ||||||
| Sansur | 2007 | ICH | S | S | NS | S | anticoagulant use, CCI score (NS) | ||
| Seijo | 2007 | AE | NS | NS | NS | ||||
| Present Study | ICH | NS | NS | NS | S | S |
Legend: AE = adverse event; CCI = Charlson comorbidity index; ICH= Intracerebral hemorrhage; NS = no statistically significant relationship; S= statistically significant relationship; blank cells indicate relationship was not examined;
relationship only seen in male patients
We were surprised that age or explicit exclusion of surgically contraindicated patients did not affect rates of ICH. A post hoc analysis failed to show a significant difference in per trajectory ICH rates for patients with Parkinson's disease versus other indications. We were also surprised that ICH rates did not consistently decline over time. The use of microelectrode recording conferred significantly greater risk, likely because this procedure involves several passes of an electrode through deep nuclei. Unfortunately, very few studies stated the number microelectrode passes, and we are unable to exclude the possibility that improvements in technique and imaging have reduced needed passes and hence the safety of MER. In our study, putaminal trajectory was associated with lower risk of ICH. This may be due to the structure's relative accessibility; alternatively, it may simply reflect the absence of MER in putaminal trajectories or a limitation in our study's power (only 13% of trajectories in our sample were putaminal). Our dataset did not support multiple regressions, and relationships (or lack) should be interpreted with caution.
A disproportionate number of procedures targeting the putamen involved inoculations rather than electrode implantations, and post hoc analysis revealed that rates of ICH for inoculations were significantly lower than electrode implantations. There are at least three interpretations. Contrary to the assumptions with which this study was initiated, inoculations might be significantly safer than implantations of electrodes. Alternatively, reporting quality of adverse events might be worse for studies involving inoculation, leading to a spurious difference in observed rates. Third, inoculations accounted for a minority of trajectories in our sample, and the low number of ICH may reflect limited powering. Supporting the first hypothesis, the outer diameter of the needle, catheter or cannula used for inoculations is often smaller (0.64 mm10 to 1.64 mm11) than the electrodes for DBS stimulation (1.27mm)12 and thus may inflict less damage to the brain tissue; as well, inoculation studies tend not to use microelectrode recording. Novel studies might also attract the attention of well established neurosurgeons and command a greater level of detail to procedures. With regards to reporting accuracy, it should be mentioned that 63% inoculation studies reported safety in general terms (e.g. “no major surgical complications were observed”). In contrast, electrode implantation studies tended to provide richer descriptions of events that specifically excluded certain types of events (e.g. “no ICH's were observed,” “no neurological deficits were observed,” etc.), and only 18% used general descriptions of safety.
We previously argued that early phase trials of Parkinson's disease interventions that require invasive delivery involve a relatively high baseline level of risk.5 Our analysis provides an estimate of the degree and nature of surgical risk in such studies. Assuming that risk of ICH is independent for each trajectory in a given session to deep nuclei and that electrode implantation and inoculation involve similar levels of risk (the latter is undermined by our post hoc analysis), our calculations suggests that protocols involving four trajectories to deep nuclei involve 0.58% risk of causing death. This risk is comparable to that for phase 1 cancer studies, which involve.4913-.54%14 risk of mortality.14 However, the ethical analysis of risk for invasive cell or gene transfer studies in the setting of PD is complicated by two differences with phase 1 cancer studies. First, total risk in PD studies is likely to be greater than the estimate above, because the gene or cell agent confers additional risk. Second, patients in PD studies may be eligible for established effective care like DBS. They may thus be exposed to surgical risk plus burdens incurred by their forgoing alternative care options like DBS.
On the other hand, our findings raise the unexpected possibility that inoculation may be considerably safer than electrode implantation. To date, four gene transfer trials involving PD have been completed, involving 400 trajectories.1, 15-17 On the basis of publications and events reported to the Recombinant DNA Advisory Committee, serious surgical complications (as defined in public adverse event registries or publications) were reported in only two volunteers. As no deaths were recorded in the inoculation group, we are unable to estimate mortality rates for inoculations. Nevertheless, gene transfer studies for other neurological disorders have involved serious adverse events related to surgical procedures. For example, in a phase 1 trial involving gene transfer and Alzheimer's disease, two of eight volunteers suffered brain hemorrhages from surgery; one died as a consequence. 18-19
Our analysis has several limitations besides those described above. Our inclusion criteria might have enriched for studies reporting surgical complications, since we excluded articles that did not actively report on surgical outcomes. However, this criterion is to be preferred, as we suspect that surgical adverse events are under-reported given that most studies are primarily directed at characterizing interventions rather than delivery methods. With respect to classifying events that were reported, our approach was conservative in that we relied entirely on self-report of severity. Thus, any report of hemiparesis was not classified as a serious adverse event unless investigators labeled it as such. Limiting our analysis of adverse events were problems with descriptions, categories, and reporting quality for adverse events in our sample. In some instances, outcomes were reported on a per trajectory basis but not on a per patient basis, and vice versa. Different terminologies were used to describe similar events (e.g. confusion vs. “altered mental status” vs. delirium), and authors often did not provide much information on outcomes following surgical complications.
We also are aware that surgical procedures used to implant electrodes may have differences with those for inoculation. For example, microelectrode recording is not used in gene transfer studies (with exception of cases receiving AAV-GAD delivery in to the subthalamic nucleus15). Yet studies involving inoculation may use delivery co-interventions like convection-enhanced delivery that confer additional risk due to distribution of infusate beyond the borders of the target structure.
Our findings have ethical implications for the design, initiation, and review of trials involving multiple trajectories to deep nuclei:
1-Surgical risk as an add-on to a clinical trial
Investigators and reviewers should not allow considerations of cell or gene transfer risk to eclipse those related to surgical procedures. Investigators and reviewers should further consider ways surgical risk can be lowered in early phase studies. One possibility would be to take an extremely restrictive position on studies that propose the performance of sham inoculations to deep nuclei, as was reported in one instance.2 Another would be to limit the number of inoculations in initial cohorts before proceeding to larger doses. Some may counter that this would worsen the risk- direct benefit balance of studies (because patients would receive subtherapeutic doses). This tenability of this rejoinder hinges on claiming anti-Parkinson's agents qualify as therapies after only preclinical testing. One of us (JK) has elsewhere argued otherwise.20-21
2- Stringent assessment of benefits vs. risk for therapies requiring intracerebral interventions
Studies involving inoculation to deep nuclei should be premised on a particularly solid preclinical evidence base. Exposing patients to a relatively high level of risk has a much more compelling ethical justification if studies are supported by a robust evidence base.5
3- Subject eligibility for first-in-human studies (FIH)
Though our findings raise the possibility that inoculation is significantly safer than electrode implantation, absent robust evidence to this effect, we think researchers should be very cautious about trials involving patients who are otherwise eligible for established effective care like DBS. In contrast, patients who are no longer responsive to standard care endure less opportunity cost. Such a cautious approach may be appropriate in the early stages of testing, when the primary objective is establishment of parameters for subsequent testing.
4- Accurate reporting of AEs
We agree with Videnovic et al that AE reporting quality in the context of surgically delivered brain interventions could be improved.7 Investigators primarily interested in the safety of novel interventions may be less focused on safety reporting for comparably mundane surgical procedures. However, the cause of complications is generally less important to patients than the complication itself. Thorough and accurate reporting of surgical safety would provide a better evidence base for clinical and patient decision-making; investigators, referees, and journal editors should include or solicit information on surgical safety when reporting or reviewing manuscripts that report outcomes following invasive brain intervention.
In sum, on the premise that inoculations to deep nuclei and electrode implantation carry similar risk, we conclude the surgical risks of participating in a study involving multiple inoculations of cellular or vector materials to deep nuclei are similar to those for phase 1 cancer studies. However, our analysis raises the possibility that risk of ICH is considerably lower for inoculation than for electrode implantation. Though our estimates pertain to interventions that target deep nuclei, cellular agents directed at Alzheimer's, epilepsy, and various pediatric neurodegenerative diseases involve analogous risks.
Supplementary Material
Acknowledgments
This work was funded by Canadian Institutes of Health Research, States of Mind: Emerging Issues in Neuroethics (NNF 80045). We wish to thank Drs. Nir Lipsmann, Mark Bernstein, and Karl Sillay for helpful discussions. The authors further thank several anonymous referees for their very thoughtful and meticulous comments.
Financial Support: All financial and material support for this manuscript, regardless of date, was from the Canadian Institutes of Health Research (NNF 80045). Support unrelated to this research held by various authors comes from the following sources: CIHR, NINDS, Andrew W. Mellon Foundation, CDC, Kinetics Foundation, M. J. Fox Foundation, Parkinson's Disease Foundation, NIH -RARC base grant RR000167, Health Canada, US EPA, Heart and Stroke Foundation of Canada.
Footnotes
Interest Declaration: We declare no competing interests.
Supplemental Data: See attached file, Movement Disorders reference list.doc
References
- 1.Marks WJ, Jr, Ostrem JL, Verhagen L, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 2008;7(5):400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 2.London AJ, Kadane JB. Placebos that harm: sham surgery controls in clinical trials. Stat Methods Med Res. 2002;11(5):413–427. doi: 10.1191/0962280202sm300ra. [DOI] [PubMed] [Google Scholar]
- 3.Freed CR, Breeze RE, Schneck SA. Transplantation of fetal mesencephalic tissue in Parkinson's disease. N Engl J Med. 1995;333(11):730–731. doi: 10.1056/NEJM199509143331112. [DOI] [PubMed] [Google Scholar]
- 4.Master Z, McLeod M, Mendez I. Benefits, risks and ethical considerations in translation of stem cell research to clinical applications in Parkinson's disease. J Med Ethics. 2007;33(3):169–173. doi: 10.1136/jme.2005.013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimmelman J, London AJ, Ravina B, et al. Launching invasive, first-in-human trials against Parkinson's disease: ethical considerations. Mov Disord. 2009;24(13):1893–1901. doi: 10.1002/mds.22712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 7.Videnovic A, Metman LV. Deep brain stimulation for Parkinson's disease: prevalence of adverse events and need for standardized reporting. Mov Disord. 2008;23(3):343–349. doi: 10.1002/mds.21753. [DOI] [PubMed] [Google Scholar]
- 8.Kleiner-Fisman G, Herzog J, Fisman DN, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21(Suppl 14):S290–304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- 9.Appleby BS, Duggan PS, Regenberg A, Rabins PV. Psychiatric and neuropsychiatric adverse events associated with deep brain stimulation: A meta-analysis of ten years' experience. Mov Disord. 2007;22(12):1722–1728. doi: 10.1002/mds.21551. [DOI] [PubMed] [Google Scholar]
- 10.Gill CE, Konrad PE, Davis TL, Charles D. Deep brain stimulation for Parkinson's disease: the Vanderbilt University Medical Center experience, 1998-2004. Tenn Med. 2007;100(4):45–47. [PubMed] [Google Scholar]
- 11.Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005;102(2):216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- 12.Medtronic. Activa RC for Deep Brain Stimulation: Leads and Extensions. 2010 In: http://professional.medtronic.com/devices/activa-rc/leads-and-extensions/index.htm.
- 13.Horstmann E, McCabe MS, Grochow L, et al. Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med. 2005;352(9):895–904. doi: 10.1056/NEJMsa042220. [DOI] [PubMed] [Google Scholar]
- 14.Roberts TG, Jr, Goulart BH, Squitieri L, et al. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. Jama. 2004;292(17):2130–2140. doi: 10.1001/jama.292.17.2130. [DOI] [PubMed] [Google Scholar]
- 15.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369(9579):2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 16.Eberling JL, Jagust WJ, Christine CW, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70(21):1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- 17.National Institute of Health. CERE-120, an Adeno-Associated Virus-Based Vector to Deliver Human Neurturin to Parkinson's Disease Patients in a Phase II Trial. 2006 http://www.gemcris.od.nih.gov/Contents/GC_CLIN_TRIAL_RPT_VIEW.asp?WIN_TYPE=R&CTID=790.
- 18.Tuszynski MH, Thal L, Pay M, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11(5):551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 19.Recombinant DNA Advisory Committee. Serious, possibly associated and unexpected adverse events reported for human gene transfer protocols. 2002 http://oba.od.nih.gov/oba/rac/SAE_rpts/Mod0602s/MODs_06_02.pdf.
- 20.Anderson JA, Kimmelman J. Extending clinical equipoise to phase 1 trials involving patients: unresolved problems. Kennedy Inst Ethics J. 2010;20(1):75–98. doi: 10.1353/ken.0.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimmelman JK, London AJ. Predicting Harms and Benefits in Translational Trials: Ethics, Evidence, and Uncertainty. PLoS Med. 2011 doi: 10.1371/journal.pmed.1001010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.