Abstract
Objective
To determine the correspondence of in vivo quantitative estimates of brain uptake of [18F]-flutemetamol with immunohistochemical estimates of amyloid levels in previously biopsied patients.
Design
Cross-sectional study of [18F]-flutemetamol positron emission tomography (PET) findings in patients with prior cortical biopsy stained for the presence or absence of amyloid plaques.
Setting
University Hospital.
Patients
Seven patients who previously had a prior right frontal cortical biopsy obtained at the site of ventriculo-peritoneal (VP) placement for presumed Normal Pressure Hydrocephalus (NPH) were recruited. Inclusion criteria included an adequate biopsy for detection and quantification of Aβ pathology and age greater than 50 years.
Intervention
All patients underwent an [18F]-flutemetamol PET scan.
Main Outcome Measures
Quantitative measures of [18F]-flutemetamol uptake (SUVR – ratio of mean target cortex activity divided by that in a cerabellar reference region) were made at a location contralateral to the biopsy site and compared to estimates of amyloid load based on immunohistochemical and histological staining.
Results
There was complete agreement between visual reads of [18F]-flutemetamol PET scans (three blinded readers with majority rule) and histology. A regression model, including time from biopsy as a covariate, demonstrated a significant relationship (p=0.011) between [18F]-flutemetamol uptake and percent area of amyloid measured by a monoclonal antibody raised against amyloid (NAB228). Similar results were found with the amyloid specific monoclonal antibody 4G8 and Thioflavin S.
Conclusions
These data are the first to demonstrate the concordance of [18F]-flutemetamol PET imaging with histopathology, supporting its sensitivity to detect amyloid and potential use in the study and detection of Alzheimer’s Disease.
Introduction
The development of molecular imaging techniques to visualize the pathology of Alzheimer’s Disease (AD) in vivo represents a major achievement for the diagnosis and study of neurodegenerative conditions. In addition to a potential role in early and accurate diagnosis in clinical practice, molecular imaging is likely to play a critical role in drug development through cohort enrichment by increasing diagnostic confidence, clarification of drug mechanism and dosage, and assessment of biological effect. Over the last decade, several positron emission tomography (PET) radioligands have displayed affinity for fibrillar β-amyloid (Aβ) aggregates (1–5), one of the hallmark pathologic lesions of AD.
To date, [11C]-Pittsburgh Compound B ([11C]-PIB; 2-[4-methylamino phenyl]-1, 3-benzothiazol-6-ol) is the most well studied of these PET ‘amyloid imaging’ ligands (6). PiB is a neutral analogue of Thioflavin T, an established histological stain for detecting Aβ. [11C]-PiB has demonstrated success in discriminating patients with probable AD from healthy controls and predicting the likelihood of clinical progression in those with Mild Cognitive Impairment (MCI) to frank AD (2, 7–13). Additionally, [11C] -PiB uptake inversely correlates with levels of Aβ42 in the cerebrospinal fluid and Aβ aggregates in a limited number of post-mortem and biopsy studies (14–18). Despite the considerable promise of [11C]-PIB, its potential for wide-spread use is severely limited by the short half-life of carbon-11 (~20 minutes), which requires on-site cyclotron production.
The radionuclide fluorine-18 has a longer half-life (~110 minutes), allowing for shipment to scanning sites after production, and has been successfully employed in other PET tracers (e.g. fluorodeoxyglucose). Several [18F]-based amyloid PET imaging ligands with divergent structural properties from [11C]-PIB are at various stages of development (1, 4, 5), including recent work demonstrating a high concordance between [18F]-florbetapir and postmortem histology (19). Alternatively, the novel ligand [18F]-flutemetamol only differs from [11C]-PIB with regard to the radionuclide. Recent work has suggested that [18F]-flutemetamol demonstrates similar imaging findings in patients with probable AD and MCI relative to healthy controls and has a high correlation with [11C]-PIB when tested in the same patients (20). However, histopathological confirmation of the in vivo findings of this compound are lacking.
Normal pressure hydrocephalus (NPH) is a progressive condition associated with a classic triad of dementia, gait abnormalities, and urinary incontinence. Despite its apparent distinctiveness, this condition can be difficult to diagnose and the presence of AD pathology may alter response to ventricular shunting. Prior work has suggested that a significant proportion of patients with a presentation consistent with NPH demonstrate AD pathological lesions with biopsy (21, 22). Indeed, in a prior series, we found that ~ 68% of NPH patients had evidence of AD pathology in a small cortical biopsy obtained at the site of ventriculo-peritoneal (VP) shunt placement (23) and that higher levels of AD pathology were associated with poorer response to the surgery. Thus, amyloid imaging may be of diagnostic and prognostic value in such patients. In the current study, we leveraged pathologic data available from these previously biopsied NPH patients to assess concordance with in vivo [18F]-flutemetamol PET imaging.
Methods
Participants
Patients were recruited from the Penn Memory Center who had undergone VP shunting with concomitant biopsy. The majority participated in a previously reported longitudinal study of the impact of AD pathology on clinical response to shunting (23). In addition to a biopsy that was adequate for detection and quantification of Aβ pathology, inclusion criteria included age > 50 years and general health appropriate for the study procedures. Patients were excluded if they had a contraindication to PET or MRI, were pregnant/lactating, or had a known or suspected hypersensitivity/allergy to [18F]-flutemetamol or to any of the excipients. Of 59 patients in our database with biopsy tissue available, 19 were ineligible (mostly due to death). Seven patients agreed (33 declined) to enter the study and underwent PET scans obtained approximately 3 to 45 months after biopsy. The study was approved by the institutional review board at the University of Pennsylvania. All patients and their designated representatives signed informed consent prior to participation.
Tissue Analysis
An approximately 0.5 cm3 piece of cortical tissue had been biopsied at the site of shunt entry in the right prefrontal cortex. Tissue was immersion fixed in neutral buffered formalin overnight, followed by paraffin embedding, sectioning and staining with hematoxylin and eosin for standard neuropathological examination and immunohistochemistry for neurodegenerative disease pathology. For this study, 6 micron-thick sections were cut from paraffin blocks and processed for immunohistochemical labeling, as previously described (24, 25), for Aβ plaques with NAB228, a monoclonal antibody raised against Aβ1-11 synthetic peptide (26), and 4G8, reactive to residues 17–24 (27). Adjacent sections were also histochemically stained with Thioflavin S.
All biopsy section slides were visually inspected for the microscopic presence or absence of Aβ plaques with the investigator (SEA) masked to case identity. For sections in which Aβ plaques were present, the amount of Aβ plaque deposition was determined using semi-automated computer-assisted microscopic image acquisition and image analysis with methods adapted from those previously described (28, 29). Briefly, high-resolution, grayscale photomontages of whole biopsy sections were acquired at 100X magnification via a Leica DMRB light microscope equipped with a Ludl MAC 2000 motorized stage and Retiga Exi/QEi digital camera driven by Image-Pro Plus software (Media Cybernetics Inc., Silver Springs, Maryland, USA). Photomontages of the entire section were acquired, and all gray matter was circumscribed manually as the region of interest. To measure the abundance of Aβ plaque deposits, we applied histogram-based optical density thresholding to delineate those tissue elements exceeding background levels of immunoreactivity by > 3 standard deviations above background, as well as a size and pixel contiguity threshold filter in order to delineate plaque-like profiles. The software calculated the total area of the plaques and expressed this as a percent of the area of the entire selected region of interest.
MRI and PET Image Acquisition
All patients underwent an MRI scan on a 3T Siemens Trio whole-body scanner (Erlangen, Germany) equipped with a product eight-channel array coil within 35 days of PET scanning. High resolution structural images were acquired with a 3D-MPRAGE (30) sequence at 1mm3 isotropic resolution.
Flutemetamol (18F) Injection was prepared and handled according to Good Manufacturing Practice at a PET manufacturing site and then transported to the University of Pennsylvania. Before administration, the suitability of each preparation was assessed by a number of quality control (QC) tests including radioactivity content and chemical purity by high performance liquid chromatography (HPLC). The radiopharmacist at the PET imaging site ensured that the correct activity was present in the injection syringe and that the product was used within the validity period. Subjects received Flutemetamol (18F) Injection under the direct supervision of study personnel. Each subject received an i.v. dose of Flutemetamol (18F) Injection (less than 10 μg of total [18F]-flutemetamol). The activity of a single administration of Flutemetamol (18F) Injection was approximately 185 MBq [(5 mCi); range: 111 MBq to 197 MBq].
Dynamic brain scanning was performed on an Allegro whole-body PET scanner (Phillips). The duration of the PET imaging was 30 minutes beginning approximately 90 minutes after the administration of Flutemetamol (18F) Injection. Dynamic PET scans of the whole brain were obtained in a single field of view. The dynamic PET data consisted of six 5-minute frames.
[18F]-flutemetamol PET Visual Image Analysis
Blinded visual reads were performed by three trained raters, two nuclear physicians and a senior medical physicist, with experience reading amyloid scans to determine a ‘positive’ or ‘negative’ scan for increased [18F]-flutemetamol uptake. Readers worked independently and visually assessed the level of [18F]-flutemetamol uptake in cortical regions with reference to the level of uptake in the cerebellum. The review methodology was to locate a sagittal view plane along the longitudinal fissure and move a few millimeters from the centre until the view plane encountered the medial gyri and sulci. Having completed one hemisphere, the opposite hemisphere was surveyed in the same manner. An axial review was then carried out working in the dorsal to ventral direction.
If readers found elevated cortical uptake in at least one region, with emphasis on frontal/anterior cinguli, posterior cinguli/precuneus and lateral temporal regions, the image would be rated as abnormal. Otherwise the image was rated as normal. In cases of disagreement, assignment was based on the majority verdict.
[18F]-flutemetamol PET Quantitative Image Analysis
From the dynamic PET data, a 30-minute summed PET image was derived. The reconstruction method was a 3D-Ramala giving an approximate spatial resolution of 6mm. The blinded visual assessment was based on the summed images in native space. For quantitative analysis, this summed PET image was co-registered with the subject’s MRI to define volumes of interest (VOIs). Two types of target VOIs were determined in the following manner: 1. a hollow VOI surrounding the excised tissue area (representing the most adjacent sampling achievable) and 2. a VOI sampling the same anatomical region on the contralateral side. Use of a contralateral site is based upon prior work demonstrating only small differences in PiB uptake for symmetrically placed VOIs in the left and right frontal cortices (31). Figure 1 shows the location of the biopsy in a [18F]-flutemetamol MPR view. A composite VOI was also calculated as the mean of several anatomic regions typically associated with significant amyloid plaque burden in AD (i.e. frontal cortex, anterior cingulate gyrus, posterior cingulate gyrus/precuneus, lateral-temporal cortex and parietal cortex), as previously described (20).
Figure 1.

MPR PET image showing location of biopsy. The volume of interest for the SUVR was taken from the equivalent position on the contralateral side.
Standardized uptake value ratios (SUVRs), defined as SUVVOI/SUVREF with SUV being the integrated activity over a given time period per unit of injected dose and body weight, were calculated for the VOIs. The reference region used for this study was the cerebellar cortex. Note that the pons was also used as a reference region to explore if the SUVR versus % area comparisons would show a higher degree of correlation. No significant improvement was observed and, as a result, these data were not included.
Statistical Analysis
To determine the relationship between [18F]-flutemetamol uptake and amyloid plaque burden on biopsy, both dichotomous (presence or absence) and continuous variable statistics were employed. A qualitative read of scans classified them as positive or negative and the microscopic inspection of tissue sections classified them as ‘normal’ (no Aβ plaques) or ‘abnormal’ (Aβ plaques present). For continuous variable analyses, regression models included percent area of Aβ plaque as the dependent variable while the PET SUVR measure for the VOI was the independent variable. A covariate for the time interval from biopsy to PET scan was included. All statistics were performed with SAS 9.2 and were pre-specified in the study protocol statistical analysis plan.
Results
Demographic data is presented in Table 1. Four patients displayed evidence of amyloid plaque pathology based on NAB228 and 4G8 immunohistochemical and Thioflavin-S histological staining (Figure 2). Corresponding [18F]-flutemetamol PET scans are also displayed.
Table 1.
Patient Characteristics
| Case | Age (yrs) | Gender | Education (yrs) | MMSE | Time from biopsy (months) | Amyloid on biopsy | Composite SUV |
|---|---|---|---|---|---|---|---|
| 1 | 78 | M | 16 | 28 | 33 | + | 2.13 |
| 2 | 75 | F | 12 | 12 | 46 | + | 2.48 |
| 3 | 82 | M | 12 | 25 | 42 | − | 1.39 |
| 4 | 75 | F | 12 | 23 | 14 | + | 2.74 |
| 5 | 66 | F | 12 | 17 | 26 | + | 2.62 |
| 6 | 56 | M | 12 | 29 | 22 | − | 1.34 |
| 7 | 66 | M | 18 | 27 | 4 | − | 1.34 |
| Mean | 70.4 | 12.4 | 23.0 | 26.6 |
Figure 2.

Representative NAB228 stained tissue is displayed for each of the seven patients in this study. Above each is an axial image of the corresponding 18F-flutemetamol PET scan. Scans from cases 1,2,4,& 5 were classified as ‘positive’ by the independent readers, while patients 3, 6 & 7 were classified as ‘negative’. These designations corresponded to ratings of the pathology.
There was high concordance for the visual reads across the three raters with disagreement on only one scan (this scan was rated as abnormal by two of the three raters). By majority verdict, 4/7 scans were considered positive. In each of these cases, the biopsy tissue contained amyloid plaque pathology. The three negative flutemetamol scans were all in patients without evidence of Aβ plaque pathology.
The regression model for NAB228 was statistically significant [F = 10.1, p < 0.05, R2 = 0.83, β = 0.63 (95% confidence interval: 0.24, 1.02)], and there was no apparent modulating influence of the interval from biopsy to the PET scan (p > 0.1). The relationship between these measures can be seen in Figure 3. Similar results were obtained when percent area estimates of Aβ were determined with 4G8 [model: F = 10.0, p < 0.05, R2 = 0.83; β = 2.00 (95% confidence interval: 0.76, 3.24)] and thioflavin S [model: F = 15.6, p < 0.05, R2 = 0.89; β = 0.56 (95% confidence interval: 0.28, 0.84)]. Ipsilateral VOI SUVR’s produced similar findings for 4G8 and thioflavin S (both p < 0.05) although the regression model with percent area of NAB228 staining did not reach significance (p=0.08). The composite VOI also produced similar results and is not presented here for brevity.
Figure 3.
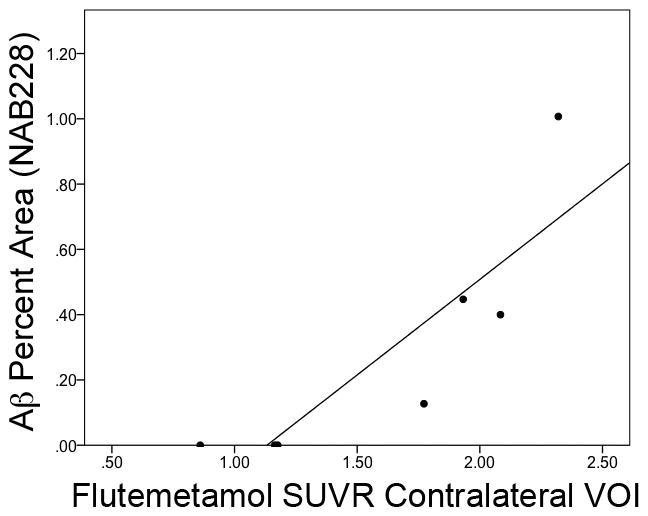
Relationship of estimated Aβ deposition in the biopsy sample (percent area of NAB228 monoclonal antibody) and 18F-flutemetamol PET uptake in the contralateral VOI.
Single doses of [18F]-flutemetamol was well tolerated. Five (71.4%) subjects experienced a total of 8 adverse events (AEs). The majority of the AEs reported were mild in intensity. Six (in 4 subjects) were considered to be possibly related/related to Flutemetamol (18F) Injection. The most frequently reported AE was increased blood pressure (5 subjects [71.4%] with 5 AEs). The remaining 3 AEs (dizziness, blood glucose decreased, and flushing) all occurred in 1 subject. The mild to moderate increases of blood pressure observed in 3 subjects resolved without treatment and were considered related to the administration of Flutemetamol (18F) Injection. No deaths, serious adverse events, or withdrawals due to adverse events occurred during the study. No clinically significant abnormalities were noted in clinical laboratory results. There were no clinically significant changes in ECG results.
Discussion
The present data represent the first study to compare the novel in vivo amyloid imaging ligand [18F]-flutemetamol with in vivo histopathological evidence of AD-related amyloid pathology. Despite variability in the delay from biopsy to PET scanning, there was a remarkable correspondence of [18F]-flutemetamol uptake and quantitative measures of amyloid pathology based on immunohistochemical and histological estimates. Additionally, masked visual assessments of these scans were consonant with the histopathology results. These findings are supportive of the potential role of [18F]-flutemetamol PET imaging in the detection of fibrillar Aβ plaques. As with [11C]-PiB, binding is expected to be more prominent to neuritic than diffuse plaques (16).
The current results are consistent with the still relatively limited, but accruing, histopathological data for [11C]-PIB PET imaging (14–17, 32, 33). For example, Ikonomovic and colleagues reported regional correlations between in vivo [11C]-PIB uptake and quantitative measures of Aβ pathology in a patient who had autopsy performed 10 months after PET imaging (16). Of most direct relevance to this report is a study in which right frontal cortical biopsies were obtained in 10 patients being evaluated for suspected NPH with intracranial pressure monitoring (17). Patients underwent [11C]-PIB scanning 2 to 36 months after biopsy. Overall, there was a reasonably high correlation of [11C]-PIB binding in the right frontal cortex and the number of Aβ aggregates on biopsy. Of the five cases without clear evidence of fibrillar amyloid pathology, none displayed elevated PiB uptake. Alternatively, four of five cases with evidence of Aβ pathology had a clearly abnormal [11C]-PiB scan, but there was one ‘false negative’ result. An additional ‘false negative’ [11C]-PiB study was recently reported in a patient whose scan was performed 2 ½ years prior to autopsy (33). The explanation for these ‘normal’ scans in the context of Aβ pathology remains unclear, but one potential explanation is that conformational variants of the secondary or tertiary structure of Aβ may influence PiB binding. Prolonged timing between imaging and autopsy may also contribute due to possible disease progression (34). To date, there have been no reports of ‘false positive’ [11C]-PiB studies based on histopathology.
Similar to this work with [11C]PiB, [18F]flutemetamol uptake also showed a strong association with quantitative measures of amyloid burden. This was the case for both a region surrounding the right frontal biopsy site and an analogous VOI in the contralateral hemisphere, consistent with the view that there generally is little asymmetry in amyloid pathology (31). These correlations are perhaps somewhat surprising given the substantial delay in the time from biopsy to the PET scan in several cases. However, this result is commensurate with the relatively slow change in amyloid levels that have been observed in prior [11C]-PiB PET longitudinal studies (35–37). Possibly of greatest relevance to potential clinical applicability of [18F]-flutemetamol PET scans was the accuracy of clinical reads relative to the presence or absence of Aβ pathology on the biopsy specimens. Such dichotomous visual reads is likely the strategy that will be employed in clinical practice, as with other imaging modalities.
Single doses of [18F] flutemetamol were well tolerated in NPH subjects. The mechanism and possible explanation of mild to moderate increases of blood pressure observed in 3 subjects, which resolved without treatment, is unknown and may be related to the patient reaction to study procedures (intravenous line placement, blood draws, etc.).
The current study has a number of limitations. Sample size was modest. The small cortical biopsy could have led to sampling error with presence of AD pathology in other brain regions despite an unremarkable biopsy. Additionally, the delay between the time from biopsy to PET scanning may have been associated with some patients developing AD pathology during that interval. The latter two issues would seemingly have resulted in ‘false positive’ scans relative to the biopsy findings, which would have presented interpretative difficulties. Nonetheless, there were no instances of such a result. As noted above, the lag between scan and biopsy also may have reduced the association between quantitative [18F]-flutemetamol uptake and biopsy determined amyloid load, particularly given differences in this delay across subjects and the potential for different rates of accumulation.
These findings provide support for the use of [18F]-flutemetamol PET in the detection of AD-related amyloid pathology. As has been suggested by work with [11C]PiB PET, this information may play an important prognostic role in patients with MCI, and, perhaps, in preclinical populations (10, 38, 39). With the potential emergence of disease specific interventions for AD, biomarkers that provide molecular specificity will likely become of greater importance in the differential diagnosis of cognitive impairment in older adults. Furthermore, amyloid imaging may serve an important role in the development of such interventions by allowing for assessment of biological effect (40). Finally, amyloid imaging agents such as [18F]-flutemetamol may be of particular relevance to the NPH population studied here given evidence that the presence of AD pathology in suspected NPH patients may be associated with a poorer outcome after ventricular shunt placement, which may impact decisions on treatment (23). For example, a ‘positive’ scan may lead to a clinical decision to avoid the expense and potential medical complications associated with shunt placement in a patient unlikely to obtain a significant benefit from the procedure. All of these potential roles for amyloid imaging agents will be greatly facilitated by the increased availability of fluorine-18 PET radioligands.
Acknowledgments
We acknowledge the staff of the i3 Statprobe, US, for biometric services, programming and statistical analyses. The authors wish to thank GE Healthcare study team members for operational support (Kim Mansfield), project management (Gill Farrar), data management (Ginger Lewis), and medical writing assistance with the study report (Ann Tate, Cris Devine).
References
- 1.Small GW, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355(25):2652–2663. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- 2.Klunk WE, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 3.Verhoeff NP, et al. In-vivo imaging of Alzheimer disease beta-amyloid with [11C]SB-13 PET. Am J Geriatr Psychiatry. 2004;12(6):584–595. doi: 10.1176/appi.ajgp.12.6.584. [DOI] [PubMed] [Google Scholar]
- 4.Rowe CC, et al. Imaging of amyloid beta in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008;7(2):129–135. doi: 10.1016/S1474-4422(08)70001-2. [DOI] [PubMed] [Google Scholar]
- 5.Lin KJ, et al. Whole-body biodistribution and brain PET imaging with [18F]AV-45, a novel amyloid imaging agent--a pilot study. Nucl Med Biol. 2010;37(4):497–508. doi: 10.1016/j.nucmedbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Wolk DA, Klunk W. Update on amyloid imaging: from healthy aging to Alzheimer’s disease. Curr Neurol Neurosci Rep. 2009;9(5):345–352. doi: 10.1007/s11910-009-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowe CC, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68(20):1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 8.Jack CR, Jr, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131(Pt 3):665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edison P, et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology. 2007;68(7):501–508. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- 10.Wolk DA, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65(5):557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowe CC, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Forsberg A, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008;29(10):1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Koivunen J, et al. PET amyloid ligand [11C]PIB uptake and cerebrospinal fluid beta-amyloid in mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;26(4):378–383. doi: 10.1159/000163927. [DOI] [PubMed] [Google Scholar]
- 14.Burack MA, et al. In vivo amyloid imaging in autopsy-confirmed Parkinson disease with dementia. Neurology. 2010;74(1):77–84. doi: 10.1212/WNL.0b013e3181c7da8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacskai BJ, et al. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch Neurol. 2007;64(3):431–434. doi: 10.1001/archneur.64.3.431. [DOI] [PubMed] [Google Scholar]
- 16.Ikonomovic MD, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131(Pt 6):1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leinonen V, et al. Assessment of beta-amyloid in a frontal cortical brain biopsy specimen and by positron emission tomography with carbon 11-labeled Pittsburgh Compound B. Arch Neurol. 2008;65(10):1304–1309. doi: 10.1001/archneur.65.10.noc80013. [DOI] [PubMed] [Google Scholar]
- 18.Fagan AM, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59(3):512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 19.Clark CM, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305(3):275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandenberghe R, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol. 2010;68(3):319–329. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- 21.Golomb J, et al. Alzheimer’s disease comorbidity in normal pressure hydrocephalus: prevalence and shunt response. J Neurol Neurosurg Psychiatry. 2000;68(6):778–781. doi: 10.1136/jnnp.68.6.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holm A, et al. Brain biopsy prior to treatment of Alzheimer’s disease. Minim Invasive Neurosurg. 2003;46(3):161–164. doi: 10.1055/s-2003-40733. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton R, et al. Lack of shunt response in suspected idiopathic normal pressure hydrocephalus with Alzheimer disease pathology. Ann Neurol. 2010;68(4):535–540. doi: 10.1002/ana.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das SR, et al. Structure specific analysis of the hippocampus in temporal lobe epilepsy. Hippocampus. 2009;19(6):517–525. doi: 10.1002/hipo.20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold SE, et al. Quantitative neurohistological features of frontotemporal degeneration. Neurobiol Aging. 2000;21(6):913–919. doi: 10.1016/s0197-4580(00)00173-1. [DOI] [PubMed] [Google Scholar]
- 26.Lee EB, et al. Secretion and intracellular generation of truncated Abeta in beta-site amyloid-beta precursor protein-cleaving enzyme expressing human neurons. J Biol Chem. 2003;278(7):4458–4466. doi: 10.1074/jbc.M210105200. [DOI] [PubMed] [Google Scholar]
- 27.Spillantini MG, et al. Different configurational states of beta-amyloid and their distributions relative to plaques and tangles in Alzheimer disease. Proc Natl Acad Sci U S A. 1990;87(10):3947–3951. doi: 10.1073/pnas.87.10.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett DA, et al. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61(3):378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 29.Soetanto A, et al. Association of anxiety and depression with microtubule-associated protein 2- and synaptopodin-immunolabeled dendrite and spine densities in hippocampal CA3 of older humans. Arch Gen Psychiatry. 2010;67(5):448–457. doi: 10.1001/archgenpsychiatry.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mugler JP, 3rd, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE) Magn Reson Med. 1990;15(1):152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- 31.Raji CA, et al. Characterizing regional correlation, laterality and symmetry of amyloid deposition in mild cognitive impairment and Alzheimer’s disease with Pittsburgh Compound B. J Neurosci Methods. 2008;172(2):277–282. doi: 10.1016/j.jneumeth.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villemagne VL, et al. 11C-PiB PET studies in typical sporadic Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry. 2009;80(9):998–1001. doi: 10.1136/jnnp.2008.171496. [DOI] [PubMed] [Google Scholar]
- 33.Cairns NJ, et al. Absence of PIttsburgh Compound B Detection of CerebralAmyloid Beta in a Patient With Clinical, Cognitive, and Cerebrospinal FluidMarkers of Alzheimer Disease. Arch Neurol. 2009;66(12):1557–1562. doi: 10.1001/archneurol.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klunk WE. Biopsy support for the validity of Pittsburgh compound B positron emission tomography with a twist. Arch Neurol. 2008;65(10):1281–1283. doi: 10.1001/archneur.65.10.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jack CR, Jr, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132(Pt 5):1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engler H, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129(Pt 11):2856–2866. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- 37.Klunk WE, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129(Pt 11):2805–2807. doi: 10.1093/brain/awl281. [DOI] [PubMed] [Google Scholar]
- 38.Jack CR, Jr, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storandt M, et al. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66(12):1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rinne JO, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9(4):363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]


