Structured Abstract
Objective
To examine the association between extent of surgery and overall survival (OS) in a large contemporary cohort of patients with papillary thyroid cancer (PTC).
Background
Guidelines recommend total thyroidectomy for PTC tumors >1 cm based on older data demonstrating an OS advantage for total thyroidectomy over lobectomy.
Methods
Adult patients with PTC tumors 1.0–4.0 cm undergoing thyroidectomy in the National Cancer Database, 1998–2006, were included. Cox proportional hazards models were applied to measure the association between extent of surgery and OS while adjusting for patient demographic and clinical factors, including comorbidities, extrathyroidal extension, multifocality, nodal and distant metastases, and radioiodine treatment.
Results
Among 61,775 PTC patients, 54,926 underwent total thyroidectomy and 6,849 lobectomy. Compared to lobectomy, total thyroidectomy patients had more nodal (7% vs. 27%), extrathyroidal (5% vs.16%), and multifocal disease (29% vs. 44%), all p<0.001. Median follow-up was 82 months (60–179 months). After multivariable adjustment, OS was similar for total thyroidectomy vs. lobectomy in patients with tumors 1.0–4.0 cm (HR 0.96 [0.84–1.09], p=0.54), and when stratified by tumor size: 1.0–2.0 cm (HR 1.05 [0.88–1.26], p=0.61) and 2.1–4.0 cm (HR 0.89 [0.73–1.07], p=0.21). Older age, male gender, black race, lower income, tumor size, and presence of nodal or distant metastases were independently associated with compromised survival (p<0.0001).
Conclusions
Current guidelines suggest total thyroidectomy for PTC tumors >1 cm. However, we did not observe a survival advantage associated with total thyroidectomy compared to lobectomy. These findings call into question whether tumor size should be an absolute indication for total thyroidectomy.
Introduction
Thyroid cancer is the most common malignancy of the endocrine system, with an estimated incidence of 60,220 in 2013 in the United States.1 Papillary thyroid cancer represents more than 90% of all thyroid cancer cases, and is the most indolent form of the disease.2 Prognosis is excellent, with 20-year survival surpassing 90% when appropriate therapy is undertaken.3 The mainstay of treatment for papillary thyroid cancer is surgical resection.
The current American Thyroid Association (ATA) guidelines recommend total or near-total thyroidectomy for papillary thyroid cancers >1 cm.4 This recommendation was supported by an analysis of population-level data from the National Cancer Database (NCDB) by Bilimoria et al. of 52,173 patients who either underwent total thyroidectomy or lobectomy for papillary thyroid cancer between 1985 and 1998. They found that total thyroidectomy was associated with better overall survival for papillary thyroid cancers tumors ≥1 cm, whereas extent of thyroid resection did not impact survival in patients with tumors <1 cm.5 However, a subsequent analysis of 22,724 papillary thyroid cancer patients (1988–2001) from the Surveillance, Epidemiology and End Results (SEER) database showed no survival difference between thyroid lobectomy vs. total thyroidectomy.6 These conflicting findings have reopened the debate regarding the issue of extent of surgery. Others have pointed out concerns with Bilimoria et al.’s multivariable analysis, as it did not account for potentially important factors such as comorbidities, multifocality, extrathyroidal extension, and completeness of resection.7, 8
Therefore, we sought to examine the association between extent of surgery and overall survival in relation to tumor size in a more contemporary NCDB cohort (1998–2006), while adjusting for patient demographic, clinical, and pathologic factors, including those that may have been confounders in prior studies.
METHODS
The National Cancer Database (NCDB) is a joint program of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The NCDB is a nationwide, facility-based, comprehensive clinical surveillance data set that currently captures 70% of all newly diagnosed malignancies in the United States. It was established in 1989 and currently contains more than 29 million cancer cases from more than 1500 CoC-accredited cancer programs from all 50 states, Puerto Rico, and the District of Columbia. More than 85% of all thyroid cancer cases in the U.S. are captured in the database.9
Data were coded according to the CoC Registry Operations and Data Standards Manual, the American Joint Committee for Cancer (AJCC) Manual for Staging of Cancer, and the International Classification of Disease for Oncology. To reduce data errors and maintain the integrity of the database, all data were extracted from medical records by trained and certified tumor registrars. Data were validated locally and at the NCDB level. Data were de-identified and submitted to the NCDB in compliance with the Health Insurance Portability and Accountability Act (HIPAA).10 The Duke University Institutional Review Board granted this study an exemption status.
The NCDB participant user file was used to identify all thyroid cancer patients who underwent thyroid surgery between 1998 and 2011. The following International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes 8050/3, 8260/3, 8340/3, 8341/3, 8342/3, 8343/3 were used to identify patients with papillary thyroid cancer. Aggressive histologic variants including columnar/tall cell, diffuse sclerosing, and insular variants were excluded from the study whenever identified. Patients <18 years and those who did not undergo surgery were excluded. Patients with multiple cancer diagnoses were excluded to ensure that outcomes were not confounded by other cancer diagnoses and/or treatment. Only patients with tumor size 1.0–4.0 cm were included. Participant registries report patient follow-up data to the NCDB in five-year intervals.11 Therefore, the study was further restricted to cases diagnosed up to 2006 to only include cases with a minimum postoperative follow-up period of 5 years.
Patient variables including age at diagnosis, race, gender, level of education, annual income, insurance status, type of insurance, year of diagnosis, distance travelled to treating institution, and comorbidity were extracted from the database. Comorbidity was represented by the modified Charlson/Deyo scoring system (1992).12 Education and annual income levels were assigned by NCDB by linking a patient’s ZIP code to the year 2000 U.S. Census data. Annual income data were extracted from the database. The NCDB reports median annual income data in the patient’s ZIP code in quartiles. The lower two quartiles represent median income of <$35,000 vs. ≥$35,000 for the top two quartiles.
The cohort was categorized based on extent of surgery into two groups: patients who underwent lobectomy and patients who underwent total thyroidectomy. The lobectomy group included patients who underwent lobectomy with or without an isthmusectomy. The total thyroidectomy group included patients who underwent total, near-total, or subtotal thyroid resection. Patients who underwent completion thyroidectomy were coded as having had a total thyroidectomy. Patients were excluded if they had removal of less than a lobe or when extent of surgery was not specified. Overall survival analyses were performed for all patients (tumors 1.0–4.0 cm) and for subgroups defined by tumor size: 1.0–2.0 cm and 2.1–4.0 cm.
Statistical Analyses
Baseline characteristics were reported using frequencies and proportions for categorical variables. Descriptive data were compared across groups using the Kruskal-Wallis test for continuous variables and Pearson χ2 or Fisher’s exact tests for categorical variables. Overall survival was defined from the time of diagnosis to time of death or last follow-up. Survival time was censored for patients alive at the end of the study period. Patients with zero months of follow-up were excluded. Estimates and 95% confidence intervals (CI) of overall survival proportions were computed using the Kaplan-Meier method, and survival distributions were compared across groups using the log-rank test.
A multivariable Cox proportional hazards model was used to examine the adjusted associations of extent of surgery and tumor size with overall survival. The model adjusted for the effects of patient, clinical, and tumor factors including age, gender, race, annual income, insurance status, hospital volume, patient comorbidities, tumor multifocality, extrathyroidal extension, lymph node involvement, distant metastases, surgical margin status, and radioactive iodine (RAI) treatment. The interaction of extent of surgery and tumor size was not included in the final model, as it was not significant after adjustment. Graphical aids, including Schoenfeld residuals, score progress plots and restricted cubic splines, were used to check the proportional hazards assumption of the variables included in the model.
In the NCDB database, subtotal thyroidectomy shares the same code as near-total thyroidectomy. Near-total thyroidectomy is an acceptable alternative to total thyroidectomy for the treatment of thyroid cancer; therefore, patients who had subtotal thyroidectomy could not be excluded. To examine the effect of including patients who had subtotal thyroidectomy on the multivariable results, a subset analysis was performed excluding patients who had subtotal or near-total thyroidectomy.
Comorbidity data were not available in the NCDB prior to 2003, and extrathyroidal extension and multifocality were not available prior to 2004. These three variables are clinically important for covariate adjustment, but have 50%–60% missing values from 1998 to 2006. To address this issue in the main analysis, an additional level was introduced for each of the three categorical variables above to represent missing data (e.g. data before 2004), and the variables were included in the model. Two sensitivity analyses were performed. The first sensitivity analysis modeled the effect of surgery on overall survival without adjusting for comorbidities, extrathyroidal extension and multifocality (i.e. all data from 1998–2006). A second sensitivity analysis was limited to complete case analysis (i.e. the three covariates were non-missing).
RESULTS
There were 171,073 thyroid cancer patients identified from the NCDB (1998–2006); 61,775 patients had papillary thyroid cancers 1.0–4.0 cm and met the study criteria for inclusion. Of these, 6,849 (11%) underwent thyroid lobectomy, and 54,926 (89%) had total thyroidectomy (Table 1). Of all study patients, 59% had tumors 1.0–2.0 cm, and 41% had tumors 2.1–4.0 cm. Compared to patients undergoing lobectomy, those who had total thyroidectomy encompassed more tumors with multifocal disease (29% vs. 44%), extrathyroidal extension (5% vs. 16%), lymph node involvement (7% vs. 27%), distant metastases (0.4% vs. 1.0%), positive surgical margin status (7% vs. 27%), and who received RAI treatment (33% vs. 65%), all p<0.01.
Table 1.
Patient demographic, clinical and pathologic characteristics by extent of surgery (1998–2006)
| Lobectomy (N=6849) | Total Thyroidectomy (N=54926) | P-value | |
|---|---|---|---|
| Female | 5556 (81.1%) | 43242 (78.7%) | <0.01 |
| Age (yrs) | <0.01 | ||
| <45 | 3498 (51.1%) | 29044 (52.9%) | |
| 45–64 | 2500 (36.5%) | 20183 (36.7%) | |
| ≥65 | 851 (12.4%) | 5699 (10.4%) | |
| Race | <0.01 | ||
| White | 5886 (87.6%) | 47471 (88.2%) | |
| Black | 463 (6.9%) | 2972 (5.5%) | |
| Asian | 270 (4.0%) | 2599 (4.8%) | |
| Other | 97 (1.4%) | 786 (1.5%) | |
| Annual income | <0.01 | ||
| <$35,000 | 1880 (29.1%) | 12594 (24.4%) | |
| ≥$35,000 | 4572 (70.9%) | 38992 (75.6%) | |
| Insurance status | NS | ||
| Not insured | 196 (2.9%) | 1384 (2.6%) | |
| Insured | 6450 (97.1%) | 51926 (97.4%) | |
| Comorbidity* | NS | ||
| 0 | 2796 (88.4%) | 26476 (89.3%) | |
| 1 | 311 (9.8%) | 2764 (9.3%) | |
| ≥2 | 56 (1.8%) | 408 (1.4%) | |
| Tumor size | NS | ||
| 1.0–2.0 cm | 4092 (59.7%) | 32541 (59.2%) | |
| 2.1–4.0 cm | 2757 (40.3%) | 22385 (40.8%) | |
| Multifocality** | <0.01 | ||
| No | 1609 (71.5%) | 12664 (56.2%) | |
| Yes | 642 (28.5%) | 9879 (43.8%) | |
| Extrathyroidal extension** | <0.01 | ||
| No | 3167 (94.7%) | 24759 (84.5%) | |
| Yes | 177 (5.3%) | 4545 (15.5%) | |
| Nodal metastasis | <0.01 | ||
| Absent*** | 6189 (90.4%) | 39468 (71.9%) | |
| Present | 479 (7.2%) | 14655 (27.1%) | |
| Distant metastasis | <0.01 | ||
| Absent | 6823 (99.6%) | 54368 (99.0%) | |
| Present | 26 (0.4%) | 558 (1.0%) | |
| Margin status | <0.01 | ||
| Negative | 1413 (21.2%) | 14191 (26.2%) | |
| Positive | 479 (7.2%) | 14655 (27.1%) | |
| RAI administered | 2165 (33.2%) | 33756 (65.3%) | <0.01 |
| Annual hospital case volume (median) | 8 (IQR 4–16) | 12 (IQR 6–22) | <0.01 |
IQRs: interquartile range.
Values are presented as percentages of given sample size. Percentages were rounded and may not add to 100 due to missing values.
Variable not collected by the NCDB before 2003; values are presented as percentages of the known (after 2003).
Variable not collected by the NCDB before 2004; values are presented as percentages of the known (after 2004).
Absent nodal disease, included those with no proven lymph node disease: either lymph nodes were not examined or negative when examined.
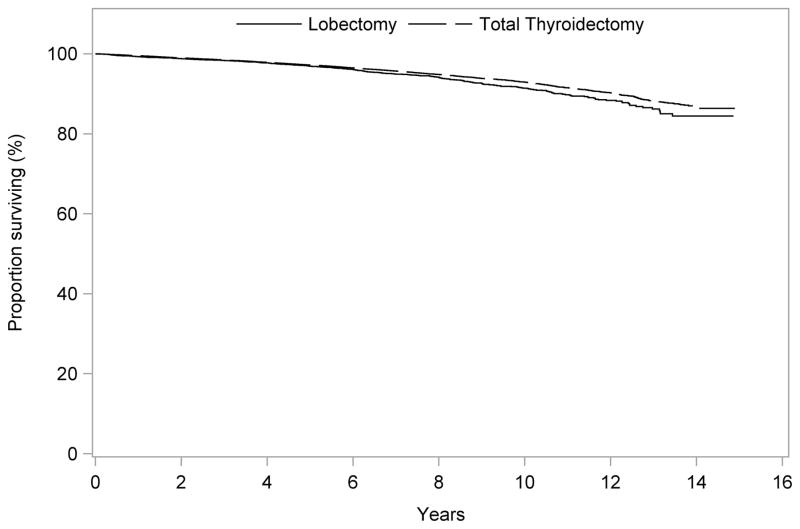
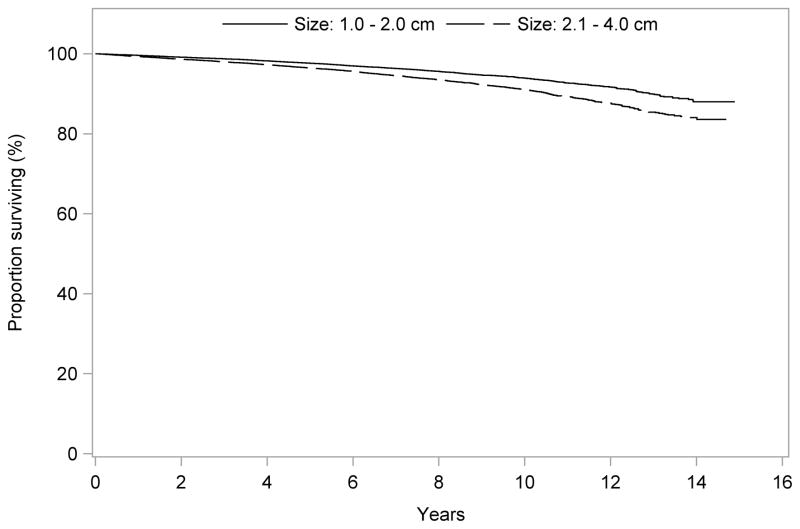
The median follow-up time was 82 months (range 60–179 months). Unadjusted overall survival was slightly better for patients who had total thyroidectomy compared to those who had lobectomy at 5 years (97.2% vs. 96.9%), 10 years (92.9% vs. 91.4%), and 14 years (86.6% vs. 84.4%), p=0.001 (Fig. 1). Overall survival was inversely related to tumor size both at 5 years (97.6% for tumors 1.0–2.0 cm, and 96.4% for tumors 2.1–4.0 cm), 10 years (94.0% for tumors 1.0–2.0 cm, and 91.0% for tumors 2.1–4.0 cm), and 14 years (88.0% vs. 84.1%), p<0.001 (Fig. 2).
Figure 1.
Unadjusted overall survival for patients undergoing total thyroidectomy vs. lobectomy for PTC tumors 1.0–4.0 cm
OS, overall survival
PTC, papillary thyroid carcinoma
Figure 2.
Unadjusted overall survival for patients with PTC tumors 1.0–2.0 cm vs. 2.1–4.0 cm
OS, overall survival
PTC, papillary thyroid carcinoma
After adjustment for patient demographic, clinical, and pathologic factors, overall survival was similar for total thyroidectomy compared to lobectomy in all patients (tumors 1.0–4.0 cm) [hazard ratio (HR) 0.96, 95% confidence interval (CI) 0.84–1.09, p=0.54]. When the analysis was stratified by tumor size, there were no significant differences in overall survival between total thyroidectomy and lobectomy for patients with tumors 1.0–2.0 cm (HR 1.05, CI 0.88–1.26, p=0.61) and 2.1–4.0 cm (HR 0.89, CI 0.73–1.07, p=0.21) (Table 2).
Table 2.
Cox Proportional Hazards model of all-cause mortality for patients with PTC tumors (1998–2006)
| Hazard ratio (95% CI) | |||
|---|---|---|---|
| Demographic factors | 1.0–2.0 cm | 2.1–4.0 cm | 1.0–4.0 cm |
| Patient age, per 10 years | 2.26 (2.16–2.36)* | 2.33 (2.23–2.43)* | 2.29 (2.22–2.36)* |
| Female gender | 0.68 (0.60–0.78)* | 0.68 (0.60–0.77)* | 0.68 (0.62–0.75)* |
| Race | |||
| White (Reference) | - | - | - |
| Black | 1.50 (1.20–1.89)* | 1.32 (1.06–1.66)* | 1.39 (1.19–1.63)* |
| Asian | 0.82 (0.59–1.15) | 0.83 (0.61–1.13) | 0.83 (0.67–1.04) |
| Other | 0.61 (0.25–1.48) | 1.01 (0.58–1.74) | 0.86 (0.54–1.38) |
| Annual household income | |||
| <$35,000 (Reference) | - | - | - |
| ≥$35,000 | 0.78 (0.69–0.89)* | 0.71 (0.63–0.81)* | 0.75 (0.68–0.82)* |
| Insurance status | |||
| Uninsured (Reference) | - | - | - |
| Insured | 0.81 (0.53–1.25) | 0.88 (0.57–1.36) | 0.86 (0.64–1.17) |
| Clinical factors | |||
| Charlson/Deyo score | |||
| 0 (Reference) | - | - | - |
| 1 | 1.69 (1.36–2.10)* | 1.57 (1.25–1.96)* | 1.64 (1.40–1.91)* |
| ≥2 | 4.16 (3.07–5.64)* | 3.40 (2.40–4.81)* | 3.83 (3.05–4.81)* |
| Tumor characteristics | |||
| Tumor size, per 0.1 cm | 1.01 (0.99–1.02) | 1.02 (1.01–1.03)* | 1.02 (1.01–1.02)* |
| Extrathyroidal extension | 1.01 (0.80–1.28) | 1.10 (0.89–1.36) | 1.05 (0.90–1.23) |
| Multifocality | 1.06 (0.86–1.31) | 1.19 (0.96–1.48) | 1.11 (0.96–1.29) |
| Nodal metastasis | 1.64 (1.36–1.98)* | 2.02 (1.67–2.45)* | 1.83 (1.61–2.10)* |
| Distant metastasis | 3.16 (2.30–4.35)* | 3.23 (2.51–4.15)* | 3.28 (2.69–3.99)* |
| Treatment characteristics | |||
| Total thyroidectomy | 1.05 (0.88–1.26) | 0.89 (0.73–1.07) | 0.96 (0.84–1.09) |
| Positive surgical margin | 1.39 (1.19–1.63)* | 1.37 (1.19–1.59)* | 1.38 (1.24–1.53)* |
| Radioiodine treatment | 0.77 (0.68–0.87)* | 0.86 (0.76–0.98)* | 0.81 (0.74–0.88)* |
| Hospital characteristics | |||
| Hospital surgery volume, per 10 cases per year | 1.00 (0.96–1.04) | 0.99 (0.95–1.04) | 0.99 (0.96–1.03) |
Indicates statistical significance with p-value ≤0.05
Reference=1
CI: Confidence interval.
CDCC: modified Charlson/Deyo score representing comorbidity; 0 indicates no comorbidity.
Model included patient age, gender, race, annual income, insurance status, hospital volume, comorbidity, nodal status, distant metastasis, extrathyroidal extension, multifocality, extent of surgery, margin status, and radioiodine treatment.
Patient factors independently associated with compromised overall survival included older patient age, male sex, black race, lower income, and higher comorbidity scores (Table 2). Tumor and treatment-related factors independently associated with compromised overall survival included increasing tumor size, presence of lymph node and distant metastases, positive surgical margins, and lack of RAI treatment.
Sensitivity Analysis
To examine the effect of subtotal thyroidectomy on the association of extent of surgery on overall survival, a subset-analysis was performed excluding those who had subtotal/near-total thyroidectomy from the total thyroidectomy cohort; 5109 patients were excluded. The findings did not change; there were no differences in overall survival between total thyroidectomy vs. lobectomy for patients with tumors 1.0–4.0 cm (HR 0.96, CI 0.84–1.09, p=0.52) or when stratified by size: 1.0–2.0 cm (HR 1.05, CI 0.88–1.27, p=0.57), and 2.1–4.0 cm (0.87, CI 0.72–1.06, p=0.17).
A second sensitivity analysis was conducted to assess the impact of modeling the missing values for comorbidity, multifocality and extrathyroidal extension on the association of extent of surgery and overall survival. The multivariable models were repeated excluding these variables. Again, there were no differences in overall survival between total thyroidectomy vs. lobectomy for patients with tumors 1.0–4.0 cm (HR 0.99, CI 0.76–1.29, p=0.94) or when stratified by size: 1.0–2.0 cm (HR 1.18, CI 0.82–1.70, p=0.36) and 2.1–4.0 cm (HR 0.76, CI 0.52–1.125, p=0.22).
A third sensitivity analysis limited to patients with no missing variables (diagnosed in 2004 or later, when all variables were collected) was performed; similarly, we found no difference in overall survival among patients with tumors 1.0–4.0 cm in size (HR 0.99, CI 0.76–1.29, p=0.94), or when stratified by size: 1.0–2.0 cm (HR 1.18, CI 0.82–1.697, p=0.36) and 2.1–4.0 cm (HR 0.76, CI 0.52–1.12, p=0.16).
DISCUSSION
In this study, we examine the association between extent of surgery and tumor size with overall survival in a contemporary cohort of patients with papillary thyroid cancer from the NCDB (1998–2006). After adjusting for patient comorbidities, tumor multifocality, extrathyroidal extension, nodal disease, distant metastasis, and completeness of resection, there was no survival advantage associated with undergoing total thyroidectomy over lobectomy for patients with tumors 1.0–4.0 cm. Upon further stratification, overall survival was similar between patients who underwent total thyroidectomy compared to lobectomy for tumors 1.0–2.0 cm and 2.1–4.0 cm. Our findings call into question whether tumor size alone should be an absolute determinant for deciding optimal extent of thyroid surgery for papillary thyroid cancer.
Before the 2009 ATA guidelines, extent of surgery for differentiated thyroid cancer was a matter of considerable debate, as there was no evidence documenting a survival advantage with either lobectomy or total thyroidectomy. Proponents of total thyroidectomy argue that complete resection of thyroid tissue affords the opportunity to use radioactive iodine for postoperative detection of residual or metastatic disease, as well as for treatment, and it facilitates use of serum thyroglobulin as a marker to detect residual disease and recurrence.13, 14 Hypothetically, employment of total thyroidectomy could also eliminate the possibility of undetected multifocal disease in the contralateral lobe.15 On the other hand, those who advocate for lobectomy point out that papillary thyroid carcinoma is an indolent disease with an excellent prognosis, and that patients should not be subjected to higher risks for thyroidectomy-related complications such as hypoparathyroidism and recurrent laryngeal nerve injury without a clear survival benefit.8
In our study, after multivariable adjustment for patient, clinical, and tumor factors, there was no survival advantage associated with total thyroidectomy vs. lobectomy in papillary thyroid cancer patients with tumors 1.0–4.0 cm at a median follow-up of 82 months (range 60–179 months). Even when performing stratified analyses for patients with tumors 1.0–2.0 cm and 2.1–4.0 cm, no significant differences in overall survival were detected between total thyroidectomy and lobectomy. Our findings are in disagreement with the previous study by Bilimoria et al. in which they analyzed data from 52,173 patients from the NCDB who either had lobectomy or total thyroidectomy for papillary thyroid cancer between 1985–1998. After a median follow-up of 70 months, they found an overall survival difference associated with total thyroidectomy over lobectomy for tumors ≥1 cm.5 In our study, in addition to having a longer median follow-up period, we were able to adjust for several important factors, such as patient comorbidities, which are known to have an impact on overall survival. In our study, comorbidity was a significant predictor of mortality. Tumor characteristics, such as extrathyroidal extension, multifocality, and completeness of surgical resection, were included in our multivariable models. These are well-known prognostic factors in thyroid cancer, and their presence correlates well with disease-specific mortality.6, 7, 16, 17 The inclusion of these factors in our multivariable models strengthens our ability to make valid comparisons between surgery types. In addition, our analysis represents a more recent cohort from the NCDB (1998–2006 vs.1985–1998); completeness of data collection by participating registries and accuracy of administrative databases are expected to improve over time. The NCDB increased data quality-assurance measures starting in 2002.18
Our findings is consistent with a more recent report from the SEER database by Mendelsohn et al. in which they analyzed data of 22,724 patients with papillary thyroid cancer (1988–2001). They demonstrated that there is no survival difference between lobectomy and total thyroidectomy.6 Single institutional data also have failed to show a significant survival difference between patients undergoing total thyroidectomy vs. lobectomy for papillary thyroid cancer.19, 20 Shah et al. analyzed 931 patients with differentiated thyroid cancer who underwent surgical treatment at a single institution. Patients undergoing lobectomy were matched to those who had total thyroidectomy based on patient age, histology, tumor size, extrathyroidal extension, nodal disease, and distant metastases. This matched-pair analysis revealed no disease-specific survival difference between total thyroidectomy and lobectomy at 20 years.20
The limitations of our study include those inherent to studies from large databases such as the potential for coding errors. However, data reporting to the NCDB is highly standardized and heavily audited.21 Given the fact that the database does not include information on disease recurrence or cause-specific mortality, these could not be included in our analysis. The strengths of our study lie in the large number of patients analyzed and the fact that it encompasses contemporary population-level data. Previous institutional studies have been limited by small numbers of patients, precluding adequate multivariable adjustment.
This study provides valuable information regarding the role of tumor size in determining extent of thyroid surgery for papillary thyroid carcinoma. Although current guidelines suggest that tumor size alone should dictate extent of surgery, we believe that tumor size is just one factor that should be considered in deciding the optimal extent of surgery. Total thyroidectomy may be indicated for many patients, including those who require radioactive iodine in the adjuvant setting, and those who have other high-risk features, such as extrathyroidal extension, nodal metastases, or distant metastases. However, total thyroidectomy is associated with higher risks of hypoparathyroidism and recurrent laryngeal nerve injury. This study may inform physicians and patients about overall survival benefits; the decision to perform lobectomy vs. total thyroidectomy should be done accounting for several important patient and disease factors, in order to optimize quality of life.
Footnotes
Data were presented at the 134th Annual Meeting of the American Surgical Association, April 11, 2014, Marriott Copley Place, Boston, Massachusetts.
The data used in the study are derived from a de-identified National Cancer Database (NCDB) file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
The authors declare no conflict of interest.
References
- 1.American Cancer Society. [Accessed March 01, 2014];Cancer Facts & Figures. 2013 Available at: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf.
- 2.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. Thyroid cancer survival in the United States: observational data from 1973 to 2005. Arch Otolaryngol Head Neck Surg. 2010;136:440–444. doi: 10.1001/archoto.2010.55. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 5.Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375–381. doi: 10.1097/SLA.0b013e31814697d9. discussion 381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendelsohn AH, Elashoff DA, Abemayor E, et al. Surgery for papillary thyroid carcinoma: is lobectomy enough? Arch Otolaryngol Head Neck Surg. 2010;136:1055–1061. doi: 10.1001/archoto.2010.181. [DOI] [PubMed] [Google Scholar]
- 7.Shah JP. Re: Extent of surgery affects papillary thyroid cancer. Ann Surg. 2008;247:1082–1083. doi: 10.1097/SLA.0b013e3181758d93. author reply 1083–1084. [DOI] [PubMed] [Google Scholar]
- 8.Shaha AR. Extent of surgery for papillary thyroid carcinoma: the debate continues: comment on “surgery for papillary thyroid carcinoma”. Arch Otolaryngol Head Neck Surg. 2010;136:1061–1063. doi: 10.1001/archotol.136.11.1061. [DOI] [PubMed] [Google Scholar]
- 9.Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488–490. doi: 10.1002/jso.21173. [DOI] [PubMed] [Google Scholar]
- 10.Phillips JKSA, editor. Facility Oncology Data Standards. Chicago, IL: Commission on Cancer; 2006. [Google Scholar]
- 11.Commission on Cancer, American College of Surgeons. [Accessed March 15, 2013];NCDB PUF Getting Started. 2010 Available at: http://www.facs.org/cancer/ncdb/pufgettingstarted.pdf.
- 12.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 13.DeGroot LJ, Kaplan EL, McCormick M, et al. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990;71:414–424. doi: 10.1210/jcem-71-2-414. [DOI] [PubMed] [Google Scholar]
- 14.Kebebew E, Clark OH. Differentiated thyroid cancer: “complete” rational approach. World J Surg. 2000;24:942–951. doi: 10.1007/s002680010165. [DOI] [PubMed] [Google Scholar]
- 15.Pasieka JL, Thompson NW, McLeod MK, et al. The incidence of bilateral well-differentiated thyroid cancer found at completion thyroidectomy. World J Surg. 1992;16:711–716. doi: 10.1007/BF02067365. discussion 716–717. [DOI] [PubMed] [Google Scholar]
- 16.Hay ID, Bergstralh EJ, Grant CS, et al. Impact of primary surgery on outcome in 300 patients with pathologic tumor-node-metastasis stage III papillary thyroid carcinoma treated at one institution from 1940 through 1989. Surgery. 1999;126:1173–81. doi: 10.1067/msy.2099.101435. discussion 1181–1182. [DOI] [PubMed] [Google Scholar]
- 17.Andersen PE, Kinsella J, Loree TR, et al. Differentiated carcinoma of the thyroid with extrathyroidal extension. Am J Surg. 1995;170:467–70. doi: 10.1016/s0002-9610(99)80331-6. [DOI] [PubMed] [Google Scholar]
- 18.Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaha AR, Shah JP, Loree TR. Low-risk differentiated thyroid cancer: the need for selective treatment. Ann Surg Oncol. 1997;4:328–333. doi: 10.1007/BF02303583. [DOI] [PubMed] [Google Scholar]
- 20.Shah JP, Loree TR, Dharker D, et al. Lobectomy versus total thyroidectomy for differentiated carcinoma of the thyroid: a matched-pair analysis. Am J Surg. 1993;166:331–335. doi: 10.1016/s0002-9610(05)80326-5. [DOI] [PubMed] [Google Scholar]
- 21.Winchester DP, Stewart AK, Phillips JL, et al. The national cancer database: past, present, and future. Ann Surg Oncol. 2010;17:4–7. doi: 10.1245/s10434-009-0771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]